Abstract
The effects of self-administered 3,4-methylenedioxymethamphetamine (MDMA) on behavior and neurochemistry have not been previously studied in laboratory primates. We investigated the capacity of MDMA and its enantiomers to maintain contingent responding over an extended duration, whether any decrements in the reinforcing effects of these compounds would be observed over time, whether such decrements would be MDMA-selective, and whether any neurochemical correlates could be identified. Animals were previously trained to self-administer cocaine, then exposed to periodic substitutions of various doses of racemic MDMA and its enantiomers; full dose–effect curves were generated for each MDMA compound repeatedly over the duration of the study. After approximately 18 months of MDMA self-administration, drug exposure was halted and after at least 2 months drug abstinence, animals were scanned using positron emission tomography (PET) with the vesicular monoamine transporter (VMAT) ligand dihydrotetrabenazine (DTBZ). Shortly thereafter, animals were euthanized, brains were dissected, and samples were assayed for brain monoamines and their metabolites using high-performance liquid chromatography (HPLC), and for VMAT using DTBZ binding. The reinforcing effects of racemic and R(−)-MDMA were reduced over a long series (months) of individual self-administration access periods; the reinforcing effects of S(+)-MDMA were more resistant to this effect, but were attenuated for one animal. The reinforcing effects of cocaine were not altered by chronic MDMA self-administration, nor was the VMAT binding potential as assessed by PET. Further, there were no measurable decrements in serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) or VMAT in any brain regions assayed. The reinforcing effects of MDMA are selectively attenuated by chronic MDMA self-administration, although this behavioral change appears to occur in the absence of any frank neurochemical correlates of toxicity.
Similar content being viewed by others
INTRODUCTION
Human ecstasy users have reported that the psychological effects of this compound seem to diminish with repeated drug exposures (Beck and Rosenbaum, 1994), a finding which may underlie the high rates (50% or higher) of spontaneous abstinence noted in several populations of youths with varying levels of MDMA use, abuse, and dependence (von Sydow et al, 2002). Potential explanations for this phenomenon have invoked several mechanisms, including loss of novelty, behavioral tolerance, and MDMA-induced neurotoxicity. With regard to this latter point, the evidence collected from multiple laboratory animal species shows that the selective effects of MDMA on brain serotonin (5-HT) systems can be quite extensive under particular dose regimens. For example, large or repeated doses of MDMA have been shown to deplete 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in primates and rats, without affecting homovanillic acid (HVA), 3-methoxy-4-hydroxy-phenethylene glycol (MHPG), dopamine (DA), or norepinephrine (NE) (DeSouza et al, 1990).
Single bolus MDMA doses as low as 5 mg/kg have been shown to produce 5-HT and/or 5-HIAA depletions in some regions of the monkey brain 2 weeks later (Ricaurte et al, 2000), however, the relation of these doses to those used by humans has been questioned (Saunders, 1995; Kish, 2002). Nonetheless, application of some formulae for interspecies scaling places some of these experimental animal doses within the range of those commonly administered by humans in recreational settings (Morgan, 2000), implying that there may be no significant margin of safety between neurotoxic and recreational doses in humans (but see Vollenweider et al (2001) for a critique of allometric interspecies scaling). Given that the reinforcing effects of MDMA in rhesus monkeys are mediated, at least in part, by brain 5-HT systems (Fantegrossi et al, 2002), it seems plausible to postulate that serotonergic neurotoxicity could alter the reinforcing effects of MDMA in this species.
Although multiple measures of serotonergic neurotoxicity (ie decreases in levels of 5-HT, 5-HIAA, 5-HT transporters, vesicular monoamine transporters (VMAT), measures of anterograde transport, and tryptophan hydroxylase (TPH) activity, as well as histological evidence of 5-HT axon degeneration and the concomitant ‘pruning effect’) have been employed to yield evidence of MDMA-induced neurochemical and neuroanatomical changes in laboratory animals, only rarely have these effects been correlated with behavioral deficits in drug-free animals. In this regard, following the standard multiple MDMA dose regimen, alterations in the acoustic startle response (Schmidt and Kehne, 1990), rates of isolation calling (Winslow and Insel, 1992), reduced anxiety-like behavior in the open field and elevated plus maze assays (Mechan et al, 2002) and impaired thermoregulation (Miller and O'Callaghan, 1995) have been noted in rodents, although some of these effects have not been particularly robust. More germane to the present experiments, the discriminative stimulus effects of S(+)-MDMA (Virden and Baker, 1999) and racemic MDMA (Schechter, 1991) were disrupted in the rat following a multiple dose regimen of racemic MDMA, as were the rewarding effects of racemic MDMA as assessed by a conditioned place preference assay (Schechter, 1991). However, it should be noted that similar dose regimens in the monkey have not previously been shown to produce any spontaneous behavioral changes in drug-free animals (Slikker et al, 1989). Thus, at least in the primate, it appears that some neurochemical consequences of MDMA administration can occur in the absence of obvious behavioral correlates, although behavioral consequences may be uncovered by subsequent drug challenges (Ricaurte et al, 2000).
Importantly, the fact that all previous experiments regarding the neurotoxic effects of MDMA in laboratory animals have relied on noncontingent MDMA administration raises the question of whether or not these effects would be obtained via self-administration of the drug. Several cases from diverse pharmacological classes serve to illustrate the point that response-dependent drug administration can have vastly different effects than one might predict based upon data gathered following noncontingent drug administration. These differences are manifested in measures as diverse as withdrawal signs (Siegel, 1988), discriminative stimulus properties (Ator and Griffiths, 1992), lethal effects (Dworkin et al, 1995), and even neurochemical and neuropharmacological measures (Hemby et al, 1997; Stefanski et al, 1999) across several species and pharmacological classes. Thus, the relationship of animal neurotoxicity studies relying on noncontingent MDMA administration to the human experience (in which MDMA is self-administered) must be regarded as unknown.
In this regard, we have been investigating the long-term maintenance of intravenous self-administration of MDMA and its stereoisomers in rhesus monkeys. Of particular interest to us were the issues of whether or not any decrements in the reinforcing effects of these compounds would be observed over time, whether or not such decrements would be MDMA-selective, and whether or not neurochemical correlates could be identified. The reinforcing effects of MDMA and its enantiomers were thus assessed in multiple dose–effect curve determinations over a period of approximately 18 months, and compared with behavior maintained by a reference dose of cocaine over this same time interval in order to gauge the selectivity of any observed response decrements when MDMA was available for self-administration.
