Key Points
-
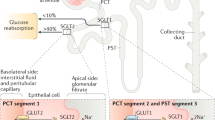
The kidneys contribute to the maintenance of normal glucose homeostasis by using glucose as a metabolic fuel, by producing glucose via gluconeogenesis, and by reabsorbing all filtered glucose
-
Under physiological conditions SGLT2 reabsorbs the majority (80–90%) of filtered glucose, while SGLT1 reabsorbs the remaining 10–20% of glucose
-
Kidneys contribute to the development of hyperglycaemia in diabetes by producing excess amounts of glucose and by increasing glucose reabsorption in response to an elevated threshold for glucosuria and an increase in the maximum glucose reabsorptive capacity (TmG)
-
SGLT2 inhibitors improve glucose tolerance by reducing both the threshold for glucosuria and the TmG and by ameliorating glucotoxicity leading to enhanced β-cell function and improved insulin sensitivity in muscle
-
The efficacy of SGLT2 inhibitors is partially offset by an increase in endogenous glucose production and enhanced glucose reabsorption by SGLT1
-
Findings from the EMPA-REG OUTCOME study suggest that the SGLT2 inhibitors might be beneficial in reducing cardiovascular events and preventing the progression of renal disease in patients with type 2 diabetes mellitus at high cardiovascular risk
Abstract
The kidney has a pivotal role in maintaining glucose homeostasis by using glucose as a metabolic fuel, by producing glucose through gluconeogenesis, and by reabsorbing all filtered glucose through the sodium–glucose cotransporters SGLT1 and SGLT2 located in the proximal tubule. In patients with diabetes, the maximum glucose reabsorptive capacity (TmG) of the kidney, as well as the threshold for glucose spillage into the urine, are elevated, contributing to the pathogenesis of hyperglycaemia. By reducing the TmG and, more importantly, the threshold of glucosuria, SGLT2 inhibitors enhance glucose excretion, leading to a reduction in fasting and postprandial plasma glucose levels and improvements in both insulin secretion and insulin sensitivity. The beneficial effects of SGLT2 inhibition extend beyond glycaemic control, however, with new studies demonstrating that inhibition of renal glucose reabsorption reduces blood pressure, ameliorates glucotoxicity and induces haemodynamic effects that lead to improved cardiovascular and renal outcomes in patients with type 2 diabetes mellitus. In this Review we examine the role of SGLT2 and SGLT1 in the regulation of renal glucose reabsorption in health and disease and the effect of SGLT2 inhibition on renal function, glucose homeostasis, and cardiovascular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998). First study to demonstrate that improved glycaemic control reduces microvascular complications in T2DM.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993). First study to demonstrate that improved glycaemic control decreases microvascular complications in T1DM.
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
Hayward, R. A. et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 372, 2197–2206 (2015).
Ferrannini, E. & DeFronzo, R. A. Impact of glucose- lowering drugs on cardiovascular disease in type 2 diabetes. Eur. Heart J. 36, 2288–2296 (2015).
DeFronzo, R. A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53, 1270–1287 (2010). Review of the relationship between insulin resistance and the development of atherosclerotic cardiovascular disease.
Action to Control Cardiovascular Risk in Diabetes Study Group. et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008).
Patel, A. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
Look Ahead Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 22, 5–13 (2014).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015). First study to demonstrate that an SGLT2 inhibitor, empagliflozin decreases MACE (Major Adverse Cardiovascular Events) and hospitalization for heart failure in patients with T2DM.
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016). First study to demonstrate that a glucagon-like peptide-1 (GLP-1) receptor agonist reduces MACE in patients with T2DM.
Wanner, C. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 374, 323–334 (2016). Kidney disease was a secondary end point in the EMPA-REG OUTCOME study. Empagliflozin significantly decreased the composite renal end point of doubling of serum creatinine; development of eGFR <45 ml/min per 1.73m2; development of macroalbuminuria; initiation of renal replacement therapy; or death from renal disease.
