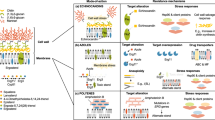
Key Points
-
Invasive fungal infections are one of the most devastating consequence of the rapidly increasing number of immunocompromised patients. Despite the rising incidence and mortality of infections with Cryptococcus neoformans, Candida albicans and Aspergillus fumigatus, the antifungal armamentarium remains limited, and novel targets focused on fundamental molecular pathogenesis are needed.
-
The calcineurin pathway is a conserved stress-response signalling pathway that has revolutionized today's immunosuppression. The calcineurin inhibitors FK506 and cyclosporine A (CsA) bind to human calcineurin and block signal transduction and T-cell activation, leading to efficient immunosuppression that can prevent organ rejection. Additionally, calcineurin inhibitors have been used clinically to treat a myriad of other conditions.
-
In this article, the authors propose that inhibiting fungal calcineurin pathways could be an effective method of halting the growth of invasive fungal pathogens, thereby preventing or treating disease. The two currently available inhibitors FK506 and CsA delivered in their present intravenous formulations will not solve this problem, as they possess inherent and well-utilized cross-reactive human immunosuppressive activity. However, molecular dissection of the fungal calcineurin pathway is yielding promising results and concepts for new drug development.
-
Current calcineurin inhibitors have been shown to possess antifungal activity against the major fungal pathogens, but under different conditions. Several calcineurin pathway genes in each of the major human fungal pathogens have also been disrupted and show effects on fungal growth and virulence. However, the effects are not uniform, highlighting that while the pathway is genetically conserved, the divergent disruption phenotypes require each individual pathogen to be studied in detail.
-
In C. neoformans, calcineurin inhibition leads to temperature-sensitive growth and fungal clearing in animal models and at the relevant temperatures in patients. Additionally, calcineurin inhibition leads to defects in hyphal elongation and therefore the inability to mate. In C. albicans, calcineurin A is required for growth under stress, growth in serum and virulence in systemic animal models. In A. fumigatus calcineurin A is not essential, in contrast to its essential role in the less pathogenic A. nidulans. Disruption of calcineurin A in A. fumigatus yielded extremely blunted hyphae that were unable to invade animal tissue and resulted in near avirulence in multiple animal models.
-
Molecular findings over the past decade have pointed to the fungal calcineurin pathway as a novel antifungal target with impressive effects at halting growth and inhibiting virulence in the three major fungal pathogens that affect immunocompromised patients. The future will hold further detailed molecular dissection of the pathway to optimize fungal killing and effectively harness the calcineurin pathway to both prevent and treat invasive fungal infections.
Abstract
The number of immunocompromised patients with invasive fungal infections continues to increase and new antifungal therapies are not keeping pace with the growing incidence of these infections and their associated mortality. Calcineurin inhibition is currently used to exert effective immunosuppression following organ transplantation and in treating various other conditions. However, the calcineurin pathway is also intricately involved in the growth and pathogenesis of the three major fungal pathogens of humans, Cryptococcus neoformans, Candida albicans and Aspergillus fumigatus, and the exploitation of fungal calcineurin pathways holds great promise for the future development of novel antifungal agents. This Review summarizes our current understanding of calcineurin biology in these fungal species, and its exciting potential role in treating invasive fungal infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kidd, S. E. et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl Acad. Sci. USA 101, 17258–17263 (2004).
Groll, A. H. et al. Trends in postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33, 23–32 (1996).
Edmond, M. B. et al. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29, 239–244 (1999).
Marr, K. A., Carter, R. A., Crippa, F., Wald, A. & Corey, L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34, 909–917 (2002).
Chayakulkeeree, M. & Perfect, J. R. Cryptococcosis. Infect. Dis. Clin. North Am. 20, 507–544 (2006).
Wilson, L. S. et al. The direct cost and incidence of systemic fungal infections. Value Heath 5, 26–34 (2002).
Cutler, J. E., Deepe, G. S. Jr & Klein, B. S. Advances in combating fungal diseases: vaccines on the threshold. Nature Rev. Microbiol. 5, 13–28 (2007).
Klee, C. B., Crouch, T. H. & Krinks, M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc. Natl Acad. Sci. USA 76, 6270–6273 (1979). The first identification of calcineurin signalling as an important signal transduction pathway.
Fox, D. S. & Heitman, J. Good fungi gone bad: the corruption of calcineurin. Bioessays 24, 894–903 (2002).
