Abstract
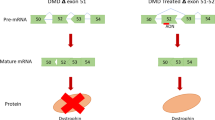
Manipulation of pre-mRNA splicing by antisense oligonucleotides (AOs) offers considerable potential for a number of genetic disorders. One of these is Duchenne muscular dystrophy (DMD), where mutations in the dystrophin gene typically result in premature termination of translation that causes a loss of functional protein. AOs can induce exon skipping such that the mutation is by-passed and the reading frame restored, producing an internally deleted protein similar to that found in the milder Becker muscular dystrophy. To date, this approach has been applied to the mdx mouse model in vitro and in vivo and in human myoblast cultures. Here, we report the application of AO-directed exon skipping to induce dystrophin expression in vitro in a canine model of DMD, golden retriever muscular dystrophy (GRMD). The efficacy of 2′-O-methyl phosphorothioate (2OMe), phosphorodiamidate morpholino oligomers (PMOs) and peptide-linked PMOs (PMO-Pep) to induce dystrophin expression was assessed. The 2OMe chemistry was only effective for short-term induction of corrected transcript and could not induce detectable dystrophin protein. The PMO chemistry generally induced limited exon skipping at only high concentrations; however, a low level of dystrophin protein was produced in treated cells. Use of the PMO-Pep, applied here for the first time to a DMD model, was able to induce high and sustained levels of exon skipping and induced the highest level of dystrophin expression with no apparent adverse effects upon the cells. The induction of dystrophin in the GRMD model offers the potential for further testing of AO delivery regimens in a larger animal model of DMD, in preparation for application in human clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoffman EP, Brown Jr RH, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Emery AE . The muscular dystrophies. Lancet 2002; 359: 687–695.
Mellies U, Ragette R, Dohna Schwake C, Boehm H, Voit T, Teschler H . Long-term noninvasive ventilation in children and adolescents with neuromuscular disorders. Eur Respir J 2003; 22: 631–636.
Alman BA . Duchenne muscular dystrophy and steroids: pharmacologic treatment in the absence of effective gene therapy. J Pediatr Orthop 2005; 25: 554–556.
Heald A, Anderson LV, Bushby KM, Shaw PJ . Becker muscular dystrophy with onset after 60 years. Neurology 1994; 44: 2388–2390.
Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci USA 2001; 98: 42–47.
Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA 2005; 102: 198–203.
van Deutekom JC, Bremmer-Bout M, Janson AA, Ginjaar IB, Baas F, den Dunnen JT et al. Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum Mol Genet 2001; 10: 1547–1554.
Aartsma-Rus A, Janson AA, Kaman WE, Bremmer-Bout M, den Dunnen JT, Baas F et al. Therapeutic antisense-induced exon skipping in cultured muscle cells from six different DMD patients. Hum Mol Genet 2003; 12: 907–914.
Aartsma-Rus A, Janson AA, Kaman WE, Bremmer-Bout M, Van Ommen GJ, Den Dunnen JT et al. Antisense-induced multiexon skipping for Duchenne muscular dystrophy makes more sense. Am J Hum Genet 2004; 74: 83–92.
Howell JM, Fletcher S, Kakulas BA, O'Hara M, Lochmuller H, Karpati G . Use of the dog model for Duchenne muscular dystrophy in gene therapy trials. Neuromuscul Disord 1997; 7: 325–328.
Sharp NJ, Kornegay JN, Van Camp SD, Herbstreith MH, Secore SL, Kettle S et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics 1992; 13: 115–121.
Crooke ST . Molecular mechanisms of action of antisense drugs. Biochim Biophys Acta 1999; 1489: 31–44.
Gebski BL, Mann CJ, Fletcher S, Wilton SD . Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum Mol Genet 2003; 12: 1801–1811.
Aartsma-Rus A, Kaman WE, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ et al. Comparative analysis of antisense oligonucleotide analogs for targeted DMD exon 46 skipping in muscle cells. Gene Therapy 2004; 11: 1391–1398.
Moulton HM, Nelson MH, Hatlevig SA, Reddy MT, Iversen PL . Cellular uptake of antisense morpholino oligomers conjugated to arginine-rich peptides. Bioconjug Chem 2004; 15: 290–299.
Reiss J, Rininsland F . An explanation for the constitutive exon 9 cassette splicing of the DMD gene. Hum Mol Genet 1994; 3: 295–298.
Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR . ESE Finder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res 2003; 31: 3568–3571.
Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med 2003; 9: 1009–1014.
Zuker M . Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31: 3406–3415.
Birney E, Kumar S, Krainer AR . Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res 1993; 21: 5803–5816.
Blencowe BJ . Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem Sci 2000; 25: 106–110.
Mann CJ, Honeyman K, McClorey G, Fletcher S, Wilton SD . Improved antisense oligonucleotide induced exon skipping in the mdx mouse model of muscular dystrophy. J Gene Med 2002; 4: 644–654.
Wu J, Lizarzaburu ME, Kurth MJ, Liu L, Wege H, Zern MA et al. Cationic lipid polymerization as a novel approach for constructing new DNA delivery agents. Bioconjug Chem 2001; 12: 251–257.
Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature 1988; 334: 154–156.
van Deutekom JC, van Ommen GJ . Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet 2003; 4: 774–783.
Acknowledgements
We thank Russell Johnson for technical assistance. This work was funded by Parent Project Muscular Dystrophy, USA, National Institute of Health, USA, National Health and Medical Research Council, Australia, Muscular Dystrophy Association, USA and the Medical and Health Research Infrastructure Fund, Western Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McClorey, G., Moulton, H., Iversen, P. et al. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther 13, 1373–1381 (2006). https://doi.org/10.1038/sj.gt.3302800
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302800
Keywords
This article is cited by
-
Induced alternative splicing an opportunity to study PCSK9 protein isoforms at physiologically relevant concentrations
Scientific Reports (2023)
-
Functionalization of acyclic xenonucleic acid with modified nucleobases
Polymer Journal (2023)
-
Pharmacology and toxicology of eteplirsen and SRP-5051 for DMD exon 51 skipping: an update
Archives of Toxicology (2022)
-
Using antisense oligonucleotides for the physiological modulation of the alternative splicing of NF1 exon 23a during PC12 neuronal differentiation
Scientific Reports (2021)
-
Animal models for researching approaches to therapy of Duchenne muscular dystrophy
Transgenic Research (2021)