DeFronzo, R. A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009). Classic review of the pathophysiology of T2DM and implications for therapy.
Stumvoll, M., Meyer, C., Mitrakou, A., Nadkarni, V. & Gerich, J. E. Renal glucose production and utilization: new aspects in humans. Diabetologia 40, 749–757 (1997).
Gustavson, S. M. et al. Effects of hyperglycemia, glucagon, and epinephrine on renal glucose release in the conscious dog. Metabolism 53, 933–941 (2004).
Stumvoll, M. et al. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J. Clin. Invest. 96, 2528–2533 (1995).
Vallon, V. et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Renal Physiol. 306, F194–F204 (2014).
Handelsman, Y. et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr. Pract. 22, 753–762 (2016). Overview of SGLT2-induced changes in intermediary metabolism and their implications for the development of ketoacidosis in T2DM patients treated with an SGLT2 inhibitor.
Vrhovac, I. et al. Localizations of Na-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 467, 1881–1898 (2014).
Wright, E. M., Loo, D. D. & Hirayama, B. A. Biology of human sodium glucose transporters. Physiol. Rev. 91, 733–794 (2011). Review of the basic physiology of SGLT transporters.
Bonner, C. et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 21, 512–517 (2015).
Kanai, Y., Lee, W. S., You, G., Brown, D. & Hediger, M. A. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J. Clin. Invest. 93, 397–404 (1994).
Merovci, A. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124, 509–514 (2014). Clinical study in patients with T2DM, demonstrating that treatment with an SGLT2 inhibitor induces glucosuria and reduces the plasma glucose concentration, leading to amelioration of glucotoxicity with improved insulin sensitivity in muscle.
Farber, S. J., Berger, E. Y. & Earle, D. P. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J. Clin. Invest. 30, 125–129 (1951).
DeFronzo, R. A. et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36, 3169–3176 (2013). Treatment with the SGLT2 inhibitor dapagliflozin reduced both the maximum tubular reabsorptive capacity for glucose (Tm G ) and the renal threshold for glucosuria. From the clinical standpoint, the reduction in the renal threshold to <2.2 mmol/l (<40 mg/dl) is the primary mechanism responsible for the glucosuria and reduction in HbA 1c with SGLT2 inhibition.
Mogensen, C. E. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand. J. Clin. Lab. Invest. 28, 101–109 (1971).
Kamran, M., Peterson, R. G. & Dominguez, J. H. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J. Am. Soc. Nephrol. 8, 943–948 (1997).
Abdul-Ghani, M. A., DeFronzo, R. A. & Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 62, 3324–3328 (2013). The amount of glucosuria induced by SGLT2 inhibitors is always less than expected based on the complete inhibition of SGLT2. This difference is explained by the ability of SGLT1 to markedly increase its reabsorption of glucose following SGLT2 inhibition.
Rahmoune, H. et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54, 3427–3434 (2005).
Freitas, H. S. et al. Na+–glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1α expression and activity. Endocrinology 149, 717–724 (2008).
Abdul-Ghani, M. A., Norton, L. & DeFronzo, R. A. Role of sodium–glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 32, 515–531 (2011).
Vallon, V. et al. Knockout of Na–glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Renal Physiol. 304, F156–F167 (2013).
Felicetta, J. V. & Sowers, J. R. Systemic hypertension in diabetes mellitus. Am. J. Cardiol. 61, 34H–40H (1988).
Rossetti, L., Shulman, G. I., Zawalich, W. & DeFronzo, R. A. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J. Clin. Invest. 80, 1037–1044 (1987). Classic physiologic study demonstrating that inhibition of renal glucose transport with phlorizin can normalize glucose tolerance and insulin sensitivity in a rodent model of diabetes. This study, along with another36, provided proof of concept for the development of SGLT2 inhibitor therapy for T2DM.
Rossetti, L., Smith, D., Shulman, G. I., Papachristou, D. & DeFronzo, R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Invest. 79, 1510–1515 (1987).