Desdouits, F., Siciliano, J. C., Greengard, P. & Girault, J. A. Dopamine- and cAMP-regulated phosphoprotein DARPP-32: phosphorylation of Ser-137 by casein kinase I inhibits dephosphorylation of Thr-34 by calcineurin. Proc. Natl Acad. Sci. USA 92, 2682–2685 (1995).
Dawson, T. M. et al. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc. Natl Acad. Sci. USA 90, 9808–9812 (1993).
Fraser, E. D. & Walsh, M. P. Dephosphorylation of calponin by type 2B protein phosphatase. Biochemistry 34, 5561–5568 (1995).
Conboy, I. M., Manoli, D., Mhaiskar, V. & Jones, P. P. Calcineurin and vacuolar-type H+-ATPase modulate macrophage effector functions. Proc. Natl Acad. Sci. USA 96, 6324–6329 (1999).
Satonaka, H. et al. Calcineurin promotes the expression of monocyte chemoattractant protein-1 in vascular myocytes and mediates vascular inflammation. Circulation Res. 94, 693–700 (2004).
Aperia, A., Ibarra, F., Svensson, L. B., Klee, C. & Greengard, P. Calcineurin mediates alpha-adrenergic stimulation of Na+, K+-ATPase activity in renal tubule cells. Proc. Natl Acad. Sci. USA 89, 7394–7397 (1992).
Clipstone, N. A. & Crabtree, G. R. Identification of calcineurin as a key signaling enzyme in T-lymphocyte activation. Nature 357, 695–697 (1992). This paper linked calcineurin to mammalian T-cell activation.
Hemenway, C. S. & Heitman, J. Calcineurin. Structure, function, and inhibition. Cell Biochem. Biophys. 30, 115–151 (1999).
Liu, J. et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815 (1991). Demonstrates that the calcineurin inhibitor–immunophilin complexes both bind to calcineurin as a common target.
Friedman, J. & Weissman, I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell 66, 799–806 (1991).
Heit, J. J. et al. Calcineurin/NFAT signalling regulates pancreatic β-cell growth and function. Nature 443, 345–349 (2006).
Graef, I. A., Chen, F., Chen, L., Kuo, A. & Crabtree, G. R. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105, 863–875 (2001).
Malleret, G. et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104, 675–686 (2001).
Yang, Y. et al. Reversible blockade of experience-dependent plasticity by calcineurin in mouse visual cortex. Nature Neurosci. 8, 791–796 (2005).
Flavell, S. W. et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synpase number. Science 311, 962–963 (2006).
Graef, I. A. et al. Neuroptrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell 113, 657–670 (2003).
Chang, C.-P. et al. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell 118, 649–663 (2004).
Liu, F. et al. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J. Biol. Chem. 280, 37755–37762 (2005).
Sussman, M. A. et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281, 1690–1693 (1998).
Shirane, M. & Nakayama, K. I. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nature Cell Biol. 5, 28–37 (2003).
Koga, T. et al. NFAT and osterix cooperatively regulate bone formation. Nature Med. 11, 880–885 (2005).
Semsarian, C. et al. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400, 576–581 (1999).
Storb, R. et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl. J. Med. 314, 729–735 (1986).
Remitz, A. et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. Acta Derm. Venereol. 87, 51–61 (2007).
Alexander, A. G., Barnes, N. C. & Kay, A. B. Trial of cyclosporin in corticosteroid-dependent chronic severe asthma. Lancet 339, 324–328 (1992).
Ponticelli, C. & Passerini, P. Alternative treatments for focal and segmental glomerulosclerosis. Clin. Nephrol. 55, 345–348 (2001).
Temekonidis, T. I. et al. Infliximab treatment in combination with cyclosporin A in patients with severe refractory rheumatoid arthritis. Ann. Rheum. Dis. 61, 822–825 (2002).
Jin, L. & Harrison, S. C. Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin. Proc. Natl Acad. Sci. USA 99, 13522–13526 (2002).
Huai, Q. et al. Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes. Proc. Natl Acad. Sci. USA 99, 12037–12042 (2002).
Kissinger, C. R. et al. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature 378, 641–644 (1995).
Griffith, J. P. et al. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell 82, 507–522 (1995).
Perfect, J. R. & Durack, D. T. Effects of cyclosporine in experimental cryptococcal meningitis. Infect. Immun. 50, 22–26 (1985).