Neurochemical effects of this extended MDMA self-administration regimen were assessed by imaging VMAT via PET with [11C]dihydrotetabenazine (DTBZ). The use of DTBZ as a radioligand suitable for studying the potential neurodegeneration of monoaminergic neurons in vivo is predicated upon this compound's demonstrated specific binding to monoaminergic terminals throughout the brain (DaSilva et al, 1993). Further, VMAT may present a more stable target than plasmalemmal proteins due to its lack of regulation according to varying levels of endogenous ligands or chronic treatment with dopaminergic drugs (Vander Borght et al, 1995; Frey et al, 1997; but see Sandoval et al, 2003). In addition, VMAT binding has been used to provide a highly reliable index of decreased monoaminergic innervation in human striatum among Parkinsonian patients (Frey et al, 1996), and has previously been shown to be sensitive to noncontingent MDMA administration in baboons (Ricaurte et al, 2000). Following euthanasia, VMAT was also measured using radioligand binding techniques in striatal membrane preparations. Finally, reverse-phase high-pressure liquid chromatography (HPLC) was used to measure levels of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT, and 5-HIAA in postmortem brain.
MATERIALS AND METHODS
Animals
MDMA self-administration
Seven adult rhesus monkeys housed at the University of Michigan served as subjects in these experiments, four of which self-administered MDMA, and three of which were MDMA-naïve controls. All subjects weighed between 6.5 and 12 kg and had extensive drug self-administration histories including exposure to various opioids and psychostimulants (including, for the experimental subjects only, racemic methamphetamine [METH], see Table 1 and Fantegrossi et al, 2002) prior to the initiation of these experiments, but, importantly, no control animals ever self-administered MDMA or METH. Throughout these studies, all self-administration animals were individually housed in 83.3 × 76.2 × 91.4 cm-deep stainless-steel cages in a colony room maintained at an ambient temperature of 22±2°C. A side-mounted panel was present in each cage, equipped with a row of three stimulus lamps (red–green–red) across the top, and two response levers (one mounted under each red light). Each self-administration animal wore a Teflon mesh jacket (Lomir, Québec, Canada) connected to a flexible stainless-steel spring arm attached to the rear of the cage. Animals were fed between 10 and 12 Purina monkey chows twice per day, and water was available ad libitum. Daily fresh fruit and other treats supplemented this diet. In accordance with IACUC requirements, environmental enrichment toys were also provided on a regular rotating basis. Owing to catheter problems, monkey 93X3579 was removed from the MDMA self-administration experiments several months early; this subject's self-administration data are therefore not shown and do not contribute to the group mean. Throughout the last few weeks of the MDMA self-administration regimen, monkey RC95 suffered chronic health problems (unrelated to drug intake) and was euthanized before any PET images could be acquired.
Positron emission tomography
Six of the previously described monkeys were imaged with [11C]DTBZ at the University of Michigan cyclotron/PET facility, and were subsequently euthanized 7–10 days postscan. One additional drug-naïve animal was also imaged with [11C]DTBZ.
Radioligand binding
Hemispheres not used for HPLC from the brains of all euthanized monkeys were further assayed for VMAT density in membrane preparations utilizing radioligand binding.
Determination of brain monoamines and their major metabolites
Brains from seven self-administration animals (four MDMA, three MDMA-naïve) were harvested and shipped to the Johns Hopkins Medical Center for HPLC.
Procedure
MDMA self-administration
The surgical preparation of all subjects and the operant schedules used to engender contingent responding for MDMA and its enantiomers were previously described in Fantegrossi et al (2002). Briefly, subjects were implanted with indwelling intravenous catheters in either an internal or external jugular, femoral, or brachial vein under ketamine (10 mg/kg i.m.) and xylazine (2 mg/kg i.m.) anesthesia. Catheters were run subcutaneously from the site of implantation to an exit site in the middle of the back. Tubing was then fed through the steel spring arm and passed to the outside rear of the cage where it was connected to drug supplies and additional infusion lines that passed through the rollers of the infusion pump. Operation of the infusion pump delivered 1 ml of drug solution over 5 s. Two self-administration sessions were conducted each day: a morning session starting at 1000 hours and an afternoon session starting at 0400 hours. The onset of each session was signaled by illumination of a red stimulus light. In the presence of this light, the 10th response on the lever beneath it resulted in the operation of the infusion pump (FR10). During the 5-s infusion duration, the red stimulus light was extinguished, the center green light was illuminated, and further lever presses had no programmed consequences. Immediately following the termination of each infusion, all stimulus lights were extinguished for a 1-min time out period (TO 1 min) during which lever presses continued to have no programmed consequences. Each TO period counted toward the total 60-min session time. Under baseline conditions, animals were maintained on a cocaine dose of 0.01 mg/kg/inj following the above outlined schedule requirements. To ensure that responding was being maintained by the presented drug, saline was randomly substituted for cocaine approximately every 3rd or 4th session, usually for two consecutive sessions. During dose–effect curve determinations, MDMA substitutions occurred approximately three times per week and no substitutions were made on weekends. All drug substitutions followed an ascending dose order within-drug, and at least three recovery sessions occurred between substitution trials. No drugs other than cocaine and MDMA were self-administered during the duration of the presently reported experiments.
Positron emission tomography
High specific activity [11C]DTBZ was prepared by the method of Jewett et al (1997). Radiochemical and chemical purity were subsequently determined by reverse-phase HPLC. The specific activity of the final product was >500 Ci/mmol at the time of injection, with a radiochemical purity >97%.
PET imaging studies were performed in three MDMA self-administration monkeys following at least 2 months abstinence from MDMA, and three MDMA-naïve monkeys following at least 2 weeks abstinence from all other drugs. Anesthetization for the PET procedure was accomplished with ketamine (15 mg/kg i.m., repeated as necessary) and xylazine (2 mg/kg i.m.). Scans were obtained using the TCC 4600 PET scanner (three-ring, five-slice tomograph) modified with custom-made collimation to provide improved resolution (FWHM=7.5 mm). To aid in the positioning of the animal, cerebral blood flow images were obtained using i.v. injections of 3–5 mCi 15O-labeled water. Subjects were then injected with 15–25 mCi of [11C]DTBZ in a bolus plus infusion protocol (60% bolus over 1 min followed by 40% constant infusion over 89 min) and imaged for a total of 90 min. Imaging frames progressed for 30 s at the start of the scan to 5 min frames late in the session.
