-
PDF
- Split View
-
Views
-
Cite
Cite
Banrida Wahlang, K. Cameron Falkner, Heather B. Clair, Laila Al-Eryani, Russell A. Prough, J. Christopher States, Denise M. Coslo, Curtis J. Omiecinski, Matthew C. Cave, Human Receptor Activation by Aroclor 1260, a Polychlorinated Biphenyl Mixture, Toxicological Sciences, Volume 140, Issue 2, August 2014, Pages 283–297, https://doi.org/10.1093/toxsci/kfu083
Close - Share Icon Share
Abstract
Polychlorinated biphenyls (PCBs) are persistent environmental toxicants, present in 100% of U.S. adults and dose-dependently associated with obesity and non-alcoholic fatty liver disease (NAFLD). PCBs are predicted to interact with receptors previously implicated in xenobiotic/energy metabolism and NAFLD. These receptors include the aryl hydrocarbon receptor (AhR), pregnane xenobiotic receptor (PXR), constitutive androstane receptor (CAR), peroxisome proliferator-activated receptors (PPARs), liver-X-receptor (LXRα), and farnesoid-X-receptor (FXR). This study evaluates Aroclor 1260, a PCB mixture with congener composition mimicking that of human adipose tissue, and selected congeners, as potential ligands for these receptors utilizing human hepatoma-derived (HepG2) and primate-derived (COS-1) cell lines, and primary human hepatocytes. Aroclor 1260 (20 μg/ml) activated AhR, and PCB 126, a minor component, was a potent inducer. Aroclor 1260 activated PXR in a simple concentration-dependent manner at concentrations ≥10 μg/ml. Among the congeners tested, PCBs 138, 149, 151, 174, 183, 187, and 196 activated PXR. Aroclor 1260 activated CAR2 and CAR3 variants at lower concentrations and antagonize CAR2 activation by the CAR agonist, CITCO, at higher concentrations (≥20 μg/ml). Additionally, Aroclor 1260 induced CYP2B6 in primary hepatocytes. At subtoxic doses, Aroclor 1260 did not activate LXR or FXR and had no effect on LXR- or FXR-dependent induction by the agonists T0901317 or GW4064, respectively. Aroclor 1260 (20 μg/ml) suppressed PPARα activation by the agonist nafenopin, although none of the congeners tested demonstrated significant inhibition. The results suggest that Aroclor 1260 is a human AhR, PXR and CAR3 agonist, a mixed agonist/antagonist for CAR2, and an antagonist for human PPARα.
Non-alcoholic fatty liver disease (NAFLD), characterized by accumulation of lipid droplets in the liver, affects up to 46% of the U.S. population (Williams et al., 2011). NAFLD may be accompanied by hepatic and systemic inflammation, leading to non-alcoholic steatohepatitis (NASH). NAFLD and NASH were traditionally associated with the inappropriate overactivation or underactivation of nuclear receptors involved in endobiotic metabolism. These receptors include the liver-X-receptor (LXR), farnesoid-X-receptor (FXR), and peroxisome proliferator-activated receptors (PPARs) that regulate cholesterol, bile acid, and lipid metabolism, respectively (Mangelsdorf et al., 1995; Novac and Heinzel, 2004). Recent studies have implicated the role of hepatic receptors involved in xenobiotic detoxification, including the pregnane xenobiotic receptor (PXR), the constitutive androstane receptor (CAR), and the aryl hydrocarbon receptor (AhR), in NAFLD/NASH. Although these receptors were initially thought to be involved only with detoxification and xenobiotic metabolism, overactivation or antagonism of these receptors may lead to metabolic disease states such as steatosis and obesity (Konno et al., 2008; Merrell and Cherrington, 2011; Moya et al., 2010).