Mody, C. H., Toews, G. B. & Lipscomb, M. F. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect. Immun. 56, 7–12 (1988).
Mody, C. H., Toews, G. B. & Lipscomb, M. F. Treatment of murine cryptococcosis with cyclosporin-A in normal and athymic mice. Am. Rev. Resp. Dis. 139, 8–13 (1989).
Odom, A., Del Poeta, M., Perfect, J. & Heitman, J. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685, 818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob. Agents Chemother. 41, 156–161 (1997).
Odom, A. et al. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16, 2576–2589 (1997). Shows that calcineurin A is needed for the virulence of C. neoformans.
Kojima, K, Bahn, Y. S. & Heitman, J. Calcineurin, MpK1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 152, 591–604 (2006).
Cruz, M. C., Fox, D. S. & Heitman, J. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20, 1020–1032 (2001).
Wang, P., Cardenas, M. E., Cox, G. M., Perfect, J. R. & Heitman, J. Two cyclophilin A homologs with shared and distinct functions for growth and virulence. EMBO Rep. 2, 511–518 (2001).
Kraus, P. R., Nichols, C. B. & Heitman, J. Calcium and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot. Cell 4, 1079–1087 (2005).
Gorlach, J. et al. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/Calcipressin, in Cryptococcus neoformans. EMBO J. 19, 3618–3629 (2000).
Fox, D. S. & Heitman, J. Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans. Eukaryot. Cell 4, 1526–1538 (2005).
Fox, D. S., Cox, G. M. & Heitman, J. Phospholipid-binding protein Cts1 controls septation and functions coordinately with calcineurin in Cryptococcus neoformans. Eukaryot. Cell 2, 1025–1035 (2003).
Kraus, P. R., Fox, D. S., Cox, G. M. & Heitman, J. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48, 1377–1387 (2003). Begins to characterize the link between the cell integrity pathway and the calcineurin pathway.
Cruz, M. C. et al. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 44, 143–149 (2000).
Del Poeta, M., Cruz, M. C., Cardenas, M. E., Perfect, J. R. & Heitman, J. Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743–873) with calcineurin inhibitors FK506 and L-685, 818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44, 739–746 (2000).
Fox, D. S. et al. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39, 835–849 (2001). Demonstrates that calcineurin A is required for hyphal elongation in C. neoformans.
Singh, N. et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J. Infect. Dis. 195, 756–764 (2007).
Singh, N. & Heitman, J. Antifungal attributes of immunosuppressive agents: new paradigms in management and elucidating the pathophysiologic basis of opportunistic mycoses in organ transplant recipients. Transplantation 77, 795–800 (2004).
Blankenship, J. R., Singh, N., Alexander, B. D. & Heitman, J. Cryptococcus neoformans isolates from transplant recipients are not selected for resistance to calcineurin inhibitors by current immunosuppressive regimens. J. Clin. Microbiol. 43, 464–467 (2005).
Martin, G. S., Mannino, D. M., Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003).
Husain, S. et al. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation 75, 2023–2029 (2003).
Cruz, M. C. et al. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21, 546–559 (2002).
Bader, T., Bodendorfer, B., Schroppel, K. & Morschhauser, J. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 71, 5344–5354 (2003).
Blankenship, J. R. et al. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2, 422–430 (2003). Shows that calcineurin is required for survival in serum in C. albicans , but displays different attributes than in C. neoformans.
Sanglard, D., Ischer, F., Marchetti, O., Entenza, J. & Bille, J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48, 959–976 (2003).
Bader, T. et al. Role of calcineurin in stress resistance, morphogenesis, and virulence of a Candida albicans wild-type strain. Infect. Immun. 74, 4366–4369 (2006). Shows that calcineurin A is required for the virulence of C. albicans.
Berman, J. & Sudbery, P. E. Candida albicans: a molecular revolution built on lessons from budding yeast. Nature Rev. Genet. 3, 918–930 (2002).
Calderone, R. A. & Fonzi, W. A. Virulence factors of Candida albicans. Trends Microbiol. 9, 327–335 (2001).
Cutler, J. E. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45, 187–218 (1991).
Felk, A. et al. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70, 3689–3700 (2002).
Filler, S. G., Swerdloff, J. N., Hobbs, C. & Luckett, P. M. Penetration and damage of endothelial cells by Candida albicans. Infect. Immun. 63, 976–983 (1995).