Brain dissection
Between 7 and 10 days postscan, monkeys were anesthetized with ketamine (15 mg/kg i.m.) and transported to the necropsy room where they were euthanized with an overdose of pentobarbital (100–150 mg/kg) administered i.v. Following removal of the skull cap, each brain was immediately placed on its ventral surface on a prechilled Plexiglas sheet over ice. Brainstems were quickly removed at the level of the superior colliculus, and the remaining forebrain was then hemisected. Samples were individually packed in mylar bags, covered with powdered dry ice, and transferred to a −80°C freezer in an adjacent room. The following day, samples were shipped on dry ice to the Johns Hopkins University Medical School for HPLC. Upon arrival, each brain was immediately stored in a −80°C freezer so that samples could be processed in parallel. On the day of HPLC assay, brains were removed from the freezer and cortical samples were dissected freehand from the frozen brains with a scalpel blade while subcortical samples were subsequently dissected out by a coronal slice blocking method followed by tissue punch. Powdered dry ice was kept on hand to prevent thawing of individual brains, and each sample was foil wrapped and bathed in liquid nitrogen immediately following collection.
Radioligand binding
In vitro [3H] dihydrotetrabenazine (DTBZ) binding studies were performed as in Villemagne et al (1998), with minor modification. Briefly, striatal tissue samples were homogenized for 15 s in 20 volumes (w/v) sodium phosphate buffer (25 mM, pH 7.7), then centrifuged in a Sorvall RC2B at approximately 45 000g for 15 min at 0–4°C. The resulting pellet was resuspended in 20 volumes (w/v) sodium phosphate buffer, then homogenized again for 15 s, and recentrifuged at approximately 45 000g for 15 min at 0–4°C. The supernatant was discarded and the resulting pellet was resuspended in buffer at a final concentration of 10 mg of original wet weight tissue per ml. Striatal membrane preparations were incubated with a predetermined saturating concentration of [3H]DTBZ (15 nM) in 25 mM sodium phosphate buffer, pH 7.7, for 90 min at 30°C in a shaking water bath. Each sample was run in triplicate, such that three tubes were used to define total binding, and three tubes were used to determine nonspecific binding. Nonspecific binding was determined in the presence of 1 μM tetrabenazine, and represented approximately 8–10% of total binding. The incubation was terminated by rapid filtration, using a 48-well cell harvester (Brandell, Gaithersburg, MD) and Whatman GFB filters soaked with 0.05% PEI. Filters were washed three times with 10 ml sodium phosphate buffer and residual radioactivity was measured using a Packard-1500 Tricarb Liquid Scintillation Analyzer. Specific binding, calculated by subtracting nonspecific binding from total binding, was expressed as DPM/mg original wet weight tissue.
Determination of brain monoamines and their major metabolites
Brains were sectioned using a Microm HM505E cryostat at −20°C. Half-hemisphere coronal sections (20 μm) at the level of the head of caudate were thaw-mounted onto gelatin-coated microscope slides and stored at −70°C until used. A 3 mm sample corer was then used to ‘punch’ a frozen sample of the caudate head. These frozen tissue samples were wrapped in foil and stored in liquid nitrogen for subsequent HPLC analysis, as below.
Brain concentrations of 5-HT, DA, 5-HIAA, and DOPAC were measured by reverse-phase HPLC coupled with electrochemical detection using a method similar to that previously described by Ricaurte et al (1992). Frozen tissue was weighed, bathed in 10 parts ice-cold 0.4 N perchloric acid, then homogenized for 15 s using a Polytron homogenizer (setting=5). The resulting homogenate was then centrifuged for 10 min at 15 000 rpm in a refrigerated Sorvall RC2B centrifuge. Fractions of the supernatant were transferred to polypropylene tubes, which were stored in liquid nitrogen until assay. Separation of monoamines and their metabolites was accomplished with a Brownlee Spheri-5 RP-18 250 × 4.6 mm column (5-μm particle size). For separating the various monoamines and their metabolites, a mobile phase consisting of 98 parts of an aqueous phase (125 mM citric acid, 125 mM sodium phosphate, 0.27 mM EDTA, and 0.12 mM sodium octane sulfate) and 2 parts methanol with a pH of 2.5 was used. The flow rate of the mobile phase was approximately 1.0 ml/min. The column was maintained in a CTO-6A column oven module (Shimadzu) at 40°C. Detection was achieved by means of an amperometric L-ECD-6A detector (Shimadzu, Columbia, MD), with a glassy carbon working electrode and a silver/silver chloride reference electrode. The fixed potential difference between the reference and working electrodes was +0.70 V. The electrochemical response was quantified using a Shimadzu Chromatopac C-R4A data processor equipped to measure the area under the curve for a given sample and compare it to that of standards processed in an identical manner. The sensitivity of this method is approximately 20 pg per 20 μl of sample. All samples were processed in parallel.
Drugs
Cocaine, racemic MDMA, and its stereoisomers were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC) and were dissolved in physiological saline prior to injection. Surgical and anesthetic drugs were purchased from commercial sources. [11C]DTBZ was synthesized and radiolabeled at the University of Michigan Medical Center, Division of Nuclear Medicine core facilities. All HPLC reagents were purchased from commercial sources.
Data Analysis
All data are presented as mean±SEM. Neurochemical data were analyzed by ANOVA, followed by Tukey's HSD post-hoc comparisons. All data analyses were performed using commercially available software. Results from all statistical analyses were considered significant when P was <0.05 using a two-tailed test.
RESULTS
MDMA Self-Administration
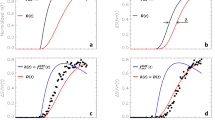
Figure 1 presents the first (filled symbols) and last (open symbols) dose–effect curve determinations for racemic MDMA self-administration in three individual animals, as well as a group average. Mean responding for racemic MDMA aggregated across all three subjects completing the timecourse study was shifted down over the 18-month period (Figure 1, bottom right panel), and this general pattern was echoed in each subject's individual data. Importantly, responding for contingent cocaine and saline were not altered over this duration, indicating a selective effect on MDMA-maintained behavior. The final dose–effect curves for monkeys RC95, 93X3577, and 96X2484 all exhibit downshifts when compared with their initial dose–effect curves, although the magnitude of this shift differed across subjects.