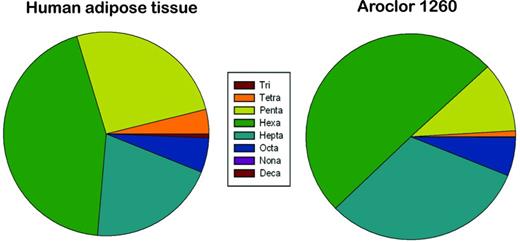
Polychlorinated biphenyls (PCBs) are persistent organic pollutants (POPs) that were commercially manufactured in the 1930s–1970s prior to being banned. Exposure to PCBs has been associated with liver disease, type 2 diabetes, and obesity (Arrebola et al., 2013; Cave et al., 2010; Silverstone et al., 2012). PCBs were manufactured and sold as mixtures rather than individual congeners. Monsanto, the only PCB manufacturer in North America, produced PCB mixtures under the brand name Aroclor at its manufacturing plant located in Anniston, Alabama (ATSDR, 2000). Based on the chlorine substitution in the two phenyl rings, PCBs are classified as either coplanar or non-coplanar (Safe, 1993). Coplanar PCBs such as PCBs 77 and 126 are AhR agonists similar to dioxin (“dioxin-like” PCBs) (Baker et al., 2013; NTP, 2006a) whereas some non-coplanar PCBs such as PCB 153 and 196 are referred to as “phenobarbital-like” PCBs, suggesting their activation of PXR and/or CAR (Kopec et al., 2010; NTP, 2006b). Moreover, a theoretical structure-activity relationship study predicted that non-coplanar PCBs can interact with PXR, estrogen receptor, androgen receptor, and thyroid receptor (Luthe et al., 2008). Aroclor 1260, a commercial PCB mixture, is heavily chlorinated and contains a limited amount of coplanar congeners (∼1%). It has predominantly non-coplanar and di-ortho substituted PCBs that have either 5, 6, 7, or 8 chlorines (Supplementary Data). As low molecular weight PCBs are often metabolized and eliminated, PCBs that bioaccumulate are typically the highly chlorinated congeners (Beyer and Biziuk, 2009). Thus the Aroclor 1260 PCB mixture was selected for this study because its composition best mimics the PCB bioaccumulation profile found in human adipose tissue (Fig. 1) rather than reflecting the actual production volume of PCBs (McFarland and Clarke, 1989).
Pie charts depicting the relative abundance of PCBs in human adipose tissue and Aroclor 1260.
Historically, PCB toxicity has been linked to cancer, endocrine disruption, and impaired cognitive development, but recent studies have shown that chronic exposure to these environmental pollutants can result in metabolic disorders associated with NAFLD, including obesity, insulin resistance/diabetes, and the metabolic syndrome (Ruzzin et al., 2010; Smith et al., 1982). Additionally, rodent studies have correlated PCB exposures with NAFLD, obesity, and the metabolic syndrome suggesting the involvement of distinct nuclear receptors in PCB-mediated toxicity (Wahlang et al., 2013). It remains unclear if the liver disease caused by PCB exposure is due to the direct involvement of receptors that regulate endobiotic metabolism such as LXR and FXR or if the disease process is linked to the activation/inhibition of xenobiotic receptors such as PXR and CAR. We hypothesized that PCBs may exert some of their toxic effects, such as NAFLD, by either interacting directly with the endobiotic nuclear receptors (LXR, FXR, PPARs) or through interaction with xenobiotic receptors that cross-talk with endobiotic receptors to otherwise modify their respective interactions with DNA response elements. Moreover, the interaction between Aroclor 1260 and these receptors that are implicated in NAFLD has never been tested. The purpose of this study is to evaluate the receptor agonism/antagonism by the PCB mixture, Aroclor 1260, and selected PCB congeners that are highly represented in this mixture. The study demonstrated selective activation by Aroclor 1260 and selected PCB congeners on human AhR, PXR, and CAR, and inhibition of PPARα. We postulate that hepatic transcription factor activation is one of the modes of action of these organo-chlorine pollutants.
MATERIALS AND METHODS
Materials
Aroclor 1260 was purchased from AccuStandard (New Haven, CN) and PCB congeners were obtained from Ultra Scientific (North Kingstown, RI). T0901317 (N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide), GW3965 (2-(3-(3-((2-chloro-3-(trifluoromethyl)benzyl)(2,2-diphenylethyl)amino)propoxy)phenyl) acetic acid hydrochloride), GW4064 (3-[2-[2-chloro-4-[[3-(2,6-dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methoxy]phenyl]ethenyl] benzoic acid), and pioglitazone were obtained from Tocris Bioscience (Bristol, UK). Dimethyl sulfoxide (DMSO) was acquired from Fisher BioReagents (Thermo Fisher Scientific, Pittsburg, PA) whereas 6-(4-chlorophenyl) imidazo[2,1-b] [1,3] thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl) oxime (CITCO), rifampicin (RIF), benz[a]anthracene (BA), and 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were from Sigma Aldrich (St Louis, MO). Restriction endonucleases and T4 DNA ligase were purchased from New England BioLabs (Ipswich, MA). Lipofectamine and Opti-MEM were obtained from Life Technologies Inc. (Carlsbad, CA). Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA).