Lo, H. J. et al. Nonfilamentous C. albicans mutants are avirulent. Cell 90, 939–949 (1997).
Mitchell, A. P. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1, 687–692 (1998).
Naglik, J., Albrecht, A., Bader, O. & Hube, B. Candida albicans proteinases and host/pathogen interactions. Cell. Microbiol. 6, 915–926 (2004).
Sundstrom, P. Adhesins in Candida albicans. Curr. Opin. Microbiol. 2, 353–357 (1999).
Sundstrom, P., Balish, E. & Allen, C. M. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J. Infect. Dis. 185, 521–530 (2002).
Karababa, M. et al. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59, 1429–1451 (2006).
Onyewu, C., Wormley, F. L. Jr, Perfect, J. R. & Heitman, J. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 72, 7330–7333 (2004). The calcineurin downstream effector Crz1 is related to azole tolerance but is not essential for the virulence of C. albicans.
Santos, M. & de Larrinoa, I. F. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 48, 88–100 (2005).
Boustany, L. M. & Cyert, M. S. Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 16, 608–619 (2002).
Cyert, M. S. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311, 1143–1150 (2003).
Matheos, D. P., Kingsbury, T. J., Ahsan, U. S. & Cunningham, K. W. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11, 3445–3458 (1997).
Mendizabal, I., Pascual-Ahuir, A., Serrano, R. & de Larrinoa, I. F. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Genet. Genom. 265, 801–811 (2001).
Polizotto, R. S. & Cyert, M. S. Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J. Cell Biol. 154, 951–960 (2001).
Stathopoulos, A. M. & Cyert, M. S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11, 3432–3444 (1997).
Stathopoulos-Gerontides, A., Guo, J. J. & Cyert, M. S. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13, 798–803 (1999).
Yoshimoto, H. et al. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277, 31079–31088 (2002).
Pardini, G. et al. The CRH family coding for cell wall glycosylphosphatidylinositol proteins with a predicted transglycosidase domain affects cell wall organization and virulence of Candida albicans. J. Biol. Chem. 281, 40399–40411 (2006).
Cowen, L. E., Carpenter, A. E., Matangkasombut, O., Fink, G. R. & Lindquist, S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell 5, 2184–2188 (2006).
Heath, V. L., Shaw, S. L., Roy, S. & Cyert, M. S. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot. Cell 3, 695–704 (2004).
Marchetti, O., Moreillon, P., Glauser, M. P., Bille, J. & Sanglard, D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44, 2373–2381 (2000).
Marchetti, O. et al. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44, 2932–2938 (2000). An animal model demonstration of the synergy between fluconazole and a calcineurin inhibitor.
Onyewu, C., Blankenship, J. R., Del Poeta, M. & Heitman, J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47, 956–964 (2003).
Vanden Bosche, H., Willemsens, G. & Marichal, P. Anti-Candida drugs — the biochemical basis for their activity. Crit. Rev. Microbiol. 15, 57–72 (1987).
Pfaller, M. A. et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. The SENTRY Participant Group. J. Clin. Microbiol. 36, 1886–1889 (1998).
Marchetti, O. et al. Fungicidal synergism of fluconazole and cyclsoporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrob. Agents Chemother. 47, 1565–1570 (2003).
Reedy, J. L. et al. Immunotherapy with tacrolimus (FK506) does not select for resistance to calcineurin inhibitors in Candida albicans isolates from liver transplant patients. Antimicrob. Agents Chemother. 50, 1573–1577 (2006).
Becker, J. W. et al. FK-506-binding protein: three dimensional structure of the complex with the antagonist L-685, 818. J. Biol. Chem. 25, 11335–11339 (1993).
Rotonda, J. et al. Improved calcineurin inhibition by yeast FKBP12-drug complexes. Crystallographic and functional analysis. J. Biol. Chem. 268, 7607–7609 (1993).
Onyewu, C., Afshari, N. A. & Heitman, J. Calcineurin promotes infection of the cornea by Candida albicans and can be targeted to enhance fluconazole therapy. Antimicrob. Agents Chemother. 50, 3963–3965 (2006).
Cowen, L. E. & Lindquist, S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309, 2185–2189 (2005). Shows that heat shock protein 90 and calcineurin appear to be linked for optimizing antifungal activity.
Roe, S. M. et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42, 260–266 (1999).
Heitman, J. Cell biology. A fungal Achilles' heel. Science 309, 2175–2176 (2005).