Kahn, B. B., Shulman, G. I., DeFronzo, R. A., Cushman, S. W. & Rossetti, L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J. Clin. Invest. 87, 561–570 (1991).
Katsuno, K. et al. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J. Pharmacol. Exp. Ther. 320, 323–330 (2007).
Sha, S. et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 13, 669–672 (2011).
Polidori, D. et al. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 98, E867–E871 (2013).
Grempler, R. et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes. Metab. 14, 83–90 (2012).
Komoroski, B. et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin. Pharmacol. Ther. 85, 520–526 (2009).
Sarashina, A. et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab. Pharmacokinet. 28, 213–219 (2013).
Gorboulev, V. et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196 (2012).
Powell, D. R. et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am. J. Physiol. Endocrinol. Metab. 304, E117–E130 (2013).
Rieg, T. et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Renal Physiol. 306, F188–F193 (2014). This study demonstrated the important role of SGLT1 in glucose reabsorption following SGLT2 inhibition and explains why SGLT2 inhibitors never produce the expected amount of glucosuria even when with complete transporter inhibition.
McKeown, J. W., Brazy, P. C. & Dennis, V. T. Intrarenal heterogeneity for fluid, phosphate, and glucose absorption in the rabbit. Am. J. Physiol. 237, F3112–F3318 (1979).
Powell, D. R. et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J. Pharmacol. Exp. Ther. 345, 250–259 (2013).
Zambrowicz, B. et al. Effects of LX4211, a dual SGLT1/SGLT2 inhibitor, plus sitagliptin on postprandial active GLP-1 and glycemic control in type 2 diabetes. Clin. Ther. 35, 273–285.e7 (2013).
Cariou, B. & Charbonnel, B. Sotagliflozin as a potential treatment for type 2 diabetes mellitus. Expert Opin. Investig. Drugs 24, 1647–1656 (2015). Discussion of combined SGLT2/SGLT1 therapy as a treatment for T2DM.
Song, P., Onishi, A., Koepsell, H. & Vallon, V. Sodium glucose contransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin. Ther. Targets 20, 1109–1125 (2016).
Komoroski, B. et al. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin. Pharmacol. Ther. 85, 513–519 (2009).
Vasilakou, D. et al. Sodium–glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann. Intern. Med. 159, 262–274 (2013).
Scheen, A. J. Pharmacodynamics, efficacy and safety of sodium–glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 75, 33–59 (2015). Review of the efficacy and safety of the currently approved SGLT2 inhibitors for the treatment of T2DM.
Abdul-Ghani, M. A., Norton, L. & DeFronzo, R. A. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Curr. Diab. Rep. 12, 230–238 (2012).
Nauck, M. A. et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 34, 2015–2022 (2011).
Nauck, M. A. et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes. Metab. 16, 1111–1120 (2014).
Lavalle-Gonzalez, F. J. et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 56, 2582–2592 (2013).
Schernthaner, G. et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 36, 2508–2515 (2013).
Del Prato, S. et al. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in patients with type 2 diabetes: 4-year data. Diabetes Obes. Metab. 17, 581–590 (2015).
Leiter, L. A. et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care 38, 355–364 (2015).
Zhang, L., Feng, Y., List, J., Kasichayanula, S. & Pfister, M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes. Metab. 12, 510–516 (2010).
Wilding, J. P. et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 32, 1656–1662 (2009). Clinical study demonstrating the efficacy of add-on SGLT2 inhibitor therapy in patients with poorly controlled T2DM treated with insulin.
Neal, B. et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) — a randomized placebo-controlled trial. Am. Heart J. 166, 217–223.e11 (2013).
Rosenstock, J. et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 37, 1815–1823 (2014).
Henry, R. R., Thakkar, P., Tong, C., Polidori, D. & Alba, M. Efficacy and safety of canagliflozin, a sodium–glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 38, 2258–2265 (2015).
