Key Points
-
Animal models are essential for testing antiretroviral drugs to treat HIV infection of humans and for acquiring the basic scientific knowledge that will ultimately be needed to develop a safe and effective vaccine against HIV-1.
-
Owing to the narrow host range of HIV, HIV-1 infection of mice that have been reconstituted with a human immune system (humanized mice) and simian immunodeficiency virus (SIV) infection of macaques are used as surrogate models for studying HIV-1 infection of humans.
-
There is no single animal model for AIDS, but rather a collection of host species and viruses that can be used depending on the question to be addressed.
-
A number of humanized mouse models have been developed using different genetic backgrounds and engraftment with various human tissues. Whereas humanized mice make it possible to model HIV-1 infection of human cells in vivo and to design studies using genetically identical animals, these models are limited in their ability to replicate the effects of HIV-1 on non-haematopoietic tissues and to recreate basic features of HIV-1 disease in humans.
-
Infection of Asian macaques with certain SIV or simian–human immunodeficiency virus (SHIV) recombinants results in gradual CD4+ T cell depletion and progression to AIDS, similar to HIV infection of humans. Thus, SIV and SHIV infection of macaques are currently the best, most widely accepted models for AIDS research.
-
Genetic differences between macaque species, and in some cases between geographically distinct populations of the same species, can dramatically affect the outcome of SIV and SHIV infections and are important considerations in the use of these models.
-
There is now considerable interest in engineering HIV-1 to overcome the barriers to HIV-1 replication in macaques. These barriers are imposed by the apolipoprotein B-editing catalytic subunit-like 3 (APOBEC3) proteins, by tripartite-motif-containing protein 5 (TRIM5) variants and by tetherin, all of which are HIV restriction factors. The development of minimally modified simian-tropic strains of HIV-1 that can reproducibly cause disease in macaques might eventually allow direct efficacy testing of antiretroviral drugs and vaccines in non-human primates.
Abstract
The AIDS pandemic continues to present us with unique scientific and public health challenges. Although the development of effective antiretroviral therapy has been a major triumph, the emergence of drug resistance requires active management of treatment regimens and the continued development of new antiretroviral drugs. Moreover, despite nearly 30 years of intensive investigation, we still lack the basic scientific knowledge necessary to produce a safe and effective vaccine against HIV-1. Animal models offer obvious advantages in the study of HIV/AIDS, allowing for a more invasive investigation of the disease and for preclinical testing of drugs and vaccines. Advances in humanized mouse models, non-human primate immunogenetics and recombinant challenge viruses have greatly increased the number and sophistication of available mouse and simian models. Understanding the advantages and limitations of each of these models is essential for the design of animal studies to guide the development of vaccines and antiretroviral therapies for the prevention and treatment of HIV-1 infection.
Similar content being viewed by others
Main
The viruses that cause AIDS — HIV-1 and HIV-2 — belong to a group of retroviruses that are endemic to African apes and Old World monkeys and are known collectively as the primate lentiviruses. HIV-1, which is responsible for the global AIDS pandemic, and HIV-2, which causes AIDS in regions of West Africa, are principally spread by heterosexual transmission and replicate in CD4+ T cells and macrophages. In the absence of treatment, HIV infection results in the depletion of CD4+ T cells, immunodeficiency and the eventual onset of life-threatening opportunistic infections. Over the past 30 years, HIV-1 has claimed more than 30 million lives, and tremendous effort and resources have been devoted to the development of drugs and vaccines for the treatment and prevention of infection. There have been some major advances, including the development of effective antiretroviral drug therapies and pre-exposure prophylaxis (PrEP) regimens, as well as frustrating failures, such as the lack of a vaccine that affords reliable protection and the inability to eradicate the virus from infected individuals.
One of the major limitations in searching for cures and vaccines for HIV-1 has been the lack of an animal model that recapitulates all of the salient features of HIV-1 infection in humans. HIV-1 is a direct descendant of SIVcpz1,2, a virus that infects Central Africa chimpanzees (Pan troglodytes troglodytes) and might have a substantial impact on wild chimpanzee communities3. Nevertheless, HIV-1 infection of chimpanzees in captivity rarely results in the development of disease4,5. Furthermore, owing to their endangered status and high maintenance costs, chimpanzees are not a practical model for AIDS.
When considering species other than humans as models for HIV-1 infection, it is evident that the cellular proteins of these species must support viral replication. The required proteins include, but are not limited to, the receptors and co-receptors involved in virus entry (CD4 and either CC-chemokine receptor 5 (CCR5) or CXC-chemokine receptor 4 (CXCR4)), transcription factors (cyclin T1), nuclear export factors (CRM1; also known as EXP1) and proteins that are recruited to mediate budding of nascent viral particles (belonging to the ESCRT (endosomal sorting complex required for transport) pathway). Cellular factors that inhibit specific steps of HIV-1 replication have also been identified in recent years (Box 1). Although HIV-1 has adapted to overcome such 'restriction factors' and to exploit the necessary cellular cofactors in humans, it has not done so in other species6. This at least partially accounts for the inability of HIV-1 to replicate or cause disease in most species other than humans.
Several alternative animal models have been developed to study HIV-1 infection in vivo. In the case of small animals that are amenable to genetic manipulation, such as mice, efforts have focused on the development of immunodeficient mice engrafted with cells or tissues from the human immune system to provide the virus with susceptible target cells for replication. In the case of larger animals, such as primates, research has focused on the use of simian immunodeficiency virus (SIV) or simian–human immunodeficiency virus (SHIV) recombinants. Thus, there is no single animal model for HIV-1 infection, but rather a variety of host species and viruses that can be used depending on the question to be addressed. Here, we review the advantages and limitations of these models with respect to their use in the preclinical development of vaccines and antiretroviral therapies.
Small-animal models
Initial attempts to infect small animals, including mice, rats and rabbits, with HIV-1 were unsuccessful7. Although cats are also not susceptible to HIV-1, feline immunodeficiency virus (FIV) infection in domestic cats can serve as a surrogate model for HIV-1 infection in humans. However, this model has many limitations and is not widely used (Box 2). As transgenic animal technologies have developed, mice, rats and rabbits expressing the proteins that are necessary for HIV-1 replication — in particular, the viral receptors CD4, CCR5 and CXCR4 — have been generated. However, none of these models supports robust viral replication or the development of disease8,9,10. It is now evident that cells from these animals do not provide essential cofactors for HIV-1 replication and might also express proteins that inhibit HIV-1 infection11,12,13. These findings also limit the utility of transgenic mice expressing HIV-1 proviruses14,15. Therefore, the best small-animal models for HIV/AIDS are based on 'humanized mice' — genetically immunocompromised mice that have been engrafted with human tissues to reconstitute the human immune system (Fig. 1). Although several different humanized mouse models have been developed, here we focus on those that are used most frequently in HIV-1 research.
a-d | The different mouse models that are used for HIV-1 research are depicted here, grouped according to the presence of human immune cells in mouse tissues, and of HIV-1 infection, as reported in the literature (indicated in red). Peyer's patches and the appendix represent the GALT (gut-associated lymphoid tissue). For simplicity, not all lymphoid organs are shown. The conjoint human thymus–liver organ formed following injection of human fetal liver and thymus cells into the mouse renal capsule is shown in parts a and d. For details of each mouse model, see main text. BLT, bone marrow–liver–thymus; Il2rg, interleukin-2 receptor common γ-chain gene; NOD, non-obese diabetic; NSG, NOD scid gamma; PBL, peripheral blood lymphocyte; Rag2, V(D)J recombination- activating gene 2; scid, severe combined immunodeficiency.
scid- hu–Thy/Liv mice. Many humanized mice have been developed using severe combined immunodeficiency (scid) genetic backgrounds. scid mice have a mutation in the gene encoding DNA-dependent protein kinase catalytic subunit (PRKDC), resulting in the absence of functional B cells and T cells16. scid-hu–Thy/Liv mice are generated by transplanting human fetal thymus and liver cells into scid mice, resulting in the formation of a conjoint organ that produces human haematopoietic (CD34+) progenitor stem cells and mature human lymphocytes17 (Fig. 1a). These mice can be infected by direct injection of HIV-1 into the human implants, which leads to CD4+ T cell depletion and high viral loads in the human tissue18,19,20,21. Applications of this model include studies of the mechanisms of CD4+ T cell loss20,22 and the efficacy of antiretroviral drugs in suppressing acute HIV-1 infection23,24. Limitations of this model include a substantial degree of 'leakiness' in certain mouse strains (leading to the development of mouse T cells and B cells in older animals)25 and the maintenance of innate immunity, including natural killer cells (NK cells), which decrease the success of engraftment26. Another disadvantage is that this model cannot be used to study mucosal transmission of HIV-1.
scid- hu–PBL mice. In scid-hu–PBL mice, peripheral blood lymphocytes (PBLs) from humans are injected into scid mice intraperitoneally and disseminate mainly to the lymph nodes, spleen, bone marrow and genital mucosa27 (Fig. 1b). Although animals with a low percentage of human cells can survive for up to 6 months, higher levels of human cells are associated with increased mortality28. These mice secrete human antibodies and can mount human antibody responses following immunization, which is an advantage over scid-hu–Thy/Liv mice. scid-hu–PBL mice can be infected with HIV-1 by intraperitoneal injection with cell-free virus or engraftment with cells from humans infected with HIV-1. Both infection routes result in the depletion of human CD4+ T cells in the mouse, and the virus can be detected in tissues that have been infiltrated by human cells29,30 (Fig. 1b). This model has been used for several different applications, such as demonstrating the effectiveness of passive immunization with monoclonal antibodies specific for the HIV-1 envelope (Env) glycoprotein31,32,33, testing of Env-based vaccines34,35 and assessing the ability of dendritic cells pulsed with inactivated HIV-1 to protect against HIV-1 infection36,37. Like the scid-hu–Thy/Liv mice, this model cannot be used for studying mucosal transmission.