Individual and aggregated (bottom right) dose–response curves for racemic MDMA. Each point represents the mean±SEM (n=3 observations for individual graphs, n=3 monkeys for group graph) for first dose–effect determination (filled symbols) and final dose–effect determination (open symbols). Abscissa: dose of self-administered drug (mg/kg/inj). Ordinate: number of injections earned (raw values for individual graphs, converted to % cocaine control responding for group graph).
Dose–effect curves for S(+)-MDMA self-administration are presented in Figure 2. The aggregated data (Figure 2, bottom right panel) suggest that responding was not altered for contingent cocaine, saline, or S(+)-MDMA over the 18-month interval, but this is likely due to the large variability observed across individual subjects. Although responding for contingent cocaine and saline were not altered in any subject, S(+)-MDMA-maintained responding did change for two animals over the 18-month period. For monkey RC95 (Figure 2, top left panel), responding for S(+)-MDMA dropped to saline-like levels across the dose range tested without a concomitant decrease in cocaine-maintained behavior. Interestingly, responding for S(+)-MDMA, but not cocaine, increased over the 18-month period for monkey 93X3577 (Figure 2, top right panel), although initial self-administration of S(+)-MDMA was quite low for this subject. Finally, no significant changes in drug-maintained responding were observed for monkey 96X2484 (Figure 2, bottom left panel).
Individual and aggregated (bottom right) dose–response curves for S(+)-MDMA. Each point represents the mean±SEM (n=3 observations for individual graphs, n=3 monkeys for group graph) for first dose–effect determination (filled symbols) and final dose–effect determination (open symbols). Abscissa: dose of self-administered drug (mg/kg/inj). Ordinate: number of injections earned (raw values for individual graphs, converted to % cocaine control responding for group graph).
Mean responding for R(-)-MDMA aggregated across all three subjects was considerably reduced over the 18-month period (Figure 3, bottom right panel), and this general pattern conforms to that observed in each subject's individual data. Again, responding for contingent cocaine and saline were not altered over this duration, indicating a selective effect on MDMA-maintained behavior. As with S(+)-MDMA, individual differences in the extent of this effect were observed across animals. The final dose–effect curve for monkey RC95 exhibits a downward shift when compared with this subject's initial dose–effect curve (Figure 3, top left panel). The final dose–effect curves for monkeys 93X3577 and 96X2484 each display a rightward shift when compared with their initial dose–effect curves (Figure 3, top right and bottom left panels, respectively).
Individual and aggregated (bottom right) dose–response curves for R(−)-MDMA. Each point represents the mean±SEM (n=3 observations for individual graphs, n=3 monkeys for group graph) for first dose–effect determination (filled symbols) and final dose–effect determination (open symbols). Abscissa: dose of self-administered drug (mg/kg/inj). Ordinate: number of injections earned (raw values for individual graphs, converted to % cocaine control responding for group graph).
Over the approximately 18-month period of intermittent MDMA access, subjects had the opportunity to self-administer MDMA and its enantiomers on 120–139 separate occasions. Total intakes, greatest single-session intakes, and average single-session intakes for racemic MDMA, its enantiomers, and METH for each subject across these sessions are presented in Table 1. Total METH self-administration (from experiments previously described in Fantegrossi et al, 2002) was quite low for all subjects, amounting to between 1 and 3 mg/kg, and greatest single-session intakes for METH (out of the 11–17 discrete sessions where it was available to these subjects) were less than 1 mg/kg in all subjects. Total intakes of racemic MDMA ranged between approximately 33 and 57 mg/kg, and similar amounts of S(+)-MDMA (approximately 28–51 mg/kg) were self-administered. Total intakes of R(−)-MDMA were greatest in all subjects (ranging between approximately 62 and 143 mg/kg) as higher doses of this enantiomer were made available for self-administration. Intakes when low unit doses of MDMA and its enantiomers were available for self-administration were artificially capped by the particulars of the operant schedule used to engender responding. As such, the overall average intakes presented in Table 1 may not be particularly representative of how much drug a given subject would self-administer under conditions of unrestricted access. When average intake is computed based on data obtained when unit dose was high (0.1 and 0.3 mg/kg/inj), the average intake for each subject is approximately double that of their overall average intake, ranging between approximately 2 and 4 mg/kg.
Positron Emission Tomography
DTBZ was rapidly taken up resulting in clear visualization of various brain structures within 5 min postinjection. Distribution volume ratios were computed at equilibrium for each subject (Koeppe et al, 1997) using the basal ganglia (readily visualized in all scans) and midline structures (corresponding to thalamic and hypothalamic nuclei) as regions of interest (ROI, Figure 4). Binding within each ROI was compared with binding in a control region within the occipital cortex for each individual subject, thus providing a quantitative measure of VMAT binding potential for each subject. Scans from animals with MDMA self-administration histories (filled symbols) were compared with those obtained from MDMA-naïve subjects (open symbols) by establishing a scatterplot of distribution volume ratios for the two ROI (Figure 5, top). No significant differences were found between groups.
Representative PET images for an MDMA-naive control animal (top row), as well as images from monkeys with the lowest (middle row) and highest (bottom row) total MDMA intakes. The first image in each row illustrates specific VMAT binding in midline structures, while the second image depicts binding in basal ganglia. MDMA intake refers to total self-administered racemic, S(+)- and R(−)-MDMA.
Top: VMAT binding in two regions of interest for MDMA self-administration history (filled symbols) and control (open symbols) monkeys as assessed by PET. Abscissa: region of interest. Ordinate: VMAT binding potential (distribution volume ratio). Bottom: VMAT binding in striatal membrane preparations from MDMA self-administration history (filled bars) and control (open bars) monkeys as assessed by radioisotope binding. Abscissa: group. Ordinate: VMAT binding potential (disintegrations/min/mg tissue).
Radioligand Binding
As previously demonstrated with PET, binding of the radioligand [3H]DTBZ to VMAT in striatal membrane preparations was similar in MDMA-history and control animals (Figure 5, bottom).
Determination of Brain Monoamines and Their Major Metabolites
Tissue concentrations of 5-HT (Figure 6, top) and 5-HIAA (Figure 6, bottom) were similar across groups in all brain regions assayed as assessed by HPLC. Similar to the results obtained with brain 5-HT and 5-HIAA, subjects with a history of MDMA self-administration also did not differ from controls in terms of DA tissue concentrations (Figure 7, top) or DOPAC tissue concentrations (Figure 7, bottom) in the caudate or putamen.