Lamos, E. M., Younk, L. M. & Davis, S. N. Empagliflozin, a sodium glucose co-transporter 2 inhibitor, in the treatment of type 1 diabetes. Expert Opin. Investig. Drugs 23, 875–882 (2014).
DeFronzo, R. A., Stonehouse, A. H., Han, J. & Wintle, M. E. Relationship of baseline HbA1c and efficacy of current glucose-loweing therapies: a meta-analysis of randomized clinical trials. Diabet. Med. 27, 309–317 (2010).
DeFronzo, R. A. et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 38, 384–393 (2015).
Rosenstock, J. et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 38, 376–383 (2015).
Ferrannini, E., Ramos, S. J., Salsali, A., Tang, W. & List, J. F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33, 2217–2224 (2010).
Ferrannini, E. et al. Metabolic response to sodium–glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124, 499–508 (2014).
Merovci, A. et al. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J. Clin. Endocrinol. Metab. 100, 1927–1932 (2015). Clinical study in patients with T2DM demonstrating that treatment with an SGLT2 inhibitor induces glucosuria and reduces the plasma glucose concentration, leading to a marked improvement in β-cell function by ameliorating glucotoxicity.
Eldor, R. et al. Discordance between central (brain) and pancreatic action of exenatide in lean and obese subjects. Diabetes Care 39, 1804–1810 (2016).
Merovci, A. et al. Effect of dapagliflozin with and without acipimox on insulin sensitivity and insulin secretion in T2DM males. J. Clin. Endocrinol. Metab. 101, 1249–1256 (2016).
Ferrannini, E. et al. Shift to fatty substrates utilization in response to sodium–glucose co-transporter-2 inhibition in nondiabetic subjects and type 2 diabetic patients. Diabetes 65, 1190–1195 (2016).
Bonadonna, R. C. et al. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J. Clin. Invest. 92, 486–494 (1993).
Groop, L. C., Bonadonna, R. C., Shank, M., Petrides, A. S. & DeFronzo, R. A. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. Clin. Invest. 87, 83–89 (1991).
Bays, H., Mandarino, L. & DeFronzo, R. A. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 89, 463–478 (2004).
Hansen, L., Iqbal, N., Ekholm, E., Cook, W. & Hirshberg, B. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr. Pract. 20, 1187–1197 (2014).
Lambers Heerspink, H. J., de Zeeuw, D., Wie, L., Leslie, B. & List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 15, 853–862 (2013).
Oliva, R. V. & Bakris, G. L. Blood pressure effects of sodium–glucose co-transport 2 (SGLT2) inhibitors. J. Am. Soc. Hypertens. 8, 330–339 (2014). Comprehensive review of the blood pressure lowering effect of SGLT2 inhibition.
Jiang, F. et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat. Rev. Cardiol. 11, 413–426 (2014).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359, 1811–1821 (2008).
Yamout, H. et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am. J. Nephrol. 40, 64–74 (2014).
Abdul-Ghani, M., Del Prato, S. & DeFronzo, R. A. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME Study. Diabetes Care 39, 1–9 (2016). Overview of the potential mechanisms by which SGLT2 inhibitors prevented cardiovascular events in the EMPA-REG OUTCOME Study.
Skrtic, M. & Cherney, D. Z. Sodium–glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 24, 96–103 (2015). Review of the potential mechanisms by which SGLT2 inhibitors prevent diabetic nephropathy.
Hostetter, T. H., Rennke, H. G. & Brenner, B. M. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am. J. Med. 72, 375–380 (1982).
Nelson, R. G. et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 335, 1636–1642 (1996).
Ruggenenti, P. et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 35, 2061–2068 (2012).
Arakawa, K. et al. Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na+–glucose cotransporter inhibitor T-1095. Br. J. Pharmacol. 132, 578–586 (2001).
Cherney, D. Z. et al. Renal hemodynamic effect of sodium–glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014). First description in man of the ability of SGLT2 inhibitors to reverse hyperfiltration in patients with diabetes.