NOD scid and NOD scid Il2rg−/−mice. scid mice can be crossed with strains of mice harbouring additional mutations to improve the efficiency of engraftment with human haematopoietic tissue. NOD scid mice are generated by crossing scid mice with non-obese diabetic (NOD) mice, which have multiple genetic deficiencies that result in low host NK cell activity and impaired complement activation38. These characteristics substantially improve the efficiency of human tissue engraftment. However, NOD scid mice suffer from a high incidence of thymic lymphomas, which limits their lifespan39. To further improve engraftment efficiency, NOD scid mice have been crossed with Il2rg−/− mice, which harbour a mutation in interleukin-2 receptor common γ-chain (IL2Rγ), a protein that is essential for immune cell growth and development. Two different versions of NOD scid Il2rg−/− mice have been generated: NOG mice, which harbour a mutation that truncates IL2Rγ in the intracellular-signalling domain40, and NOD scid gamma (NSG) mice, which harbour a mutation that deletes the intracytoplasmic domain of the IL2Rγ chain41. These mice completely lack mature B cells and T cells, have extremely low NK cell activity and have the highest success rate of human cell engraftment of all scid strains42. Furthermore, the Il2rg−/− mutation prevents the development of lymphomas, improving the lifespan of NOG and NSG mice compared to that of NOD scid mice.
Engraftment of NOD scid, NOG and NSG mice is achieved by injecting (using several routes) human haematopoietic (CD34+) stem cells derived from cord blood, fetal liver or adult blood41,43,44. These mice produce human immune cells that disseminate to numerous sites, among which are the peripheral blood, liver, lung, vagina and rectum (Fig. 1c). When inoculated with HIV-1 or SHIV, NOD scid mice engrafted with human CD34+ stem cells can develop high levels of plasma viraemia and HIV-specific antibodies, with HIV-infected cells invading multiple tissues45. NSG mice have been used as a model for HIV-1-induced neuropathogenesis46,47, and NOG and NSG mice have been used to explore the potential of various gene therapy approaches to inhibit HIV-1 replication48,49,50,51.
BLT mice. NOD scid and NSG mice have been implanted with human fetal thymus and liver cells followed by sublethal irradiation and bone marrow transplantation with human CD34+ cells from the same donor to generate BLT (bone marrow–liver–thymus) mice52,53. Human cells are found in the same tissues as in the parental mice, including the gut-associated lymphoid tissue (GALT) (Fig. 1d). One of the main advantages of BLT mice over other mouse models is that human T cells develop within a human thymus, thus mimicking T cell development in humans. Intraperitoneal inoculation of BLT mice with HIV-1 results in high levels of sustained viraemia, CD4+ T cell depletion and virus-specific humoral and cellular immune responses54. Another advantage of this model is the ability to infect animals with HIV-1 by mucosal routes of inoculation55,56. Thus, BLT mice provide a useful model to study antiretroviral treatments and have been used to demonstrate that antiretroviral drugs, whether delivered intravenously or topically, can prevent HIV-1 infection55,57.
Rag2−/−Il2rg−/−mice. A humanized mouse model based on Rag2−/−Il2rg−/− mice has also been used as a model for HIV-1 infection. V(D)J recombination-activating gene 2 (Rag2) is essential for immunoglobulin and T cell receptor gene rearrangement; Rag2−/− mice therefore lack mature T cells and B cells58. Unlike the mutation in scid mice, the Rag2 mutation is not leaky and does not result in a tendency to develop tumours. Crossing Rag2−/− mice with Il2rg−/− mice results in the Rag2−/−Il2rg−/− model, which lacks functional lymphocytes and NK cells58. Engraftment of these mice with human CD34+ stem cells is enhanced and results in human immune cells that disseminate mainly in the peripheral blood, liver, spleen, bone marrow, vagina and GALT (Fig. 1c). This model shares the limitation of NOG and NSG mice: when engrafted with human CD34+ stem cells, T cells develop in the mouse thymus, rather than a human organ. Rag2−/−Il2rg−/− mice engrafted with human CD34+ stem cells can be infected with HIV-1 by intraperitoneal injection, which results in plasma viraemia that can persist for up to 1 year, viral dissemination to multiple organs and CD4+ T cell depletion59,60. Several studies have used this model to demonstrate the effectiveness of antiretroviral therapy in suppressing viraemia, to test new therapeutic approaches and to investigate the effects of viral replication on the immune system61,62,63. However, humoral responses to HIV-1 are generally not detectable59,60, and there are conflicting reports regarding the ability to infect these animals by mucosal inoculation64,65,66.
Overall, advances in the generation of humanized mice that improve the ability to engraft them with human tissues also improve their utility as models for HIV-1 infection. It is possible to infect BLT mice with HIV-1 by mucosal routes of inoculation, and BLT mice can be used to assess antiretroviral treatment regimens. Indeed, this model has recapitulated early results obtained in human trials evaluating a single antiretroviral drug for PrEP67 (although the results from subsequent human trials have been inconsistent).
The human haematopoietic system is extremely complex. Although it originates from pluripotent haematopoietic cells in the bone marrow, numerous interactions throughout the body guide the differentiation and maturation of haematopoietic cells, ultimately resulting in cells of multiple lineages (reviewed in Refs 68, 69). It is imperative to recognize that only parts of the human immune system can be reconstituted in different humanized mouse models, resulting in important limitations. For example, any interactions between introduced human cells and other components of the mouse immune system, or even other types of mouse cells, will not accurately reflect lymphoid tissue development in humans. The development of BLT mice aimed to address such limitations, and new approaches are currently being pursued by various laboratories to improve multiple-lineage haematopoiesis70. For instance, to overcome the bias towards lymphoid cell development in current models, BLT mice are developed using knock-in mice expressing human cytokines that are important for human myeloid cell development71. Nevertheless, the effects of HIV-1 infection of non-haematopoietic tissues cannot be studied using these models.
Notably, humanized mice live in an artificially sterile environment and do not require a functional immune system to survive; thus, they cannot recapitulate basic features of HIV-1 pathogenesis. Furthermore, humanized mice are not trivial to generate. As they cannot be bred, they must be generated de novo for each experiment by surgical engraftment of human tissues, a technically challenging procedure. In addition, because these mice are genetically immunocompromised, they require specialized housing and handling procedures. These considerations substantially increase the costs associated with establishing and maintaining humanized mice.
Non-human primate models
Many species of African monkeys and apes are natural hosts for SIV, but generally do not develop disease as a consequence of infection. By contrast, infection of Asian macaques, which are not natural hosts for primate lentiviruses, with certain strains of SIV results in high viral loads, progressive CD4+ T cell depletion and opportunistic infections.
Natural SIV hosts. More than 40 different African primate species are endemically infected with their own unique strain of SIV. Owing to thousands of years of virus–host co-evolution, these natural SIV hosts typically do not develop disease as a result of their infections and are thus not useful as pathogenic models. However, comparisons of non-pathogenic versus pathogenic SIV infection in African versus Asian monkeys have led to a number of important insights into AIDS pathogenesis (reviewed in Ref. 72). Of the natural SIV hosts, SIVsmm infection of the sooty mangabey (Cercocebus atys) and SIVagm infection of the African green monkey (Chlorocebus aethiops) have been studied in detail owing to the availability of these primate species at primate centres in the United States and Europe. The sooty mangabey model is of particular interest, as cross-species transmissions of SIVsmm gave rise to HIV-2 in humans and SIVmac in macaques73. Similar to pathogenic lentiviral infections, SIVsmm and SIVagm infections of their natural hosts result in high levels of sustained viral replication, rapid turnover of productively infected lymphocytes (including severe depletion of mucosal CD4+ T cells) during acute infection and the activation of innate and adaptive immunity72. However, these viruses do not result in chronic immune activation, progressive depletion of mucosal or peripheral CD4+ T cells, or the destruction of lymph node architecture in their respective hosts72. Moreover, whereas HIV-1 and SIVmac infections are associated with a substantial depletion of central memory CD4+ T cells, SIVsmm preferentially infects effector memory CD4+ T cells in tissues72. Although the underlying genetic differences between pathogenic and non-pathogenic hosts have not been defined, differences in the cellular tropism of SIV in natural hosts, which spares central memory CD4+ T cells, might be an important factor contributing to the lower levels of both chronic immune activation and progressive CD4+ T cell depletion observed in these species.
Non-natural SIV hosts. In contrast to infection of African monkeys, infection of Asian macaques with certain strains of SIV closely resembles HIV-1 infection of humans, and together with the use of recombinant SHIV viruses (described below), Asian macaques have become the most commonly used and widely accepted models for HIV/AIDS. Numerous advances in our understanding of viral transmission, pathogenesis and latency can be attributed to the use of macaque models, and they are used extensively in studies aimed at the development of vaccines and microbicides for the prevention of HIV-1 infection. Rather than provide an exhaustive list of the advances made possible by macaque models, we focus on the differences among the macaque species and challenge viruses used, and the practical implications of these differences.