Aggregated tissue concentrations of 5-HT (top) or 5-HIAA (bottom) in several brain regions. Open bars represent group mean±SEM for all control animals, filled bars represent group mean±SEM for all MDMA monkeys. Abscissa: brain region assayed (FC=frontal cortex, PC=parietal cortex, TC=temporal cortex, OC=occipital cortex, HPC=hippocampus, TH=thalamus, HYP=hypothalamus, Cd=caudate nucleus, GP=globus pallidus. Ordinate: 5-HT concentration (ng/mg tissue).
DISCUSSION
The erratic content of racemic MDMA in ecstasy pills has rendered estimates of average recreational doses in humans somewhat difficult. For example, ecstasy pills in the UK have been shown to vary up to 70-fold in terms of their MDMA content (Sherlock et al, 1999). Another source of variation seems to be temporal in nature, with older estimates of MDMA content per pill typically ranging between 75 and 150 mg (Kirsch, 1996; Wolf et al, 1995), while more recent studies have found average levels between 60 and 80 mg (Cole et al, 2002). Finally, Winstock et al (2001) have previously reported MDMA usage trends collected from a survey of dance music enthusiasts in the UK; in this account, approximately 75% of the population surveyed report using three or fewer pills per session. Assuming a 75 kg human, the average total dose self-administered by humans may then be somewhere between 1 and 6 mg/kg, although some binge users might ingest significantly more than that. Interestingly, the average single-session intakes of any form of MDMA for the monkeys in the present studies (when unit dose was high enough to minimize schedule-induced constraints on intake) were between ∼2 and 3 mg/kg—quite similar to what the average human users might be expected to ingest in a given night.
The presently reported results for long-term self-administration of racemic MDMA and its enantiomers by rhesus monkeys seem to confirm the anecdotal reports from human ecstasy users that the effects of this compound diminish over repeated administrations (Beck and Rosenbaum, 1994). That the observed reduction in MDMA-maintained behavior occurred without a concomitant change in cocaine self-administration indicates that there is some degree of behavioral and pharmacological specificity to this effect. This was especially pronounced for racemic and R(−)-MDMA, but was also true, in at least one animal, for S(+)-MDMA. Further studies on the MDMA enantiomers should therefore be conducted to extend these findings and perhaps elucidate this intriguing stereoselective effect.
The absence of any significant differences in VMAT binding or tissue levels of 5-HT, 5-HIAA, DA, or DOPAC in the MDMA-history animals suggest that low doses of contingent MDMA, self-administered over an extended duration, do not produce the sorts of neurochemical effects that have previously been observed following repeated acute exposures to noncontingent MDMA in laboratory monkeys. Interestingly, previous research with 3,4-methylenedioxyamphetamine (MDA), a structurally similar drug of abuse and active metabolite of MDMA, has revealed significant 5-HT depletion in the hippocampus following 7 days of MDA self-administration in rats (Markert and Roberts 1991). No behavioral effects were noted in the rats following MDA self-administration in this study, but the short (7 day) MDA access period might have precluded their development. Whether this represents a species difference or a pharmacodynamic distinction between MDA and MDMA should be investigated further. A single report demonstrating DA depletion following MDMA administration in squirrel monkeys and baboons (Ricaurte et al, 2002) was later retracted (Ricaurte et al, 2003) due to methodological flaws. The results of the present studies thus agree with previous research suggesting that MDMA does not alter DA content in primates (DeSouza et al, 1990; Ricaurte et al, 2000).
Although not significant, downward trends in 5-HT content were present in several brain regions following long-term MDMA self-administration; depletions of 40–50% were evident in frontal, parietal, and temporal cortex, while lesser depletions (approximately 25%) were apparent in occipital cortex, thalamus, and hypothalamus. These reductions are consistent with previous studies involving noncontingent MDMA administration in Old World macaques, although they are of a lesser magnitude. DeSouza et al (1990), for example, found 5-HT depletions of 90–95% in frontal, parietal, and temporal cortex following 4 days of subcutaneous injections (twice per day) of 10 mg/kg MDMA. Similarly, Taffe et al (2002) reported 5-HT reductions of 80–95% in these same brain regions after administering 10 mg/kg MDMA twice daily for four consecutive days via the intramuscular route. Thus, the possibility remains that our results (especially those obtained from frontal, parietal, and temporal cortex) failed to reach significance due to the relatively small n presently employed. However, an interesting finding of the present studies was the complete lack of any detectable change in hippocampal 5-HT following long-term MDMA self-administration. In previous studies involving noncontingent MDMA administration in monkeys, marked (60–90%) depletions of hippocampal 5-HT have been reported (Kleven et al, 1989; Slikker et al, 1988,1989; DeSouza et al, 1990; Ali et al, 1993; Frederick et al, 1998; Taffe et al, 2002). The sum of the present imaging and neurochemical observations in monkeys that had self-administered MDMA thus implies that, despite the selective behavioral effects on self-administration, no apparent neurotoxicity resulted from the extended MDMA history.
Why then should it be the case that the dose regimens commonly administered noncontingently to induce neurotoxicity in laboratory primates are largely without spontaneous behavioral consequences, while the present self-administration regimen produced a clear behavioral change in the absence of any frank neurotoxicity? Several possibly interacting factors seem relevant. As mentioned in the introduction, numerous experiments have revealed large differences in drug effects depending on the contingency of their administration. For example, rats that had self-administered morphine exhibited more severe withdrawal symptoms than did those that received identical morphine infusions noncontingently (the so-called ‘yoked-controls’) (Siegel, 1988). Further, a history of midazolam self-administration resulted in potentiated sensitivity to the drug's discriminative stimulus properties, while a history of response-independent midazolam decreases sensitivity to these same effects (Ator and Griffiths, 1992). Similarly, the lethal effects of cocaine are profoundly attenuated in rats receiving the drug on a response-dependent basis, as compared with a yoked control group receiving the same pattern of infusions noncontingently (Dworkin et al, 1995). Finally, there is evidence that the contingencies governing drug administration can even affect neurochemical measures. Hemby et al (1997) have demonstrated significantly higher extracellular dopamine concentrations in the nucleus accumbens, despite equivalent brain concentrations of drug, in rats self-administering cocaine, as compared with a yoked-control group. Further, Stefanski et al (1999) observed region-specific downregulations of D1 and D2 receptors in rat brain following a 5 week regimen of methamphetamine (METH) self-administration, but not following equivalent response-independent drug exposure using a yoked control design. Although no such experiments have dealt specifically with MDMA, it seems reasonable to assume that the contingencies surrounding MDMA administration might also be important variables to study in terms of the drug's neurotoxic potential.