Hostetter, T. H., Troy, J. L. & Brenner, B. M. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 19, 410–415 (1981).
Tuttle, K. R. et al. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N. Engl. J. Med. 324, 1626–1632 (1991).
Cherney, D. Z. et al. Empagliflozin reduces microalbuminura and macroalbuminuria in patients with type 2 diabetes. Poster SA-PO-1109 presented at American Society of Nephrology Kidney Week. November 7, 2015: San Diego, CA.
Yale, J. F. et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 15, 463–473 (2013).
Barnett, A. H. et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2, 369–384 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02065791 (2016). Well-designed, large, prospective, placebo-controlled study to examine whether SGLT2 inhibitors can prevent or slow the progression of diabetic renal disease.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01989754 (2016).
Bolinder, J. et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J. Clin. Endocrinol. Metab. 97, 1020–1031 (2012).
Cefalu, W. T. et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382, 942–950 (2013).
Devenny, J. J. et al. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring) 20, 1645–1652 (2012).
Ferrannini, G. et al. Energy balance after sodium–glucose cotransporter 2 inhibition. Diabetes Care 38, 1730–1735 (2015).
Bode, B. et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes. Metab. 17, 294–303 (2015).
Stenlof, K. et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr. Med. Res. Opin. 30, 163–175 (2014).
Briand, F. et al. Empagliflozin, via switching metabolism toward lipid utilization, moderately increases LDL cholesterol levels through reduced LLD catabolism. Diabetes 65, 2032–2038 (2016). This study provides a potential explanation as to why SGLT2 inhibitors cause a small rise in LDL cholesterol.
Di Angelantonio, E. et al. Association of cardiometabolic multimorbidity with mortality. JAMA 314, 52–60 (2015).
The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 339, 1349–1357 (1998).
Patel, A. et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370, 829–840 (2007).
Sattar, N., McLaren, J., Kristensen, S. L., Preiss, D. & McMurray, J. J. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia 59, 1333–1339 (2016).
Scheen, A. J. Reduction in cardiovascular and all-cause mortality in the EMPA-REG OUTCOME trial: a critical analysis. Diabetes Metab. 42, 71–76 (2016).
Ferrannini, E., Mark, M. & Mayoux, E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 39, 1108–1114 (2016). Novel hypothesis suggesting that a switch in myocardial metabolism from glucose to ketones may reduce myocardial oxygen consumption and enhance myocardial contractility.
Mudaliar, S., Alloju, S. & Henry, R. R. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME Study? A unifying hypothesis. Diabetes Care 39, 1115–1122 (2016).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013).
White, W. B. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013).
Green, J. B. et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242 (2015).
Daniele, G. et al. Dapagliflozin enhances fat oxidation but decreases mitochondrial ATP synthesis rate in type 2 diabetes patients. Diabetes Care 39, 2036–2041 (2016).
Ferrannini, E. The theoretical bases of indirect calorimetry: a review. Metabolism 37, 287–301 (1988).
Cotter, D. G., Schugar, R. C. & Crawford, P. A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 304, H1060–H1076 (2013).
Xie, X. et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 387, 435–443 (2016).
Cruickshank, K. et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 106, 2085–2090 (2002).
Roman, M. J. et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 50, 197–203 (2007).
Scheen, A. J. Reappraisal of the diuretic effect of empagliflozin in the EMPA-REG OUTCOME trial: comparison with classic diuretics. Diabetes Metab. 42, 224–233 (2016). This review argues against an important role for the diuretic effect of SGLT2 inhibitors in the prevention of cardiovascular events in the EMPA-REG OUTCOME study.
McMurray, J. EMPA-REG — the “diuretic hypothesis”. J. Diabetes Complications 30, 3–4 (2016).
Kimura, G. Importance of inhibiting sodium–glucose cotransporter and its compelling indication in type 2 diabetes: pathophysiological hypothesis. J. Am. Soc. Hypertens. 10, 271–278 (2016).