Plasmid construction
The reporter plasmids for human (h) PXR (pGL3-DR4-Luc), hFXR (pGL3-IR1-Luc), and hPPARα (pGL3-DR1-Luc) were constructed by using two copies of a direct repeat 4 (DR4), an inverted repeat 1 (IR1), and a direct repeat 1 (DR1) response element (RE), respectively. The top strand oligonucleotide was 5′ AGAGTTCATGAGAGTTCATGAGAGTTCATGAGAGTTCATG 3′ for pGL3-DR4-Luc, 5′ AGAGGTCATTGACCTTTAGAGGTCATTGACCTTT 3′ for pGL3-IR1-Luc, and 5′ AACTAGGTCAAAGGTCAAACTAGGTCAAAGGTCAAA 3′ for pGL3-DR1-Luc. Both the bottom complementary strands had Kpn1 and Xho1 overhangs at the 5′ and 3′ positions, respectively. The oligonucleotides were annealed and inserted into Xho1 and Kpn1 restriction sites in the polycloning region of a modified version of pGL3 promoter vector (Promega, Madison, WI). Reporter plasmid for AhR (pXRE-SV40-Luc) was synthesized using the oligonucleotide 5′ TCAGGCATGTTGCGTGCATCCCTGAGGCCAGCC 3′ inserted into the EcoR1 site of a modified version of pGL3 promoter vector. Expression vectors pSG5-hLXRα, pSG5-hPXR, and pSG5-hFXR, and reporter plasmid pTK-LXRE-Luc were a generous gift from John Y. Chiang (Department of Integrative Medical Sciences, Northeast Ohio Medical University). Expression vectors pCMV6-hPPARα, pCMV6-hPPARγ, and pCMV6-hCAR (CAR2) were purchased from Origene (Rockville, MD). The vectors, pTracer CMV2-hCAR1, pTracer CMV2-hCAR2, pcDNA 3.1-RXRα, and pGL3- basic/TK CYP2B6-dervied XREM/PBREM were described previously (Auerbach et al., 2007). pTracer CMV2-CAR3 was also reported previously (Auerbach et al., 2005). pRL-CMV, the expression plasmid encoding Renilla luciferase, was purchased from Promega and used in the Dual-Glo (Promega) assay system according to the manufacturer's protocol to normalize for transfection efficiencies in cultured cells. Prior to transfection, plasmids were prepared using the Qiagen Plasmid Plus Midi Kit (Qiagen, Valencia, CA).
Cell culture
HepG2 cells: The human hepatoma-derived cell line (HepG2) was obtained from the American Type Culture Collection (ATCC, Manassas, MD). Cells were grown in Dulbecco's modified Eagle's medium (DMEM; HyClone Laboratories Inc., Thermofisher, Waltham, MA) supplemented with 10% fetal bovine serum (FBS) and 1% antimycotic/antibiotic solution (Mediatech, Manassas, VA). The cells were incubated in a 5% carbon dioxide atmosphere and 95% humidity at 37°C and subcultured every 2 days. COS-1 cells: COS-1 cells (Simian virus-40–transformed African green monkey kidney cells) were obtained from the ATCC and maintained in DMEM plus GlutaMAX-I with 10% FBS, 10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1mM sodium pyruvate, 13 non-essential amino acids, and 1% penicillin/streptomycin. COS-1 cells were cultured at 37°C in a humidified atmosphere containing 5% carbon dioxide. All cell culture reagents were purchased from Life Technologies (Grand Island, NY).