McNeil, M. M. et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin. Infect. Dis. 33, 641–647 (2001).
Talbot, G. H. et al. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42, 657–668 (2006).
Rasmussen, C. et al. The calmodulin-dependent protein phosphatase catalytic subunit (calcineurin A) is an essential gene in Aspergillus nidulans. EMBO J. 13, 2545–2552 (1994).
Rasmussen, C., Means, R. L., Lu, K. P., May, G. S. & Means, A. R. Characterization and expression of the unique calmodulin gene of Aspergillus nidulans. J. Biol. Chem. 265, 13767–13775 (1990).
Lu, K. P., Rasmussen, C. D., May, G. S. & Means, A. R. Cooperative regulation of cell proliferation by calcium and calmodulin in Aspergillus nidulans. Mol. Endocrinol. 6, 365–374 (1992).
Juvvadi, P. R., Arioka, M., Nakajima, H. & Kitamoto, K. Cloning and sequencing analysis of cnaA gene encoding the catalytic subunit of calcineurin from Aspergillus oryzae. FEMS Microbiol. Lett. 204, 169–174 (2001).
Juvvadi, P. R., Kuroki, Y., Arioka, M., Nakajima, H. & Kitamoto, K. Functional analysis of the calcineurin-encoding gene cnaA from Aspergillus oryzae: evidence for its putative role in stress adaptation. Arch. Microbiol. 179, 416–422 (2003).
Derkx, P. M. F. & Madrid, S. M. The Aspergillus niger cypA gene encodes a cyclophilin that mediates sensitivity to the immunosuppressant cyclosporin A. Mol. Genet. Genom. 266, 527–536 (2001).
Jayashree, T., Rao, J. P. & Subramanyam, C. Regulation of aflatoxin production by Ca2+ /calmodulin-dependent protein phosphorylation and dephosphorylation. FEMS Microbiol. Lett. 183, 215–219 (2000).
Steinbach, W. J. et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5, 1091–1103 (2006). Shows that calcineurin A is not essential in A. fumigatus , in contrast to A. nidulans (Ref. 106 ), but is crucial for hyphal growth and virulence.
Ferreira, M. E. et al. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44, 219–230 (2007).
da Silva Ferreira, M. E. et al. The akuBKU80 mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5, 207–211 (2005).
High, K. P. The antimicrobial activities of cyclosporine, FK506, and rapamycin. Transplantation 57, 1689–1700 (1994).
Bell, N. P., Karp, C. L., Alfonso, E. C., Schiffman, J. & Miller, D. Effects of methylprednisolone and cyclosporine A on fungal growth in vitro. Cornea 18, 306–313 (1999).
Lugardon, K. et al. Structural and biological characterization of chromofungin, the antifungal chromogranin A-(47–66)-derived peptide. J. Biol. Chem. 276, 35875–35882 (2001).
Kontoyiannis, D. P., Lewis, R. E., Osherov, N., Albert, N. D. & May, G. S. Combination of caspofungin with inhibitors of the calciuneurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 51, 313–316 (2003).
Steinbach, W. J. et al. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48, 1664–1669 (2004). Showed that antifungal activity against A. fumigatus can be rendered fungicidal by the addition of a calcineurin inhibitor.
Steinbach, W. J. et al. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob. Agents Chemother. 48, 4922–4925 (2004).
Singh, N. et al. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin. Infect. Dis. 36, 46–52 (2003). Demonstrated the epidemiological observation that the use of calcineurin inhibitors in transplant recipients can lead to decreased invasive fungal infections (see also Ref. 57).
Berenguer, J. et al. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Resp. Crit. Care Med. 152, 1079–1086 (1995).
High, K. P. & Washburn, R. G. Invasive aspergillosis in mice immunosuppressed with cyclosporin A, tacrolimus (FK506), or sirolimus (rapamycin). J. Infect. Dis. 175, 222–225 (1997).
Drew, R. H. et al. Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Transplantation 77, 232–237 (2004).
Iacono, A. T. et al. A randomized trial of inhaled cyclosporine in lung-transplant recipients. N. Engl. J. Med. 354, 141–150 (2006).
Iacono, A. T. et al. Aerosol cyclosporin therapy in lung transplant recipients with bronchiolitis obliterans. Eur. Respir. J. 23, 384–390 (2004).
Blankenship, J. R. & Heitman, J. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect. Immun. 73, 5767–5774 (2005).