Although most, if not all, macaques can be infected with SIV, three species are routinely used as animal models for AIDS: the rhesus macaque (Macaca mulatta), the pig-tailed macaque (Macaca nemestrina) and the cynomolgus macaque (Macaca fascicularis) (Fig. 2). Each of these species has a unique evolutionary history and population structure that can profoundly affect the outcome of SIV infection, particularly for viruses that are not fully adapted to a given model. Understanding how differences among these species, and in some cases among populations of the same species, can affect the pathogenesis of SIV infection is essential if we are to avoid pitfalls that can lead to confusing or uninterpretable results when using these models.
a | The geographical range of the rhesus macaque (Macaca mulatta) exceeds that of all other primate species except humans, extending from western India and Pakistan across China. Distinct populations of rhesus macaques can be differentiated on the basis of mitochondrial DNA sequences or SNPs203,204. Captive-breeding programmes in the United States were initially established using animals imported from India, contributing to the widespread use of Indian-origin rhesus macaques in AIDS research. However, owing to the increasing demand for rhesus macaques and an embargo on the exportation of these animals from India since 1978, there has been a substantial decline in their availability and a sharp increase in their cost. This has led to greater dependence on rhesus macaques imported from China and Burma. b | The pig-tailed macaque (Macaca nemestrina) is native to Southeast Asia, Malaysia and Indonesia, and last shared a common ancestor with rhesus macaques approximately 3.5 million years ago205. c | The cynomolgus macaque (Macaca fascicularis), also known as the long-tailed or crab-eating macaque, is native to regions of Indochina, Malaysia, Indonesia and the Philippines. Genetic evidence suggests that cynomolgus and rhesus macaques diverged from a common ancestor approximately 1.9 million years ago204,205. Distribution data from Ref. 206.
Rhesus macaques. Rhesus macaques of Indian origin are by far the best characterized and most utilized non-human primate model for AIDS. The two most common SIV challenge strains, SIVmac251 and SIVmac239, were initially isolated from rhesus macaques of Indian descent and are thus particularly well adapted to these animals, with infections resulting in high viral loads with minimal animal-to-animal variation (Table 1). Similarly to HIV-1 replication, ongoing SIV replication results in the turnover and progressive loss of CD4+ T cells, particularly in the GALT74. However, the rate of disease progression for SIV-infected animals is considerably more rapid than for HIV-1-infected humans; Indian-origin rhesus macaques typically progress to AIDS within 1–2 years of SIV infection, compared with 8–10 years for humans who are infected with HIV-1 and not receiving antiretroviral therapy.
The major histocompatibility complex (MHC) genes of the rhesus macaque are highly polymorphic and encode molecules that present virus-derived peptides on the surface of infected cells for recognition by T cells. Polymorphisms in the peptide-binding pocket of MHC molecules determine the repertoire of peptides presented by these complexes and can have a profound effect on the ability of the immune system to control SIV infection. Compared to the human leukocyte antigen (HLA) genes of humans (the human MHC is also known as the HLA complex), the MHC genes of macaques and other Old World monkeys differ not only in sequence, but also in copy number. For instance, whereas humans have three classical HLA class I genes (HLA-A, HLA-B and HLA-C), macaques have an expanded set of genes corresponding to HLA-A and HLA-B but not to HLA-C, because HLA-C represents a duplication of an ancestral B locus that occurred after the divergence of apes and Old World monkeys75. There are up to four Mamu-A genes (corresponding to HLA-A) and an undefined and variable number of Mamu-B genes (corresponding to HLA-B) for any given haplotype in the rhesus macaque76. However, it is important to recognize that not all of these genes are functional or well expressed. Typically, only one Mamu-A gene and up to three Mamu-B genes are highly expressed per haplotype. Thus, it is presently unclear how differences in MHC class I gene copy number affect virus-specific CD8+ T cell responses in humans versus macaques.
Numerous SIV epitopes that are recognized by CD8+ T cells, and a few epitopes that are recognized by CD4+ T cells, along with the MHC class I and class II molecules, respectively, that present these epitopes, have been defined in Indian-origin rhesus macaques (reviewed in Ref. 77). As in humans, certain MHC class I alleles are associated with better containment of viral replication. Mamu-A1*001 (formerly Mamu-A*01) is associated with a five-fold reduction in chronic-phase viral loads compared to the loads in Mamu-A1*001-negative animals78, and Mamu-B*008 and Mamu-B*017 (formerly Mamu-B*08 and Mamu-B*17) are also significantly over-represented among animals that are able to contain plasma viral loads to below 1,000 RNA copies per ml79,80. Because of the protective effects of these alleles, their over-representation in experimental or control groups can confound our interpretation of the outcome of SIV infection in rhesus macaques, especially for studies involving small numbers of animals, and is thus an important consideration in the use of this model.
Tripartite-motif-containing protein 5α (TRIM5α) was initially identified as a post-entry block to HIV-1 infection of rhesus macaque cells81 and is now recognized more broadly as an important host-range determinant for retroviruses (Box 1). In contrast to humans, for which only a handful of minor variants of TRIM5α have been identified, rhesus macaques encode highly polymorphic TRIM5 proteins82 (Fig. 3). Although there have been conflicting reports regarding the susceptibility of SIVmac251 to different rhesus macaque TRIM5 variants83,84, dramatic differences have been observed in the susceptibility of SIVsmE543-3 to these proteins. Viral loads in SIVsmE543-3-infected macaques are 100- to 1,000-fold lower in animals expressing restrictive TRIM5α variants (with 339-TFP-341 in the SPRY domain) than in animals expressing only non-restrictive variants (with Q339 in the SPRY domain)85 (Fig. 3). The susceptibility of SIVsmE543-3 to certain rhesus macaque TRIM5α proteins probably reflects the incomplete adaptation of this virus to this host, because SIVsmE543-3 was cloned after sequential passage in only two rhesus macaques85. These observations have important practical implications, as SIVsmE543-3 and the closely related SIVsmE660 strain are widely used as heterologous-challenge viruses for rhesus macaques immunized with SIVmac239-derived antigens.
a | Numerous alleles encoding tripartite-motif-containing protein 5α (TRIM5α) and TRIMCyp have been identified in macaques. Although several amino acid differences have been found throughout the proteins, the encoding alleles can be grouped into two main variants depending on the amino acids at position 339–341 (TFP or ΔΔQ) in TRIM5α and at position 369 (N or D) in TRIMCyp. These crucial residues determine the specificity of these variants against lentiviruses. b | A summary of the restriction factors of macaques and the effects of the different variants on viral replication, as demonstrated in vivo for the simian immunodeficiency virus (SIV) strain SIVsm-E543-3 and in vitro for HIV-1. The description of TRIM5α polymorphism in cynomolgus macaques is very recent. Although the alleles described so far belong to group 2 and are not predicted to restrict SIV or HIV-1, amino acid differences at other locations in these proteins might affect restriction activity. There are also allelic variations for each APOBEC3 gene family member in macaques, but these are not described here. The extent of tetherin polymorphism in macaques is presently unclear. The number of variants listed is based on samples from 1–2 animals or cell lines (it is sometimes unclear whether cell lines were derived from animals of Indian or Chinese origin). The single tetherin variants reported for Chinese rhesus macaques and cynomolgus macaques is predicted to restrict HIV-1, but this has not been tested. +, restriction of replication; −, no significant restriction of replication (less than twofold); CypA, cyclophilin A-like.
Chinese and Burmese rhesus macaques are also sometimes used in AIDS research owing to the limited availability of animals of Indian ancestry. However, the pathogenesis of common SIV and SHIV challenge strains adapted for replication in Indian rhesus macaques differs in animals of Chinese and Burmese origin. SIVmac239, SIVmac251 and SHIV89.6P are less pathogenic in Chinese than in Indian rhesus macaques. Although the differences in peak viraemia are small, and were only significant in one study, set-point viral loads are significantly lower in Chinese rhesus macaques in multiple comparisons86,87,88 (Table 1). These differences in viral loads are reflected by higher CD4+ T cell counts and prolonged survival of animals of Chinese descent88. SIVmac239 also seems to be less pathogenic in Burmese animals than in Indian animals, on the basis on chronic-phase viral loads in unvaccinated control animals89. The reasons for these differences are unclear at present, but they probably reflect underlying immunogenetic differences90,91.
Pig-tailed macaques. Next to rhesus macaques, pig-tailed macaques are the most commonly used non-human primate model for AIDS. Although viral loads and the rate of CD4+ T cell depletion do not differ substantially in pig-tailed versus rhesus macaques (Table 1), pig-tailed macaques progress to disease more rapidly92,93. On average, pig-tailed macaques develop AIDS within 42 weeks of SIV infection compared to 70 weeks for rhesus macaques92. The reason for this difference in pathogenesis is unknown, but it might reflect a higher basal rate of lymphocyte turnover in pig-tailed macaques that is independent of SIV infection.
The MHC genes of pig-tailed macaques are not as well characterized as those of rhesus macaques. However, several optimal SIV epitopes that are recognized by CD8+ T cells, and the MHC class I molecules that present these epitopes, have been defined77. Of particular interest is Mane-A1*084 (formerly Mane-A*10). Like Mamu-A1*001, this molecule restricts an immunodominant epitope of the SIV Gag protein and is associated with decreased viral loads following SIV infection, compared to viral loads in Mane-A1*084-negative animals94,95.
A peculiar and perhaps unique feature of pig-tailed macaques is that they do not express TRIM5α. Instead, TRIM5 of pig-tailed macaques encodes a TRIMCyp isoform that does not restrict HIV-1 (Refs 96, 97, 98, 99, 100) (Fig. 3). The absence of TRIM5α in pig-tailed macaques might explain previous reports that HIV-1 can establish a low-level transient infection in these animals; it is probably also the reason why engineering simian-tropic strains of HIV-1 for replication in these monkeys has been more successful than for other macaque species (see below)101,102.