Cell viability assay
HepG2 cells were seeded in 96-well tissue culture plates at a seeding density of 10,000 cells per well. Cells were treated with graded concentrations of Aroclor 1260 made up as 500X stocks in DMSO. Controls received DMSO only. After 24 h incubation, MTT (0.2 mg/ml) was added to the cells and incubated for 3–4 h. The media were removed from the plates by aspiration, and formazan dye was liberated by adding 50 μl DMSO. MTT, a yellow tetrazole, was reduced to an insoluble product formazan by mitochondrial reductases, indicative of cell viability. Presence of formazan, a purple precipitate, was determined spectrophotometrically at 540 nm using a Bio-Tek Synergy HT multi-mode micro plate reader (Winooski, VT).
Transfection
HepG2 cells were plated in Thermo Scientific Nunc 24-well plates and transfected at 40–60% confluence. Unless otherwise specified, the transfection mix per well contained 150 ng β-galactosidase expression plasmid (pCMV-β, Stratagene, CA) as a transfection control, 50 ng receptor expression plasmid, and 150 ng reporter plasmid. All cells were cotransfected by lipofection using Lipofectamine reagent according to the manufacturer's instructions and Opti-MEM (reduced serum medium) as the transfecting medium. After 4 h incubation, the medium was changed to DMEM supplemented with 10% FBS and 1% antimycotic/antibiotic solution and cells were allowed to recover overnight. DMEM supplemented with charcoal/dextran treated FBS (HyClone Laboratories Inc., Thermofisher) was used for PPAR activation assays. Compounds of interest were then added to the cells (n = 4) and cells were incubated for 24 h. DMSO was used as a carrier for all compounds (final concentration <0.5%). For COS-1 cells, all media components remained the same except that FBS was replaced with 10% dextran/charcoal-treated FBS (HyClone, Logan, UT). The details of the luciferase reporter assays were described previously (Auerbach et al., 2005, 2007; Omiecinski et al., 2011). Briefly, approximately 1 h prior to transfection, cells were trypsinized and plated onto 48-well plates (∼50,000 cells per well). For determination of transcriptional activity of the hCAR constructs, cells were transfected using Fugene 6 (Promega) according to the manufacturer's recommendations with a cotransfection plasmid mix consisting of 10 ng pRL-CMV (Renilla luciferase) for normalization, 25 ng pcDNA 3.1-RXRα, 100 ng of pGL3-basic/TK XREM/PBREM luciferase reporter plasmid, and 25 ng of pTracer vectors containing the various CMV2-CAR expression constructs. Each condition was performed in quadruplicate. Aroclor 1260 and each of the PCBs were evaluated at 10μM. DMSO was used as a solvent control. CITCO, 5μM, was used as a positive control. Androstanol (ANDR), a known inverse agonist of human CAR, was used as a control for CAR1.
Reporter assay
HepG2 cells: Cells were washed twice with phosphate buffered saline (1X), harvested using 50 μl cell lysis buffer (Promega) and subjected to a single freeze-thaw event. For β-galactosidase assays, cell extracts (5 μl) were incubated with chlorophenol red β-galactopyranoside (Roche Diagnostics, Indianapolis, IN) at 37°C for 30–60 min. The enzyme activity was determined spectrophotometrically at 595 nm using the Bio-Tek Synergy HT multi-mode micro plate reader. Luciferase activity assays were performed on cell extracts (5 μl) using the Luciferase Assay System (Promega). Luminescence was measured using the Orion L micro plate luminometer (Berthold Detection Systems, Pforzheim, Germany) over a 10 s period. Receptor activation was measured by luciferase activity and results were normalized to the amount of β-galactosidase expressed. COS-1 cells: Luciferase assays were performed using the Dual-Glo Reporter Assay System (Promega) and a Veritas Microplate Luminometer (Turner Biosystems, Sunnyvale, CA). Firefly (Photinus pyralis) luminescence data values were recorded for each replicate and normalized luciferase activities were then calculated by dividing the raw luciferase values by the Renilla luciferase signals to correct for any differences in transfection efficiency among the assay wells.