Another possible explanation for the lack of concordance between the neurochemical results of the present study and those of previous experiments involves pharmacokinetic parameters. Most studies of MDMA neurotoxicity utilize a drug regimen involving multiple doses over a period of days. For example, Ricaurte et al (2000) describes a dosing routine consisting of 5 mg/kg racemic MDMA subcutaneously, twice per day, for four consecutive days, resulting in a total dose of 40 mg/kg MDMA in baboons. This dosing regimen produced significant reductions in VMAT density within the caudate, putamen, and frontal cortex that did not seem to be attributable to a frank loss of DA or NE axons but was likely associated with the noted significant reductions in 5-HT axonal markers. Similarly, rats injected with 10 mg/kg MDMA subcutaneously every 2 h for four total injections were shown to have 25–30% decrements in VMAT binding in the striatum 1 h after the final injection, although these effects largely ameliorated within 24 h (Hansen et al, 2002). In contrast, the rhesus monkeys in the present studies each self-administered a total amount of MDMA between approximately 120 and 250 mg/kg (the sum of all self-administered intakes of racemic MDMA or its enantiomers) over the 18-month duration of the experiment. Thus, the presently reported lack of VMAT effects or monoamine depletions in the MDMA self-administration animals cannot simply be due to insufficient MDMA intake.
The major difference here across experimental protocols seems to be one of pattern of MDMA exposure. Although no pharmacokinetic measurements were made in the present experiments, it seems doubtful that our animals would have attained peak MDMA blood levels as great as those of the animals in standard multiple dose paradigms, or at least they would not have done so as frequently. In this regard, Bowyer et al (2003) have recently demonstrated significant increases in peak plasma levels of MDMA and its metabolite MDA, as well as a concomitant decrease in 5-HT markers, following a dose regimen consisting of injections of 10 mg/kg S(+)-MDMA, twice per day for four consecutive days in rhesus monkeys. Similar experiments utilizing less frequent MDMA administration have not been conducted, making a comparison with the present studies difficult. Nevertheless, we did observe acute self-administration sessions for each rhesus monkey where MDMA intakes over the 1 h session approached or exceeded (6–15 mg/kg) the amounts typically administered to primates in other studies in a single day. If the effects of noncontingent MDMA exposure are cumulative, as seems to be assumed by the broadly used 4-day dosing regimen (ie Battaglia et al, 1988; Kleven et al, 1989; Ricaurte et al, 1988; Slikker et al, 1988,1989; DeSouza et al, 1990; Ali et al, 1993; Colado et al, 1993; Frederick et al, 1998; O'Shea et al, 1998; Taffe et al, 2002; Bowyer et al, 2003), one may presume that the effects of contingent MDMA administration are cumulative as well. On the other hand, the present results might argue that a key toxic level of MDMA (or a metabolite) must be achieved each administration in order to induce neurotoxicity. Thus, with regards to frank neurotoxicity, total amount of MDMA administered may be a less important factor than duration and pattern of MDMA exposure, although a definitive statement in this regard would require more empirical data than is presented here.
It could also be argued that the lack of observable neurotoxicity in the MDMA self-administration animals is due to any of a myriad of methodological problems with the current studies. For example, the relatively long (at least 2 months) MDMA abstinence period imposed before the acquisition of the PET images and subsequent brain harvesting might have allowed significant recovery, thereby masking the true neurotoxic insults responsible for the behavioral change. However, the fact that lasting neurochemical and neuroanatomical effects of MDMA were evident in squirrel monkeys up to 7 years after drug exposure (Hatzidimitriou et al, 1999) seems to argue against the possibility of significant recovery during this abstinence period. Further, it could be argued that our control animals—drawn from a population of monkeys with significant drug self-administration histories—provide an invalid comparison group for the MDMA self-administration subjects. However, the distribution volume ratios for both ROI in the MDMA-naïve control animals were within the range of values commonly obtained for drug-naïve rhesus monkeys (DaSilva et al, 1993), suggesting that the presently observed lack of effect on VMAT was not simply due to abnormally low levels of this protein in these control subjects. Similarly, the range of levels of DA, 5-HT, and their metabolites in the control population are consistent with those previously reported in the literature for drug-naïve rhesus monkeys (Frederick et al, 1995). The presently reported control animals thus appear to be quite appropriate, and the comparisons with the MDMA animals seem to be valid.
A related criticism of the present studies is the use of experimental subjects with extensive drug self-administration histories prior to initiation of MDMA access. Many of the previously self-administered drugs (cocaine, METH, etc) have long-term effects on neurochemistry that may overlap those of MDMA (Schifano et al, 1998). Thus, it cannot be definitively stated that the results obtained in these animals are due to self-administered MDMA, as opposed to prior self-administration of drugs other than MDMA. However, several factors serve to refute this criticism and support the validity of the present experiments.
First, it should be noted that human ecstasy users are notorious for their polydrug abuse (Pederson and Skrondal, 1999). For example, Milani et al (2000) found extensive and frequent abuse of alcohol, cigarettes, stimulants, and hallucinogens among a population of ‘heavy’ ecstasy users. Indeed, the current body of research describing the neurotoxic effects of MDMA in humans has been criticized for its general lack of inclusion of polydrug abuser control subjects (Schifano et al, 1998; Kish, 2002). Thus, the use of rhesus monkeys with extensive drug self-administration histories may actually have more face validity with the human ecstasy user population than would use of drug-naïve subjects.
Additionally, the fact that all animals initially acquired MDMA self-administration argues that previous self-administration regimens did not produce any behaviorally relevant neurotoxicity. Indeed, no response decrements were detected for any self-administered drugs until many months of MDMA self-administration had passed. Similarly, the finding that cocaine-maintained behavior remained unaltered for all subjects, even while responding for MDMA fell to saline-like levels, further suggests that such selective response decrements would not have been observed simply following continued cocaine access.