Cynomolgus macaques. Cynomolgus macaques have not been used as widely in AIDS research as rhesus or pig-tailed macaques, in part owing to historical reasons pertaining to SIV isolation and the less extensive immunogenetic characterization of this species. A comparison of viral loads and CD4+ T cell turnover revealed that SIVmac251 and SHIV89.6P are generally less pathogenic in cynomolgus macaques than in Indian or Chinese rhesus macaques88 (Table 1). For Chinese rhesus macaques, these differences probably reflect, at least to some extent, the incomplete adaptation of these viruses to this species owing to their passage history in Indian rhesus macaques. Comparisons of the MHC genes of cynomolgus macaques from multiple locations have revealed extraordinary sequence diversity among geographically distinct populations103,104. Furthermore, the TRIM5 genes of cynomolgus macaques are also the most diverse of any primate species characterized thus far, encoding polymorphisms in both the TRIM5α and TRIMCyp isoforms, including TRIMCyp proteins that differentially restrict HIV-1 (Ref. 105) (Fig. 3).
The limited MHC diversity of cynomolgus macaques from the remote Indian Ocean island Mauritius has greatly increased interest in the use of these animals in AIDS research106,107. These animals descended from a small founder population that was brought to the island approximately 400–500 years ago by European sailors. Only seven MHC haplotypes have been identified in Mauritian cynomolgus macaques, three of which are present in approximately 90% of these animals104. Thus, unlike other outbred primate models, for which it is only possible to select MHC-defined animals by genotyping for specific alleles, studies can be carried out in Mauritian cynomolgus macaques using haplotype-matched animals (that is, animals that share a set of MHC genes inherited on the same chromosome) or fully MHC-identical animals. This unique feature was used to demonstrate that there is an MHC heterozygote advantage for the ability to control SIV infection108.
SIV and recombinant SHIV challenge strains
We now understand that the inability of HIV-1 to replicate in Old World monkeys reflects, at least in part, intrinsic cellular blocks that are imposed by the restriction factors TRIM5α, the apolipoprotein B-editing catalytic subunit-like 3 (APOBEC3) proteins and perhaps also tetherin (Box 1). Thus, SIV rather than HIV-1 was used to develop non-human primate models for AIDS. The SIV strains used for pathogenic models were all derived after accidental or experimental infection of macaques with SIVsmm. Consequently, these SIV strains differ from HIV-1 in a number of important ways. For instance, SIVsmm and SIVmac do not have a vpu gene, but have another accessory gene, vpx, that is not present in HIV-1 (Fig. 4). The product of the vpx gene was recently shown to counteract SAM domain- and HD-domain-containing protein 1 (SAMHD1), a newly identified restriction factor in macrophages and dendritic cells (Box 1). Moreover, HIV-1 and SIVsmm/SIVmac have only approximately 53% nucleotide identity and differ in the organization of their overlapping ORFs (Fig. 4). Thus, although an assortment of cloned and uncloned SIV and recombinant SHIV challenge strains have been generated and shown to have diverse biological properties, these viruses are surrogates for modelling HIV-1 infection. Hence, there is now considerable interest in engineering minimally modified HIV-1 strains that are capable of replicating and causing disease in macaques.
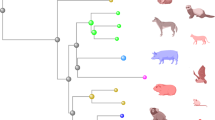
a | Monkey cartoons indicate animals from which simian immunodeficiency virus (SIV) isolates were derived. Green boxes represent uncloned biological isolates, and yellow boxes represent infectious molecular clones. Solid arrows represent direct viral transmission or isolation steps, and dotted arrows indicate passage and/or cloning steps omitted for simplicity. b | Schematic representation of HIV-1, SIV and chimeric viral genomes. Maroon and blue boxes indicate HIV-1- and SIV-derived sequences, respectively. Ca, Cercocebus atys (sooty mangabey); CNPRC, California National Primate Research Center (Davis, California, USA); Env, envelope glycoprotein; LTR, long terminal repeat; Mm, Macaca mulatta (rhesus macaque); Mn, Macaca nemestrina (pig-tailed macaque); SHIV, simian–human immunodeficiency virus; SIVmac, macaque SIV; SIVmne, pig-tailed macaque SIV; SIVsmm, sooty mangabey SIV; stHIV-1, simian-tropic HIV-1; RT, reverse transcriptase.
SIVs. SIV was first isolated at the New England Primate Research Center, Southborough, Massachusetts, USA, from rhesus macaques with a transmissible form of immunodeficiency characterized by opportunistic infections and tumours109. The source of the virus was later traced to an outbreak of lymphoma in the 1970s among macaques that were housed at the California National Primate Research Center, Davis, California USA110; these macaques might have received tissues from SIV-infected sooty mangabeys during experiments aiming to develop a non-human primate model for prion disease111.
SIVmac251 and SIVsmE660 are independent viral isolates that are commonly used as uncloned challenge viruses (Box 3; Fig. 4). Although the genetic heterogeneity of uncloned SIV strains is desirable for certain applications, such as the analysis of mucosally transmitted Env variants112, it can complicate comparisons of results obtained using challenge stocks prepared in different laboratories, as these stocks vary in composition depending on their passage history. Infectious molecular clones of SIV were therefore derived to obtain genetically defined challenge viruses and proviral DNAs that were amenable to genetic manipulation.
SIVmac239 and SIVsmE543-3 are pathogenic molecular clones that are related in their passage history to SIVmac251 and SIVsmE660, respectively113,114 (Box 3; Fig. 4). Like most naturally transmitted HIV-1 isolates, these viruses use CCR5 as a co-receptor, replicate predominantly in memory CD4+ T cells and express Env glycoproteins that are resistant to neutralizing antibodies. The genetic distance between the SIVmac251 and SIVmac239 lineage and the SIVsmE660 and SIVsmE543-3 lineage is comparable to the genetic distance between HIV-1 isolates of the same clade. Heterologous protection can therefore be assessed by vaccinating animals with SIVmac239-derived antigens and then challenging those animals with SIVsmE660 (Refs 115, 116), or by vaccinating with SIVsmE543-3-derived antigens and then challenging with SIVmac251 (Ref. 117). As mentioned above, the susceptibility of SIVsmE543-3, and probably also SIVsmE660 (Ref. 118), to certain rhesus macaque TRIM5 proteins is an important consideration in the use of these challenge viruses. However, a number of investigators are now working to adapt SIVsmE543-3 and SIVsmE660 to restrictive rhesus macaque TRIM5 variants to obtain challenge viruses with more uniform resistance in this model.
SHIVs. Despite the valuable insights into lentiviral pathogenesis that have been obtained from studies of SIV infection in macaques, there are fundamental differences between SIV and HIV-1 that limit the use of SIV–macaque models for certain applications. For example, HIV-based vaccine immunogens cannot be tested directly by challenging with SIV, and SIV is not sensitive to many of the drugs designed to inhibit the HIV-1 protease, reverse transcriptase (RT) and integrase enzymes. In addition, although most HIV-1 and SIV isolates use CCR5, other co-receptors used by these viruses may differ. Whereas HIV-1 can acquire the ability to use CXCR4, SIV rarely gains this ability, but is able to use alternative co-receptors that are not used by HIV-1 (Ref. 119). These differences in co-receptor use have the potential to complicate the testing of certain virus entry inhibitors in SIV-infected macaques. To address these limitations, efforts have focused on the development of SHIVs that can replicate and cause disease in macaques.
SHIVs expressing HIV-1 Env. A number of SHIVs expressing HIV-1 Env glycoproteins have been constructed to test Env-specific vaccines and drugs in non-human primates (Fig. 4). Most of these recombinants were generated by replacing the rev, tat and env genes of SIVmac239 with the corresponding rev, tat, vpu and env genes of HIV-1 (Ref. 120). Although initially these chimeric viruses replicated poorly in macaques, after extensive passage in animals they acquired the ability to replicate efficiently and cause disease. Indeed, the first generation of serially passaged SHIVs were highly pathogenic, replicating to high levels and causing rapid, nearly complete depletion of CD4+ lymphocytes as early as 3 months after infection121,122,123.
The prototype of this group is SHIV89.6P, which was widely used as a challenge virus until it was recognized that it is paradoxically easy to protect against by vaccination. Indeed, a range of T cell-based vaccines provide robust protection against SHIV89.6P, as measured by dramatic reductions in post-challenge viral loads relative to the loads in unvaccinated control animals, but these vaccines afford little or no protection against primary SIV isolates, such as SIVmac239 and SIVmac251. This has been attributed to several phenotypic differences between the viruses (reviewed in Ref. 124). Whereas most SIVs use CCR5 as a co-receptor (such viruses are referred to as R5-tropic viruses) and replicate predominantly in memory CD4+ T cells, SHIV89.6P and other highly pathogenic SHIVs have evolved to primarily use CXCR4 (and are referred to as X4-tropic viruses), resulting in an expanded cellular tropism that includes naive CD4+ T cells125. Thus, unlike SIV, which preferentially infects CCR5+ lymphocytes, causing an acute depletion of memory CD4+ T cells in mucosal tissues, SHIV89.6P can infect CXCR4+CCR5− lymphocytes, which constitute the majority of naive CD4+ T cells in peripheral blood and lymph nodes. The rapid and dramatic loss of these cells appears to undermine the development of the effector CD4+ T cells that are needed to contain viral replication, which may explain why SHIV89.6P-infected animals often progress to disease without making virus-specific antibodies124. Compared to the Env proteins of most primary HIV-1 and SIV isolates, SHIV89.6P Env is also more sensitive to neutralizing antibodies. Moderately effective vaccines that can partially preserve CD4+ T cells might therefore allow infected animals to generate Env-specific antibodies that can suppress viral replication124. Finally, in contrast to SIV replication, the ability to contain SHIV replication is highly dependent on the challenge dose, which might account for much of the protection observed in SHIV challenge experiments. For these reasons, X4-tropic SHIVs, such as SHIV89.6P, are no longer considered appropriate challenge viruses for evaluating T cell-based vaccines.