Acknowledgements
The authors thank the many postdoctoral fellows, students and technicians who have assisted over the years in deciphering this important pathway in pathogenic fungi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (figure)
Clustal W analysis of calcineurin A proteins in major human fungal pathogens and humans. (PDF 60 kb)
Related links
Glossary
- Dopamine and cAMP-regulated phosphoprotein
-
Involved in regulating the state of phosphorylation and the activity of a large number of phosphoproteins, ion channels and neurotransmitter receptors.
- N-methyl-D-aspartatic acid receptor
-
(NMDA receptor). NMDA receptors mediate excitatory effects in the brain when they are stimulated by endogenous ligands such as glutamic acid.
- Immunophilin
-
A family of cis–trans peptidylprolyl isomerases that includes cyclophilins and FK506-binding proteins (FKBPs). These proteins were originally discovered as cellular receptors for immunosuppressive drugs, including cyclosporine A and FK506. The complexes that form between immunophilins and their cognate ligands are the functional modules for immunosuppression. Immunophilins are now known to function at the crossroads of protein folding and trafficking, and signal transduction.
- Graft-versus-host disease
-
Tissue damage in a recipient of allogeneic tissue (usually a bone-marrow or solid-organ transplant) that results from the activity of donor cytotoxic T lymphocytes recognizing the tissues of the recipient as foreign. Graft-versus-host disease varies markedly in extent, but it can be life-threatening in severe cases.
- Segmental glomerulosclerosis
-
Renal disease with an unknown etiology or mechanism leading to injury of renal cells and collapse of renal glomerular capillaries, sclerosis and ultimately clinical renal failure.
- Calmodulin
-
A small Ca2+-binding protein, the most important transducer of intracellular Ca2+ signals. It interacts with, and regulates the activity of, a range of proteins that control many cellular processes, including protein phosphorylation and dephosphorylation, cyclic-nucleotide formation and breakdown, cytoskeletal rearrangement, gene transcription and membrane potential.
- Echinocandins
-
A class of intravenous antifungal agents that interfere with fungal cell-wall biosynthesis by non-competitive inhibition of β-1,3-D-glucan synthase, an enzyme present in fungi but absent in mammalian cells. β-1,3-glucan, an essential cell-wall polysaccharide, forms a fibril of three helically entwined linear polysaccharides and provides structural integrity for the fungal cell wall.
- Azole
-
Class of intravenous and oral antifungal agents that inhibit the fungal cytochrome P45014DM (also known as lanosterol 14α-demethylase), which catalyzes a late step in ergosterol biosynthesis in the fungal cell membrane. The drugs bind to the heme group in the target protein and block demethylation of the C-14 of lanosterol, leading to substitution of methylated sterols in the membrane and depletion of ergosterol.
- Ergosterol
-
The main sterol in the fungal cell membrane. Ergosterol is responsible, and essential, for structural and regulatory membrane features such as fluidity and permeability (equivalent to cholesterol in mammalian cells).
- Aflatoxin
-
A naturally occuring mycotoxin, most notably produced by Aspergillus flavus and Aspergillus parasiticus, which can destroy agricultural crops as well as function as a carcinogen in humans.
- Hydrophobin
-
A small hydrophobic protein found in the surface rodlet layer of Aspergillus spp. conidia that has been associated with virulence.
- Non-homologous end-joining
-
A pathway that rejoins DNA strand breaks without relying on significant homology.
- Bronchiolitis obliterans
-
A disease of the lungs with a fixed airway obstruction and inflammation in which the bronchioles are plugged with granulation tissue, leading to shortness of breath and cough.
Rights and permissions
About this article
Cite this article
Steinbach, W., Reedy, J., Cramer, R. et al. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 5, 418–430 (2007). https://doi.org/10.1038/nrmicro1680
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1680
This article is cited by
-
Architecture of the dynamic fungal cell wall
Nature Reviews Microbiology (2023)
-
Molecular mechanisms governing antifungal drug resistance
npj Antimicrobials and Resistance (2023)
-
A critical role of calcineurin in stress responses, hyphal formation, and virulence of the pathogenic fungus Trichosporon asahii
Scientific Reports (2022)
-
Antifungal activity of dendritic cell lysosomal proteins against Cryptococcus neoformans
Scientific Reports (2021)
-
Calcineurin signaling pathway influences Aspergillus niger biofilm formation by affecting hydrophobicity and cell wall integrity
Biotechnology for Biofuels (2020)