A potentially important point of contention in this regard is the brief period of METH self-administration previously established for these monkeys (Fantegrossi et al, 2002). Noncontingent METH administration has been shown to deplete DA and DOPAC in monkeys (Seiden et al, 1976), however, the doses used in these experiments to produce these depletions are quite large (typically 5.0 mg/kg, twice daily for 4 consecutive days, for a total dose of 40.0 mg/kg). In contrast, the monkeys in the current studies self-administered total METH doses between 0.89 and 3.34 mg/kg over a period of approximately 6 weeks, and single-session intakes were never higher than 0.9 mg/kg (see Table 1). Additionally, one animal was removed from the self-administration experiments before initiation of METH access, but yielded neurochemical data consistent with the rest of the MDMA-history subjects. These findings imply that the brief METH exposure employed in the previous experiments (Fantegrossi et al, 2002) is not likely to have influenced the present results.
The data presented in this report provide evidence that the reinforcing effects of racemic and R(−)-MDMA in rhesus monkeys decrease over an extended duration of contingent drug exposure, and hint that a similar effect may be obtained with S(+)-MDMA. This attenuation of the reinforcing effects of the MDMA compounds occurs without a concomitant reduction in the reinforcing effects of cocaine, indicating that this effect is specific to MDMA-maintained responding. The results of the PET ROI analysis and the radioligand binding results indicate that the decrement in MDMA-maintained behavior is not attributable to frank neuronal loss in the basal ganglia or midline structures, while the HPLC results further argue that DA and 5-HT levels were unaffected by this extended MDMA self-administration regimen. Whether these provocative results are due to behavioral or pharmacokinetic variables, or something else entirely, requires further research.
References
Ali SF, Newport GD, Scallet AC, Binienda Z, Ferguson SA, Bailey JR et al (1993). Oral administration of 3,4-methylenedioxymethamphetamine (MDMA) produces selective serotonergic depletion in the nonhuman primate. Neurotoxicol Teratol 15: 91–96.
Ator NA, Griffiths RR (1992). Differential sensitivity to midazolam discriminative-stimulus effects following self-administered versus response-independent midazolam. Psychopharmacology 110: 1–4.
Battaglia G, Yeh SY, DeSouza EB (1988). MDMA-induced neurotoxicity: parameters of degeneration and recovery of brain serotonin neurons. Pharmacol Biochem Behav 29: 269–274.
Beck J, Rosenbaum M (1994). Pursuit of Ecstasy: The MDMA Experience. State University of New York Press: Albany.
Bowyer JF, Young JF, Slikker W, Itzak Y, Mayorga AJ, Newport GD et al (2003). Plasma levels of parent compound and metabolites after doses of either d-fenfluramine or d-3,4-methylenedioxymethamphetamine (MDMA) that produce long-term serotonergic alterations. NeuroToxicology 24: 379–390.
Colado MI, Murray TK, Green AR (1993). 5-HT loss in rat brain following 3,4-methylenedioxymethamphetamine (MDMA), p-chloroamphetamine, and fenfluramine administration and effects of chlormethiazole and dizocilpine. Br J Pharmacol 108: 583–589.
Cole JC, Bailey M, Sumnall HR, Wagstaff GF, King LA (2002). The content of ecstasy tablets: implications for the study of their long-term effects. Addiction 97: 1531–1536.
DaSilva JN, Kilbourn MR, Domino EF (1993). In vivo imaging of monoaminergic nerve terminals in normal and MPTP-lesioned primate brain using positron emission tomography (PET) and [11C]tetrabenazine. Synapse 14: 128–131.
DeSouza EB, Battaglia G, Insel TR (1990). Neurotoxic effects of MDMA on brain serotonin neurons: evidence from neurochemical and radioligand binding studies. Ann NY Acad Sci 600: 682–698.
Dworkin SI, Mirkis S, Smith JE (1995). Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology 117: 262–266.
Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G (2002). 3,4-methylenedioxy-methamphetamine (MDMA, ‘Ecstasy’) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology 161: 356–364.
Frederick DL, Ali SF, Gillam MP, Gossett J, Slikker W, Paule MG (1998). Acute effects of dexfenfluramine (d-FEN) and methylenedioxymethamphetamine (MDMA) before and after short-course, high-dose treatment. Ann NY Acad Sci 844: 183–190.
Frederick DL, Ali SF, Slikker Jr W, Gillam MP, Allen RR, Paule MG (1995). Behavioral and neurochemical effects of chronic methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neurotoxicol Teratol 17: 531–543.
Frey K, Koeppe R, Kilbourne M, Vander Borght T, Albin R, Gilman S et al (1996). Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging. Ann Neurol 40: 873–884.
Frey K, Kilbourne M, Robinson T (1997). Reduced striatal vesicular monoamine transporter after neurotoxic but not after behaviourally-sensitizing doses of methamphetamine. Eur J Pharmacol 334: 273–279.
Hansen JP, Riddle EL, Sandoval V, Brown JM, Gibb JW, Hanson GR et al (2002). Methylenedioxymethamphetamine decreases plasmalemmal and vesicular dopamine transport: mechanisms and implications for neurotoxicity. J Pharmacol Exp Ther 300: 1093–1100.
Hatzidimitriou G, McCann U, Ricaurte G (1999). Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/−)-3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci 19: 5096–5107.
Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI (1997). Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology 133: 7–16.
Jewett DM, Kilbourn MR, Lee LC (1997). A simple synthesis of [11C]dihydrotetrabenazine. Appl Rad Isot 24: 197–199.
Kirsch MM (1996). Ecstasy. Designer Drugs. CompCare Publications: Minneapolis (Minn). pp 75–97.
Kish SJ (2002). How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol Biochem Behav 71: 845–855.
Kleven MS, Woolverton WL, Seiden LS (1989). Evidence that both intragastric and subcutaneous administration of methylenedioxymethylamphetamine (MDMA) produce serotonin neurotoxicity in rhesus monkeys. Brain Res 488: 121–125.
Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE (1997). Equilibrium versus compartmentalized analysis for assessment of the vesicular monoamine transporter using (+)-α-[11C]dihydrotetrabenazine (DTBZ) and PET. J Cerebral Blood Flow Metab 17: 919–931.
Markert LE, Roberts DS (1991). 3,4-methylenedioxyamphetamine (MDA) self-administration and neurotoxicity. Pharmacol Biochem Behav 39: 569–574.
Mechan AO, Moran PM, Elliott M, Young AJ, Joseph MH, Green R (2002). A study of the effect of a single neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA; ‘ecstasy’) on the subsequent long-term behaviour of rats in the plus maze and open field. Psychopharmacology 159: 167–175.