Given that most primary HIV-1 isolates use CCR5, there has been an arduous effort to develop R5-tropic SHIVs that are consistently pathogenic in macaques. However, the number of R5-tropic SHIVs is still limited. SHIVSF162P3 was the first pathogenic R5-tropic SHIV to be developed, and like SIV, preferentially replicates in memory CD4+ T cells126. SHIVSF162P3 has been especially valuable for evaluating treatments to block viral transmission, such as microbicides and passively administered Env-specific antibodies127,128,129. However, the high frequency of animals that spontaneously contain SHIVSF162P3 replication has limited the utility of this virus for testing vaccines or other treatments that are designed to reduce viral loads during chronic infection. Nevertheless, a late-stage viral isolate derived from this strain, SHIVSF162P3N, appears to be better adapted to rhesus macaques and was recently shown to result in less variable chronic-phase viral loads in infected animals130. Other pathogenic R5-tropic SHIVs have recently been developed: SHIVAD8 expresses a clade B Env131, and SHIV1157ipd3N4 expresses a clade C Env, which is typical of the most prevalent HIV-1 subtype in sub-Saharan Africa132. Current efforts are also underway to generate SHIVs expressing Env proteins from early-transmitted or founder HIV-1 isolates that represent the most relevant targets for Env-based intervention strategies.
SHIVs expressing HIV-1 pol . One of the major limitations of SIVs and of SHIVs expressing HIV-1 Env is that they are insensitive to many of the drugs that target HIV-1 enzymes. To overcome this limitation, a new generation of SHIVs is being developed by substituting SIV polymerase (pol) sequences with the corresponding sequences from HIV-1. Several of these recombinant viruses contain sequences encoding the HIV-1 RT (Fig. 4). Unlike parental SIVs, these RT-SHIVs are sensitive to non-nucleoside reverse-transcriptase inhibitors (NNRTIs) commonly used against HIV-1, and treatment of RT-SHIV-infected macaques with antiretroviral drugs effectively suppresses viraemia133,134. Importantly, these animal models have demonstrated that suboptimal treatment results in the selection of signature drug resistance mutations that are found in HIV-1 from patients receiving antiretroviral treatment135,136. These models allow much more extensive investigations of viral replication, and recent data suggest that during highly active antiretroviral therapy (HAART), viral replication persists in multiple tissues137,138. Additional SHIVs expressing HIV-1 protease139 or a combination of HIV-1 RT and Env140 are also being developed.
stHIV. One could envisage that the progressive substitution of SIV genes with those from HIV-1 would eventually lead to the generation of a virus that replicates in macaques but is more closely related to HIV-1 than to SIV. However, the opposite approach, engineering HIV-1 to replicate in macaques, has shown more promise. Although early attempts to generate such viruses were unsuccessful, our understanding of how viruses interact with host cells, and in particular with restriction factors, has led to rational approaches for engineering simian-tropic HIV-1 (stHIV-1) to overcome these barriers.
Pig-tailed macaques represent an especially promising model, as they lack TRIM5 proteins that can block HIV-1 infection (Fig. 3). Substitution of the HIV-1 vif gene with SIVmac vif results in an stHIV-1 (Fig. 4) that is resistant to macaque APOBEC3 proteins and can replicate in pig-tailed macaques during acute infection to generate viral levels similar to those in humans infected with HIV-1 (Refs 101, 102, 141). This model has been used to demonstrate the effectiveness of PrEP in providing apparent sterilizing protection with a drug cocktail commonly used against HIV-1 in humans101. Although the replication of stHIV-1 in pig-tailed macaques is eventually controlled, it is conceivable that additional engineering to overcome other restriction factors, such as tetherin, perhaps in combination with additional passage in animals, would lead to a minimally modified HIV-1 that can reproducibly cause disease in macaques. The development of stHIV-1 strains might eventually allow direct efficacy testing of HIV-1 vaccine immunogens and antiretroviral drugs in macaques.
Conclusions
Although small-animal models, such as mice, rabbits and cats, offer obvious advantages in terms of high reproductive rates, low maintenance costs and the ability to conduct studies using inbred, genetically identical animals, these species are distantly related to humans. Therefore, extrapolating results obtained in these models to human disease is often not straightforward. Many of these limitations have been overcome by advances in the development of transgenic mice that have been reconstituted, at least in part, with a human immune system. However, humanized mice lose some of the practical advantages of small-animal models, as they cannot be bred and have higher maintenance costs. Nevertheless, given that HIV-1 is highly specific for humans and faces multiple barriers to replication in most other animals, it seems unlikely that a more convenient small-animal model will be developed in the foreseeable future. Currently, though, humanized mice are not yet widely accepted as a testing platform for new therapeutics or vaccines, which are more likely to be tested in non-human primates prior to human trials.
Non-human primates have a number of important advantages over small-animal models. SIV or SHIV infection of macaques closely resembles HIV-1 infection of humans with respect to the cell types that are susceptible to viral infection, the progressive depletion of CD4+ T cells (particularly in the GALT) and the development of opportunistic infections typical of AIDS. The tissue architecture of the reproductive and gastrointestinal mucosa is also similar in macaques and humans, providing an opportunity to test vaccines and treatments designed to prevent the mucosal transmission of HIV-1. By virtue of the close phylogenetic relationship between macaques and humans, many of the macaque genes controlling the immune response to SIV are similar, if not directly orthologous, to the human genes controlling the immune response to HIV-1. However, as with humans, macaques are outbred and exhibit considerable animal-to-animal variation in the outcome of SIV or SHIV infection. This feature of macaque models is a double-edged sword. On the one hand, genetic variation among animals affords an opportunity to model specific aspects of host–pathogen interactions, such as the role of protective MHC class I alleles in controlling viral infection. On the other hand, this genetic diversity can greatly complicate studies using small numbers of animals, making it necessary to balance the frequency of protective MHC class I and TRIM5 genotypes between experimental and control groups.
Infection of macaques with SIVs and SHIVs has been a mainstay of AIDS research in animal models for more than 20 years, but SIV and HIV-1 are nevertheless different viruses. The development of stHIV strains that more accurately reflect the features of the virus responsible for the AIDS pandemic would overcome many of the remaining limitations of current models. Recent advances towards this goal have been made possible by significant basic research accomplishments, particularly in deciphering how HIV-1 overcomes intrinsic cellular barriers to viral replication. Although the development of stHIV-1 strains is still in its infancy, this approach has the potential to transform non-human primate models for anti-HIV-1 drug and vaccine development. It is possible that no animal model will ever fully capture all of the features of human HIV-1 infection. Nonetheless, the power and sophistication of systems for the preclinical evaluation of treatment and prevention strategies for HIV-1 infection continue to improve, with the refinement of existing models and the development of new ones.
References
Gao, F. et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397, 436–441 (1999).
Keele, B. F. et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313, 523–526 (2006).
Keele, B. F. et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460, 515–519 (2009).
Alter, H. J. et al. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science 226, 549–552 (1984).
O'Neil, S. P. et al. Progressive infection in a subset of HIV-1-positive chimpanzees. J. Infect. Dis. 182, 1051–1062 (2000).
Hatziioannou, T. & Bieniasz, P. D. Antiretroviral restriction factors. Curr. Opin. Virol. 1, 526–532 (2011).
Morrow, W. J., Wharton, M., Lau, D. & Levy, J. A. Small animals are not susceptible to human immunodeficiency virus infection. J. Gen. Virol. 68, 2253–2257 (1987).
Browning, J. et al. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl Acad. Sci. USA 94, 14637–14641 (1997).
Dunn, C. S. et al. Human immunodeficiency virus type 1 infection of human CD4-transgenic rabbits. J. Gen. Virol. 76, 1327–1336 (1995).
Keppler, O. T. et al. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195, 719–736 (2002).
Bieniasz, P. D. & Cullen, B. R. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74, 9868–9877 (2000).
Mariani, R. et al. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74, 3859–3870 (2000).
Tervo, H. M. & Keppler, O. T. High natural permissivity of primary rabbit cells for HIV-1, with a virion infectivity defect in macrophages as the final replication barrier. J. Virol. 84, 12300–12314 (2010).
Leonard, J. M. et al. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 242, 1665–1670 (1988).
Sun, J. et al. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J. Virol. 80, 1850–1862 (2006).
Blunt, T. et al. Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc. Natl Acad. Sci. USA 93, 10285–10290 (1996).
McCune, J. M. et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241, 1632–1639 (1988). This is the first report to describe the engraftment of scid mice with human thymus and liver tissue to establish the scid -hu–Thy/Liv mouse model.
Namikawa, R., Kaneshima, H., Lieberman, M., Weissman, I. L. & McCune, J. M. Infection of the SCID-hu mouse by HIV-1. Science 242, 1684–1686 (1988). This study demonstrates productive HIV-1 infection and CD4+ T cell depletion in human tissues of hu- scid mice.
Aldrovandi, G. M. et al. The SCID-hu mouse as a model for HIV-1 infection. Nature 363, 732–736 (1993).
Bonyhadi, M. L. et al. HIV induces thymus depletion in vivo. Nature 363, 728–732 (1993).
Stanley, S. K. et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 178, 1151–1163 (1993).
Jenkins, M., Hanley, M. B., Moreno, M. B., Wieder, E. & McCune, J. M. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood 91, 2672–2678 (1998).
McCune, J. M., Namikawa, R., Shih, C. C., Rabin, L. & Kaneshima, H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science 247, 564–566 (1990).
Shih, C. C. et al. Postexposure prophylaxis with zidovudine suppresses human immunodeficiency virus type 1 infection in SCID-hu mice in a time-dependent manner. J. Infect. Dis. 163, 625–627 (1991).
Nonoyama, S., Smith, F. O., Bernstein, I. D. & Ochs, H. D. Strain-dependent leakiness of mice with severe combined immune deficiency. J. Immunol. 150, 3817–3824 (1993).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nature Rev. Immunol. 7, 118–130 (2007).