Thuesen, L., Christiansen, J. S., Sorensen, K. E., Orskov, H. & Henningsen, P. Low-dose intravenous glucagon has no effect on myocardial contractility in normal man. An echocardiographic study. Scand. J. Clin. Lab. Invest. 48, 71–75 (1988).
Ali, S. et al. Cardiomyocyte glucagon receptor signaling modulates outcomes in mice with experimental myocardial infarction. Mol. Metab. 4, 132–143 (2015).
Levelt, E. et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65, 44–52 (2016).
Dziuba, J. et al. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes. Metab. 16, 628–635 (2014).
Peters, A. L. et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care 38, 1687–1693 (2015). First report describing the development of diabetic ketoacidosis in diabetic patients treated with a SGLT2 inhibitor.
Farxiga highlights of prescribing information. azpicentral http://www1.astrazeneca-us.com/pi/pi_farxiga.pdf (accessed 9 March 2016).
Ptaszynska, A. et al. Assessing bladder cancer risk in type 2 diabetes clinical trials: the dapagliflozin drug development program as a 'case study'. Diabetes Ther. 6, 357–375 (2015).
US Food and Drug Administration. FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. FDA http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm (2016).
Ljunggren, O. et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes. Metab. 14, 990–999 (2012).
US Food and Drug Administration. FDA Drug Safety Communication: interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate. FDA http://www.fda.gov/Drugs/DrugSafety/ucm500965.htm (2016).
Acknowledgements
R.A.D. is supported by NIH grants RO1DK24093-33 and R01DK103841-01A1. M.A.-G. is supported by NIH grant RO1-DK-097554-3. We are thankful to Ernest Wright and Chiara Ghezzi, University of California Los Angeles, who were helpful in reviewing and commenting on the manuscript before submission.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, discussion of the content, and revising or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.A.D. has consulted for AstraZeneca, Janssen, and Boehringer Ingelheim, is a member of the Speaker's Bureau for AstraZeneca and Novo Nordisk, and has received grant support from AstraZeneca, Janssen, and Boehringer Ingelheim. His salary is supported in part by the South Texas Veterans Health Care System. The other authors declare no competing interests.
Glossary
- Maximum renal glucose reabsorptive capacity
-
(TmG). The TmG represents the maximum capacity of the renal tubule to reabsorb glucose that is filtered by the glomerulus, and is expressed in mg per minute.
- Threshold for glucosuria
-
Represents the plasma glucose concentration at which glucose spillage into the urine is first observed.
- Stepped hyperglycaemic clamp
-
A technique in which the plasma glucose concentration is raised by a fixed amount (for example, ∼2.2 mmol/l) over a fixed time (for example, 30 minutes) to sequentially raise the plasma glucose concentration to 28–33 mmol/l (500–600 mg/dl). This technique enables the TmG and threshold for glucosuria to be calculated.
- First phase insulin secretion
-
The early response of insulin (within 0–10 minutes) to an acute intravenous injection of glucose.
- Second phase insulin secretion
-
The late response of insulin (within 10–120 minutes) to a sustained rise in plasma glucose concentration brought about by a continuous intravenous infusion of glucose.
Rights and permissions
About this article
Cite this article
DeFronzo, R., Norton, L. & Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 13, 11–26 (2017). https://doi.org/10.1038/nrneph.2016.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.170
This article is cited by
-
Risk of CKD among patients with DM taking diuretics or SGLT2i: a retrospective cohort study in Taiwan
BMC Pharmacology and Toxicology (2024)
-
Potential use of sodium glucose co-transporter 2 inhibitors during acute illness: a systematic review based on COVID-19
Endocrine (2024)
-
The SGLT2 inhibitor empagliflozin inhibits skeletal muscle fibrosis in naturally aging male mice through the AMPKα/MMP9/TGF-β1/Smad pathway
Biogerontology (2024)
-
Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment
Journal of Ovarian Research (2023)
-
The cardio-renal-metabolic connection: a review of the evidence
Cardiovascular Diabetology (2023)