Milani R, Turner JJD, Parrot AC, Parmar R (2000). Recreational drug use and psychobiological problems, collaborative UK/Italy study (5): ecstasy (MDMA) polydrug findings. J Psychopharmacol 14: a15.
Miller D, O'Callaghan J (1995). The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol 11: 177–192.
Morgan MJ (2000). Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology 152: 230–248.
O'Shea E, Granados R, Esteban B, Colado MI, Green AR (1998). The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘Ecstasy’). Neuropharmacology 37: 919–926.
Pederson W, Skrondal A (1999). Ecstasy and new patterns of drug use: a normal population study. Addiction 94: 1695–1706.
Ricaurte GA, DeLanney LE, Irwin I, Langston JW (1988). Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration. Brain Res 446: 165–168.
Ricaurte G, Martello A, Katz J, Martello M (1992). Lasting effects of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) on central serotonergic neurons in nonhuman primates: neurochemical observations. J Pharmacol Exp Ther 261: 616–622.
Ricaurte GA, Yuan J, McCann UD (2000). Methylenedioxymethamphetamine (MDMA, ‘ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42: 5–10.
Ricaurte GA, Yuan J, Hatzidimitriou G, Cord BJ, McCann UD (2002). Severe dopaminergic neurotoxicity in primates after a common recreational dose regimen of MDMA (‘Ecstasy’). Science 297: 2260–2263.
Ricaurte GA, Yuan J, Hatzidimitriou G, Cord BJ, McCann UD (2003). Retraction. Science 301: 1479 (letter).
Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE (2003). Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J Pharmacol Exper Ther 304: 1181–1187.
Saunders N (1995). Ecstasy and the Dance Culture. Neal's Yard Desktop Publishing: London.
Schechter MD (1991). Effect of MDMA neurotoxicity upon its conditioned place preference and discrimination. Pharmacology, Biochemistry & Behavior 38: 539–544.
Schifano F, DiFuria L, Forza G, Minicuci N, Bricolo R (1998). MDMA (‘ecstasy’) consumption in the context of polydrug abuse: a report on 150 patients. Drug Alcohol Depend 52: 85–90.
Schmidt CJ, Kehne JH (1990). Neurotoxicity of MDMA: neurochemical effects. Ann NY Acad Sci 600: 665–681.
Seiden LS, Fischman MW, Schuster CR (1976). Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend 1: 215–219.
Semple DM, Ebmeier KP, Glabus MF, O'Carroll RE, Johnstone EC (1999). Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br J Psychiatry 175: 63–69.
Sherlock K, Wolff K, Hay AW, Conner M (1999). Analysis of illicit ecstasy tablets: implications for clinical management in the accident and emergency department. J Accid Emerg Med 16: 194–197.
Siegel S (1988). Drug anticipation and drug tolerance. In Lader M (ed). The Psychopharmacology of Addiction. Oxford University Press: New York.
Slikker Jr W, Ali SF, Scallet AC, Frith CH, Newport GD, Bailey JR (1988). Neurochemical and neurohistological alterations in the rat and monkey produced by orally administered methylenedioxymethamphetamine (MDMA). Toxicol Appl Pharmacol 94: 448–457.
Slikker Jr W, Holson RR, Ali SF, Kolta MG, Paule MG, Scallet AC et al (1989). Behavioral and neurochemical effects of orally administered MDMA in the rodent and nonhuman primate. Neurotoxicology 10: 529–542.
Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR (1999). Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol 371: 123–135.
Taffe MA, Davis SA, Yuan J, Schroeder R, Hatzidimitriou G, Parsons LH et al (2002). Cognitive performance of MDMA-treated rhesus monkeys: sensitivity to serotonergic challenge. Neuropsychopharmacology 27: 993–1005.
Vander Borght TM, Kilbourn MR, Desmond TJ, Kuhl DE, Frey KA (1995). The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol 294: 577–583.
Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB et al (1998). Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci 18: 419–427.
Virden TB, Baker LE (1999). Disruption of the discriminative stimulus effects of S(+)-3,4-methylenedioxymethamphetamine (MDMA) by (+/−)-MDMA neurotoxicity: protection by fluoxetine. Behav Pharmacol 10: 195–204.
Vollenweider FX, Jones RT, Baggott MJ (2001). Caveat Emptor: editors beware (reply). Neuropsychopharmacology 24: 461–463.
von Sydow K, Lieb R, Pfister H, Hofler M, Wittchen HU (2002). Use, abuse and dependence of ecstasy and related drugs in adolescents and young adults—a transient phenomenon? Results from a longitudinal community study. Drug Alcohol Depend 66: 147–159.
Winslow JT, Insel TR (1992). Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylenedioxymethamphetamine (MDMA). J Pharmacol Exp Ther 254: 212–220.
Winstock AR, Griffiths P, Stewart D (2001). Drugs and the dance music scene: a survey of current drug use patterns among a sample of dance music enthusiasts in the UK. Drug Alcohol Depend 64: 9–17.
Wolf K, Hay AWM, Sherlock K, Conner M (1995). Contents of ‘ecstasy’. Lancet 346: 1100–1101.
Acknowledgements
Expert technical assistance with the monkey self-administration experiments was provided by Debbie Huntzinger, Sarah Pilkington, and Jolan Terner at the University of Michigan. The authors express their gratitude to Kyle Kuzspit and Leslie Doherty at the University of Michigan PET facility for their skilled aid in the conduct of the imaging procedures. Insightful comments on an earlier draft of this manuscript (presented as a chapter in the doctoral thesis of WEF) were provided by Theresea Lee and Terry Robinson at the University of Michigan Department of Psychology, and by Una McCann at the Johns Hopkins Medical Institutions. These studies were supported by USPHS Grants DA09161, DA05923, DA05707, and DA00206.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fantegrossi, W., Woolverton, W., Kilbourn, M. et al. Behavioral and Neurochemical Consequences of Long-Term Intravenous Self-Administration of MDMA and Its Enantiomers by Rhesus Monkeys. Neuropsychopharmacol 29, 1270–1281 (2004). https://doi.org/10.1038/sj.npp.1300442
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300442
Keywords
This article is cited by
-
Effects of cocaine on the discriminative stimulus and reinforcing effects of mephedrone in male rats
Psychopharmacology (2019)
-
The effects of ecstasy on neurotransmitter systems: a review on the findings of molecular imaging studies
Psychopharmacology (2016)
-
MDMA effects consistent across laboratories
Psychopharmacology (2014)