Mosier, D. E., Gulizia, R. J., Baird, S. M. & Wilson, D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335, 256–259 (1988). This article describes the generation of scid -hu–PBL mice by engraftment of scid mice with human PBLs.
Hoffmann-Fezer, G., Gall, C., Zengerle, U., Kranz, B. & Thierfelder, S. Immunohistology and immunocytology of human T-cell chimerism and graft-versus-host disease in SCID mice. Blood 81, 3440–3448 (1993).
Mosier, D. E. et al. Human immunodeficiency virus infection of human-PBL-SCID mice. Science 251, 791–794 (1991).
Mosier, D. E., Gulizia, R. J., MacIsaac, P. D., Torbett, B. E. & Levy, J. A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260, 689–692 (1993).
Gauduin, M. C. et al. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nature Med. 3, 1389–1393 (1997).
Parren, P. W. et al. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS 9, F1–6 (1995).
Safrit, J. T. et al. hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope gp120. AIDS 7, 15–21 (1993).
Delhem, N. et al. Primary Th1 cell immunization against HIVgp160 in SCID-hu mice coengrafted with peripheral blood lymphocytes and skin. J. Immunol. 161, 2060–2069 (1998).
Mosier, D. E., Gulizia, R. J., MacIsaac, P. D., Corey, L. & Greenberg, P. D. Resistance to human immunodeficiency virus 1 infection of SCID mice reconstituted with peripheral blood leukocytes from donors vaccinated with vaccinia gp160 and recombinant gp160. Proc. Natl Acad. Sci. USA 90, 2443–2447 (1993).
Lapenta, C. et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-α. J. Exp. Med. 198, 361–367 (2003).
Yoshida, A. et al. Induction of protective immune responses against R5 human immunodeficiency virus type 1 (HIV-1) infection in hu-PBL-SCID mice by intrasplenic immunization with HIV-1-pulsed dendritic cells: possible involvement of a novel factor of human CD4+ T-cell origin. J. Virol. 77, 8719–8728 (2003).
Shultz, L. D. et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154, 180–191 (1995).
Prochazka, M., Gaskins, H. R., Shultz, L. D. & Leiter, E. H. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc. Natl Acad. Sci. USA 89, 3290–3294 (1992).
Ito, M. et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182 (2002). This is one of the first reports to describe the development of a human immune system in NOD scid Il2gr−/− mice following engraftment with human CD34+ stem cells derived from cord blood.
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005). This is an early study showing the development of a nearly complete human immune system following the engraftment of NOD scid Il2rg−/− mice with human CD34+ stem cells derived from peripheral blood.
McDermott, S. P., Eppert, K., Lechman, E. R., Doedens, M. & Dick, J. E. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 116, 193–200 (2010).
Brehm, M. A. et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγnull mutation. Clin. Immunol. 135, 84–98 (2010).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 106, 1565–1573 (2005).
Watanabe, S. et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rγnull mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109, 212–218 (2007).
Dash, P. K. et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J. Neurosci. 31, 3148–3157 (2011).
Gorantla, S. et al. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am. J. Pathol. 177, 2938–2949 (2010).
Holt, N. et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nature Biotech. 28, 839–847 (2010).
Joseph, A. et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J. Virol. 84, 6645–6653 (2010).
Kumar, P. et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 134, 577–586 (2008).
Balazs, A. B. et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481, 81–84 (2011).
Melkus, M. W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature Med. 12, 1316–1322 (2006). This paper is the first to describe the generation of BLT mice by the transplantation of CD34+ stem cells into NOD scid mice that had previously been implanted with human thymus and liver tissues.
Wege, A. K., Melkus, M. W., Denton, P. W., Estes, J. D. & Garcia, J. V. Functional and phenotypic characterization of the humanized BLT mouse model. Curr. Top. Microbiol. Immunol. 324, 149–165 (2008).
Brainard, D. M. et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83, 7305–7321 (2009).
Denton, P. W. et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5, e16 (2008).
Sun, Z. et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007).
Denton, P. W. et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS ONE 5, e8829 (2010).
Goldman, J. P. et al. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor γ chain. Br. J. Haematol. 103, 335–342 (1998). This research demonstrates efficient engraftment of human CD34+ stem cells in Rag2−/−Il2rg−/− mice, and shows the advantages of these mice over scid models.
Baenziger, S. et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice. Proc. Natl Acad. Sci. USA 103, 15951–15956 (2006).
Berges, B. K., Wheat, W. H., Palmer, B. E., Connick, E. & Akkina, R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−γc−/− (RAG-hu) mouse model. Retrovirology 3, 76 (2006).
Choudhary, S. K. et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/−γc−/− mouse. J. Virol. 83, 8254–8258 (2009).
Neff, C. P. et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+ T cell decline in humanized mice. Sci. Transl. Med. 3, 66ra6 (2011).
Van Duyne, R. et al. Effect of transcription peptide inhibitors on HIV-1 replication. Virology 376, 308–322 (2008).
Hofer, U. et al. RAG2−/−γc−/− mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. J. Virol. 82, 12145–12153 (2008).
Akkina, R. et al. Humanized Rag1−/−γc−/− mice support multilineage hematopoiesis and are susceptible to HIV-1 infection via systemic and vaginal routes. PLoS ONE 6, e20169 (2011).
Berges, B. K., Akkina, S. R., Folkvord, J. M., Connick, E. & Akkina, R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2−/−γc−/− (RAG-hu) mice. Virology 373, 342–351 (2008).
Abdool Karim, Q. et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329, 1168–1174 (2010).
Doulatov, S., Notta, F., Laurenti, E. & Dick, J. E. Hematopoiesis: a human perspective. Cell Stem Cell 10, 120–136 (2012).
Takizawa, H., Boettcher, S. & Manz, M. G. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood 119, 2991–3002 (2012).
Garcia, S. & Freitas, A. A. Humanized mice: current states and perspectives. Immunol. Lett. 146, 1–7 (2012).
Rathinam, C. et al. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood 118, 3119–3128 (2011).
Chahroudi, A., Bosinger, S. E., Vanderford, T. H., Paiardini, M. & Silvestri, G. Natural SIV hosts: showing AIDS the door. Science 335, 1188–1193 (2012).
Hirsch, V. M., Olmsted, R. A., Murphey-Corb, M., Purcell, R. H. & Johnson, P. R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339, 389–392 (1989).
Veazey, R. S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998). This work identifies the GALT of rhesus macaques as a major site of SIV replication and CD4+ T cell depletion.
Boyson, J. E. et al. The MHC class I genes of the rhesus monkey: different evolutionary histories of the MHC class I and II genes in primates. J. Immunol. 156, 4656–4665 (1996).
Otting, N. et al. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc. Natl Acad. Sci. USA 102, 1626–1631 (2005).
Loffredo, J. T., Valentine, L. E. & Watkins, D. I. in HIV Molecular Immunology 2006/2007 (eds Korber, B.T. et al.) 29–51 (Los Alamos National Laboratory, 2007).
Mothe, B. R. et al. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77, 2736–2740 (2003).
Yant, L. J. et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80, 5074–5077 (2006).
Loffredo, J. T. et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81, 8827–8832 (2007).
Stremlau, M. et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 (2004). This investigation pinpoints TRIM5α as the cellular protein that accounts for the post-entry block to HIV-1 infection in cells from macaques and other Old World monkeys.
Newman, R. M. et al. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5α. Proc. Natl Acad. Sci. USA 103, 19134–19139 (2006).
Lim, S.-Y. et al. TRIM5α modulates immunodeficiency virus control in rhesus monkeys. PLoS Pathog. 6, e1000738 (2010).
Fenizia, C. et al. TRIM5α does not affect simian immunodeficiency virus SIVmac251 replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J. Virol. 85, 12399–12409 (2011).
Kirmaier, A. et al. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 8, e1000462 (2010). This study finds that differences in susceptibility to TRIM5 variants account for the highly variable outcome of SIVsmE543-3 infection in rhesus macaques.
Marthas, M. L., Lu, D., Penedo, M. C. T., Hendrickx, A. G. & Miller, C. J. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res. Hum. Retroviruses 17, 1455–1466 (2001).
Ling, B. et al. SIVmac pathogenesis in rhesus macaques of Chinese and Indian orgin compared with primary HIV infections in humans. AIDS 16, 1489–1496 (2002).
Reimann, K. A. et al. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgous macaques and associated with early T-lymphocyte responses. J. Virol. 79, 8878–8885 (2005).
Yamamoto, H., Kawada, M., Takeda, A., Igarashi, H. & Matano, T. Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS ONE 2, e540 (2007).
Karl, J. A. et al. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics 60, 37–46 (2008).
Naruse, T. K. et al. Diversity of MHC class I genes in Burmese-origin rhesus macaques. Immunogenetics 62, 601–611 (2010).
Klatt, N. R. et al. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J. Virol. 86, 1203–1213 (2012).
Batten, C. J. et al. Comparative evaluation of simian, simian–human, and human immunodeficiency virus infection in pigtail macaque (Macaca nemestrina) model. AIDS Res. Hum. Retroviruses 22, 580–588 (2006).
Fernandez, C. S. et al. Screening and confirmatory testing of MHC class I alleles in pig-tailed macaques. Immunogenetics 63, 511–521 (2011).
Smith, M. Z. et al. Analysis of pigtail macaque major histocompatibility complex class I molecules presenting immunodominant simian immunodeficiency virus epitopes. J. Virol. 79, 684–695 (2005).
Liao, C. H., Kuang, Y. Q., Liu, H. L., Zheng, Y. T. & Su, B. A novel fusion gene, TRIM5-cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. AIDS 21 (Suppl. 8), S19–S26 (2007). This investigation is the first to show that pig-tailed macaques express a TRIM5–cyclophilin A fusion protein that does not restrict HIV-1, providing a molecular basis for the susceptibility of pig-tailed macaques to HIV-1 infection.
Wilson, S. J. et al. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl Acad. Sci. USA 105, 3557–3562 (2008).
Virgen, C. A., Kratovac, Z., Bieniasz, P. D. & Hatziioannou, T. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc. Natl Acad. Sci. USA 105, 3563–3568 (2008).
Newman, R. M. et al. Evolution of a TRIM5-CypA spice isoform in Old World monkeys. PLoS Pathog. 4, e1000003 (2008).
Brennan, G., Kozyrev, Y. & Hu, S.-L. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl Acad. Sci. USA 105, 3569–3574 (2008).
Hatziioannou, T. et al. A macaque model of HIV-1 infection. Proc. Natl Acad. Sci. USA 106, 4425–4429 (2009). This is an important proof-of-concept study demonstrating that a minimally modified strain of HIV-1, differing from HIV-1 only in vif , can replicate in pig-tailed macaques and is sensitive to a combination of antiretroviral drugs used to treat HIV-1 infection in humans.
Igarashi, T. et al. Human immunodeficency virus type 1 derivative with 7% simian immunodeficiency virus genetic content is able to establish infections in pig-tailed macaques. J. Virol. 81, 11549–11552 (2007).
Pendley, C. J. et al. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics 60, 339–351 (2008).
Budde, M. L. et al. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics 62, 773–780 (2010).
Dietrich, E. A. et al. Variable prevalence and functional diversity of the antiretroviral restriction factor TRIMCyp in Macaca fascicularis. J. Virol. 85, 9956–9963 (2011).
Krebs, K. C., Jin, Z., Rudersdorf, R., Hughes, A. L. & O'Connor, D. H. Unusually high frequency MHC class I alleles in Mauritian origin cynomolgus macaques. J. Immunol. 175, 5230–5239 (2005).
Wiseman, R. W. et al. Simian immunodeficiency virus SIVmac239 infection of major histocompatibility complex-identical cynomolgus macaques from Mauritius. J. Virol. 81, 349–361 (2007).
O'Connor, S. L. et al. MHC heterozygote advantage in simian immunodeficiency virus-infected Mauritian cynomolgus macaques. Sci. Transl. Med. 2, 22ra18 (2010). This research makes elegant use of the limited number of MHC haplotypes in Mauritian cynomolgus macaques to demonstrate the heterozygote advantage in the control of SIV infection.
Daniel, M. D. et al. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 228, 1201–1204 (1985). This is the first report to describe the isolation of SIV from rhesus macaques.
Mansfield, K. G., Lerch, N. W., Gardner, M. B. & Lackner, A. A. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 24, 116–122 (1995).
Apetrei, C. et al. Kuru experiments triggered the emergence of pathogenic SIVmac . AIDS 20, 317–321 (2006).
Keele, B. F. et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206, 1117–1134 (2009).
Kestler, H. et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248, 1109–1112 (1990). This is the first study to show that an infectious molecular clone of SIV can cause AIDS in rhesus macaques.
Hirsch, V. et al. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71, 1608–1620 (1997).
Reynolds, M. R. et al. Macaques vaccinate with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. 205, 2537–2550 (2008).
Wyand, M. S. et al. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73, 8356–8363 (1999).
Barouch, D. H. et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482, 89–94 (2012).
Reynolds, M. R. et al. The TRIM5α genotype of rhesus macaques affects acquisition of simian immunodeficiency virus SIVsmE660 infection after repeated limiting-dose intrarectal challenge. J. Virol. 85, 9637–9640 (2011).
Riddick, N. E. et al. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 6, e1001064 (2010).
Thakallapally, R., Rose, P., Vasil, S., Pillai, S. & Kuiken, C. L. in Human Retroviruses and AIDS ( eds Kuiken, C. L. et al.) 506–516 (Theoretical Biology and Biophysics Group, 1999).
Reimann, K. A. et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70, 3198–3206 (1996). This report describes the passage and initial isolation of the highly pathogenic chimaera SHIV89.6P.
Joag, S. V. et al. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 70, 3189–3197 (1996).
Igarashi, T. et al. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl Acad. Sci. USA 96, 14049–14054 (1999).
Feinberg, M. B. & Moore, J. P. AIDS vaccine models: challenging challenge viruses. Nature Med. 8, 207–210 (2002).
Nishimura, Y. et al. Higly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl Acad. Sci. USA 101, 12324–12329 (2004).
Harouse, J. M., Gettie, A., Tan, R. C., Blanchard, J. & Cheng-Mayer, C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284, 816–819 (1999). This article describes the first pathogenic SHIV found to use CCR5 as a co-receptor.
Veazey, R. S. et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature 483, 99–102 (2005).
Ng, C. T. et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nature Med. 16, 1117–1119 (2010).
Hessel, A. J. et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449, 101–105 (2007).
Shakirzyanova, M. et al. Pathogenic consequences of vaginal infection with CCR5-tropic simian-human immunodeficiency virus SHIVSF162P3N . J. Virol. 86, 9432–9442 (2012).
Nishimura, Y. et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J. Virol. 84, 4769–4781 (2010).
Song, R. J. et al. Molecularly cloned SHIV-1157ipd3N4: a highly replication-competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C env. J. Virol. 80, 8729–8738 (2006).
Ambrose, Z. et al. In vitro characterization of a simian immunodeficiency virus-human immunodeficiency virus (HIV) chimera expressing HIV type 1 reverse transcriptase to study antiviral resistance in pigtail macaques. J. Virol. 78, 13553–13561 (2004).
Uberla, K. et al. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc. Natl Acad. Sci. USA 92, 8210–8214 (1995).
Ambrose, Z. et al. Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J. Virol. 81, 12145–12155 (2007).
North, T. W. et al. Suppression of virus load by highly active antiretroviral therapy in rhesus macaques infected with a recombinant simian immunodeficiency virus containing reverse transcriptase from human immunodeficiency virus type 1. J. Virol. 79, 7349–7354 (2005).
Kearney, M. et al. Genetic diversity of simian immunodeficiency virus encoding HIV-1 reverse transcriptase persists in macaques despite antiretroviral therapy. J. Virol. 85, 1067–1076 (2011).
North, T. W. et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 84, 2913–2922 (2010).
Ishimatsu, M. et al. Construction of a novel SHIV having an HIV-1-derived protease gene and its infection to rhesus macaques: a useful tool for in vivo efficacy tests of protease inhibitors. Microbes Infect. 9, 475–482 (2007).
Smith, J. M. et al. Generation of a dual RT Env SHIV that is infectious in rhesus macaques. J. Med. Primatol. 39, 213–223 (2010).
Thippeshappa, R. et al. Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1. J. Virol. 85, 3767–3779 (2011).
McNatt, M. W. et al. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5, 1–12 (2009).
Sawyer, S. L., Wu, L. I., Emerman, M. & Malik, H. S. Positive selection of primate TRIM5α identifies a critical species-specific retroviral restriction domain. Proc. Natl Acad. Sci. USA 102, 2832–2837 (2005).
Sawyer, S. L., Emerman, M. & Malik, H. S. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol. 2, 1278–1285 (2004).
Sayah, D. M., Sokolskaja, E., Berthoux, L. & Luban, J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430, 569–573 (2004).
Stremlau, M. et al. Species recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proc. Natl Acad. Sci. USA 103, 5514–5519 (2006).
Sebastian, S. & Luban, J. TRIM5α selectively binds a restriction-sensitive retroviral capsid. Retrovirology 2, 40 (2005).
Chiu, Y. L. & Greene, W. C. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu. Rev. Immunol. 26, 317–353 (2008).
Schafer, A., Bogerd, H. P. & Cullen, B. R. Specific packaging of APOBEC3G into HIV-1 virions is mediated by the nucleocapsid domain of the gag polyprotein precursor. Virology 328, 163–168 (2004).
Svarovskaia, E. S. et al. Human apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) is incorporated into HIV-1 virions through interactions with viral and nonviral RNAs. J. Biol. Chem. 279, 35822–35828 (2004).
Zennou, V., Perez-Caballero, D., Gottlinger, H. & Bieniasz, P. D. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J. Virol. 78, 12058–12061 (2004).
Sheehy, A. M., Gaddis, N. C., Choi, J. D. & Malim, M. H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 (2002). This work identifes APOBEC3G (also known as CEM15) as the first known restriction factor for HIV-1; this protein family is now recognized as an important block to HIV-1 replication in macaques.
Harris, R. S. et al. DNA deamination mediates innate immunity to retroviral infection. Cell 113, 803–809 (2003).
Mangeat, B. et al. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424, 99–103 (2003).
Zhang, H. et al. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424, 94–98 (2003).
Marin, M., Rose, K. M., Kozak, S. L. & Kabat, D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nature Med. 9, 1398–1403 (2003).
Sheehy, A. M., Gaddis, N. C. & Malim, M. H. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nature Med. 9, 1404–1407 (2003).
Yu, X. et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 vif-cul5-SCF complex. Science 302, 1056–1060 (2003).
Mariani, R. et al. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114, 21–31 (2003).
Virgen, C. A. & Hatziioannou, T. Antiretroviral activity and Vif sensitivity of rhesus macaque APOBEC3 proteins. J. Virol. 81, 13932–13937 (2007).
Neil, S. J. D., Zang, T. & Bieniasz, P. D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430 (2008).
Van Damme, N. et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 1–8 (2008). References 161 and 162 identify tetherin as a restriction factor for HIV-1.
Perez-Caballero, D. et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139, 499–511 (2009).
Fitzpatrick, K. et al. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6, e1000701 (2010).
Hammonds, J., Wang, J.-J., Yi, H. & Spearman, P. Immunoelectron microscopic evidence for tetherin/BST2 as a the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 6, e1000749 (2010).
Jia, B. et al. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5, e1000429 (2009).
Zhang, F., Lanford, W., Ng, M., Bieniasz, P. & Hatziioannou, T. SIV Nef proteins antagonize tetherin by AP-2-dependent removal from sites of virion budding. 18th Conference on Retroviruses and Opportunistic Infections Abstract 85 (2011).
Sauter, D. et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6, 409–421 (2009).
Hauser, H. et al. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7, 51 (2010).
LeTortorec, A. & Neil, S. J. D. Antagonism and intracellular sequestration of human tetherin by the HIV-2 envelope glycoprotein. J. Virol. 83, 11966–11978 (2009).
Douglas, J. L. et al. Vpu directs the degradation of the human immunodeficiency virus retriction factor BST-2/tetherin via a βTrCP-dependent mechanism. J. Virol. 83, 7931–7947 (2009).
Goldstone, D. C. et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480, 379–382 (2011).
Powell, R. D., Holland, P. J., Hollis, T. & Perrino, F. W. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J. Biol. Chem. 286, 43596–43600 (2011).
Hrecka, K. et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 (2011).
Laguette, N. et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 (2011).
Martin-Serrano, J. & Neil, S. J. D. Host factors involved in retroviral budding and release. Nature Rev. Microbiol. 9, 519–531 (2011).
Pedersen, N. C., Ho, E. W., Brown, M. L. & Yamamoto, J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235, 790–793 (1987).
Torten, M. et al. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 65, 2225–2230 (1991).
Dow, S. W., Poss, M. L. & Hoover, E. A. Feline immunodeficiency virus: a neurotropic lentivirus. J. Acquir. Immune Defic. Syndr. 3, 658–668 (1990).
Podell, M., March, P. A., Buck, W. R. & Mathes, L. E. The feline model of neuroAIDS: understanding the progression towards AIDS dementia. J. Psychopharmacol. 14, 205–213 (2000).
Wongsrikeao, P., Saenz, D., Rinkoski, T., Otoi, T. & Poeschla, E. Antiviral restriction factor transgenesis in the domestic cat. Nature Methods 8, 853–859 (2011).
Arai, M., Earl, D. D. & Yamamoto, J. K. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet. Immunol. Immunopathol. 85, 189–204 (2002).
Hayes, K. A., Lafrado, L. J., Erickson, J. G., Marr, J. M. & Mathes, L. E. Prophylactic ZDV therapy prevents early viremia and lymphocyte decline but not primary infection in feline immunodeficiency virus-inoculated cats. J. Acquir. Immune Def. Syndr. 6, 127–134 (1993).
Hayes, K. A., Wilkinson, J. G., Frick, R., Francke, S. & Mathes, L. E. Early suppression of viremia by ZDV does not alter the spread of feline immunodeficiency virus infection in cats. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9, 114–122 (1995).
Smyth, N. R. et al. Effect of 3'azido-2',3'-deoxythymidine (AZT) on experimental feline immunodeficiency virus infection in domestic cats. Res. Vet. Sci. 57, 220–224 (1994).
Egberink, H. et al. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonomethoxyethyl)adenine. Proc. Natl Acad. Sci. USA 87, 3087–3091 (1990).
Hartmann, K. et al. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet. Immunol. Immunopathol. 35, 167–175 (1992).
Puras Lutzke, R. A., Eppens, N. A., Weber, P. A., Houghten, R. A. & Plasterk, R. H. Identification of a hexapeptide inhibitor of the human immunodeficiency virus integrase protein by using a combinatorial chemical library. Proc. Natl Acad. Sci. USA 92, 11456–11460 (1995).
Mazumder, A. et al. Inhibition of the human immunodeficiency virus type 1 integrase by guanosine quartet structures. Biochemistry 35, 13762–13771 (1996).
Savarino, A. et al. Human immunodeficiency virus integrase inhibitors efficiently suppress feline immunodeficiency virus replication in vitro and provide a rationale to redesign antiretroviral treatment for feline AIDS. Retrovirology 4, 79 (2007).
Auwerx, J., Esnouf, R., De Clercq, E. & Balzarini, J. Susceptibility of feline immunodeficiency virus/human immunodeficiency virus type 1 reverse transcriptase chimeras to non-nucleoside RT inhibitors. Mol. Pharmacol. 65, 244–251 (2004).
Slee, D. H. et al. Selectivity in the inhibition of HIV and FIV protease: inhibitory and mechanistic studies of pyrrolidine-containing α-keto amide and hydroxyethylamine core structures. J. Am. Chem. Soc. 117, 11867–11878 (1995).
Sparger, E. E. in In Vivo Models of HIV Disease and Control (eds Friedman, H., Specter, S. & Bendinelli, M.) 149–237 (Springer, 2006).
Shimojima, M. et al. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 303, 1192–1195 (2004).
Dean, G. A., Reubel, G. H., Moore, P. F. & Pedersen, N. C. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J. Virol. 70, 5165–5169 (1996).
Rud, E. W. et al. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J. Gen. Virol. 75, 529–543 (1994).
Benveniste, R. E. et al. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV/LAV and other lentiviruses. J. Virol. 60, 483–490 (1986).
Morton, W. R. et al. Transmission of the simian immunodeficiency virus SIVmne in macaques and baboons. J. Med. Primatol. 18, 237–245 (1989).
Rudensey, L. M., Kimata, J. T., Long, E. M., Chackerian, B. & Overbaugh, J. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVmne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J. Virol. 72, 209–217 (1998).
Chackerian, B., Rudensey, L. M. & Overbaugh, J. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71, 7719–7727 (1997).
Kimata, J. T., Kuller, L., Anderson, D. B., Dailey, P. & Overbaugh, J. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nature Med. 5, 535–541 (1999).
Fultz, P. N., McClure, H. M., Anderson, D. C. & Switzer, W. M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res. Hum. Retroviruses 5, 397–409 (1989).
Smith, D. G. & McDonough, J. Mitochondrial DNA variation in Chinese and Indian rhesus macaques (Macaca mulatta). Am. J. Pirmatol. 65, 1–25 (2005).
Hernandez, R. D. et al. Demographic histories and patterns of linkage disequilibrium in Chinese and Indian rhesus macaques. Science 316, 240–243 (2007).
Morales, J. C. & Melnick, D. J. Phylogenetic relationships of the macaques (Ceropithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal gens. J. Hum. Evol. 34, 1–23 (1998).
Wolfheim, J. H. Primates of the World: Distribution, Abundance, and Conservation (Univ. of Washington Press, 1983).
Acknowledgements
The authors are grateful to P. Bieniasz and R. Desrosiers for critically reviewing this manuscript. The authors also thank P. Marx for sharing the data included in table 1, and A. Halper-Stromberg and R. Liberatore for help with figure 1 and lessons in mouse anatomy. The authors acknowledge and regret that a number of important studies are not cited owing to space constraints. T.H. is supported by US Department of Health and Human Services Public Health Service (PHS) grants AI078788 and AI093255. D.T.E. is supported by PHS grants AI087498, AI095098, AI098485 and RR000168/OD011103, and is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- ESCRT
-
(Endosomal sorting complex required for transport). A conserved cellular machinery for the sorting of ubiquitylated cargo proteins into vesicles and the subsequent scission of the membrane neck. The recruitment of ESCRT proteins through retroviral late domains is essential for viral budding.
- Proviruses
-
The DNA forms of retroviral genomes that are integrated into host chromosomal DNA.
- Haematopoietic (CD34+) progenitor stem cells
-
A population of multipotent cells that can differentiate into any type of blood cell, including the major cell lineages of the immune system. These cells are typically defined by the cell surface marker CD34.
- Natural killer cells
-
Cytotoxic lymphocytes that do not express the B cell or T cell receptor and are capable of responding to virus-infected cells or tumour cells without prior antigenic stimulation.
- Passive immunization
-
The transfer of immunoglobulins.
- Complement
-
A system of >25 serum proteins that is activated by a number of triggers responding to foreign antigen on the surface of a cell; complement activation results in a proteolytic cascade leading to cell lysis or enhanced phagocytosis.
- Gut-associated lymphoid tissue
-
(GALT). Secondary lymphoid tissue associated with the digestive tract, including the Peyer's patches, mesenteric lymph nodes and the appendix. For the purpose of this Review, the GALT also refers to effector sites of the lamina propria and intestinal epithelium that are rich in memory lymphocytes which support HIV and simian immunodeficiency virus (SIV) replication.
- Central memory CD4+ T cells
-
Antigen-experienced resting T cells that express cell surface receptors which are required for homing to secondary lymphoid organs. These cells are generally long-lived and can serve as the precursors for effector T cells during recall responses.
- Effector memory CD4+ T cells
-
Terminally differentiated T cells that lack lymph node-homing receptors but express receptors that enable the cells to home to inflamed tissues. Effector memory T cells can exert immediate effector functions without the need for further differentiation.
Rights and permissions
About this article
Cite this article
Hatziioannou, T., Evans, D. Animal models for HIV/AIDS research. Nat Rev Microbiol 10, 852–867 (2012). https://doi.org/10.1038/nrmicro2911
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2911
This article is cited by
-
Towards a better preclinical cancer model – human immune aging in humanized mice
Immunity & Ageing (2023)
-
Modelling host–microbiome interactions in organ-on-a-chip platforms
Nature Reviews Bioengineering (2023)
-
Microphysiological model reveals the promise of memory-like natural killer cell immunotherapy for HIV± cancer
Nature Communications (2023)
-
Advances in HIV therapeutics and cure strategies: findings obtained through non-human primate studies
Journal of NeuroVirology (2023)
-
Subsequent malaria enhances virus-specific T cell immunity in SIV-infected Chinese rhesus macaques
Cell Communication and Signaling (2022)