Validation of receptor activation in primary human hepatocytes
Human hepatocytes were obtained from BioreclamationIVT (Baltimore, MD). Hepatocytes were thawed and plated in 12-well plates according to the supplier's protocols, and the compounds of interest were added. The cells were incubated for 24 h and RNA was extracted using RNA STAT-60 protocol (Tel-test, Austin, TX). RNA purity and quantity were assessed with the Nanodrop (ND-1000, Thermo Scientific, Wilmington, DE) using the ND-1000 V3.8.1 software. cDNA was synthesized from total RNA using the QuantiTect Reverse Transcription Kit (Qiagen). Polymerase chain reaction (PCR) was performed on the Applied Biosystems StepOne Plus Real-Time PCR Systems using the Taqman Universal PCR Master Mix (Life Technologies). Each PCR mix (20 μl) contained: Taqman Universal PCR Master Mix (10 μl), 20X Gene Expression Assay Mix (1 μl), cDNA sample (2 μl), and nuclease-free water (7 μl). Primer sequences from Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA) were as follows: cytochrome P450s [CYP3A4 (Hs00604506_m1), CYP2B6 (Hs04183483_g1), CYP1A1 (Hs01054797_g1)], CD36 (Hs01567185_m1), fatty acid synthase (FAS) (Hs01005622_m1), carnitine palmitoyl transferase 1A (CPT1A), (Hs00912671_m1), small heterodimeric partner (SHP) (Hs00222677_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Cycle conditions were maintained according to the Applied Biosystems guide. The levels of mRNA were normalized relative to the amount of GAPDH mRNA, and expression levels in DMSO-exposed cells were set at 1%. Gene expression levels were calculated according to the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.01 (San Diego, CA). In general for all assays, data are expressed as means ± SEM. Quantitative data for two group comparisons were assessed using an unpaired t-test. Multiple group data were examined by one way analysis of variance followed by the Dunnett's post hoc test to compare all groups with the control sample. Multiplicity adjusted p values are reported in the Results section. p < 0.05 was considered statistically significant.
RESULTS
Cell Viability Assay for Aroclor 1260
MTT assays were performed to determine the optimal concentration for Aroclor 1260 that does not cause toxicity in HepG2 cell culture experiments. Cells were exposed to this PCB mixture at concentrations ranging from 1.25 to 250 μg/ml. The optical density for formazan, representative of cell viability, was plotted against Aroclor 1260 concentrations. The toxicity threshold (concentration that caused 50% cell death) was determined to be 26.0 ± 3.7 μg/ml (data not shown). Concentrations of Aroclor 1260 at 5, 10, 15, and 20 μg/ml were selected for subsequent experiments.
Aroclor 1260 Activation of the Human Aryl Hydrocarbon Receptor (AhR)
Coplanar PCBs (PCBs 126 and 77) activate AhR in rodents and hence are classified as “dioxin-like” PCBs. Coplanar congeners comprise only ∼1% of the total composition in Aroclor 1260. HepG2 cells, cotransfected with pXRE-SV40-Luc, were exposed to various concentrations of Aroclor 1260 for 24 h and the normalized luciferase activity was measured. As anticipated, AhR was activated by its polycyclic aromatic hydrocarbon ligand BA (10μM), and Aroclor 1260 exposure resulted in a significant increase in luciferase activity (3.5-fold at 20 μg/ml, p < 0.0001) compared with DMSO-exposed cells (Fig. 2A). However, this fold induction was relatively low when compared with that of BA. These results indicate that Aroclor 1260 has a weak AhR agonistic activity that is likely due to the presence of coplanar congeners at lower concentrations in the mixture. AhR activation by BA was assessed in the presence of increasing concentrations of Aroclor 1260 to evaluate for any potentiation or antagonism effects. Cells co-exposed to BA and Aroclor 1260 showed no difference versus BA-exposed cells only (Fig. 2B). Next, we selected 10 non-coplanar congeners that are more highly represented in Aroclor 1260 mixture (≥1%) as well as two coplanar congeners (PCBs 126 and 118) and tested the ability of these compounds to individually activate AhR at a concentration of 10μM. Although PCB 126 (no ortho) constitutes ∼0.002% of the total PCB composition in Aroclor 1260, it was selected for this study because it is a good AhR activator (Rushneck et al., 2004). PCB 118 (mono-ortho) was chosen because it was one of the coplanar congeners with the highest composition in Aroclor 1260 (0.48%). Among the selected congeners, coplanar PCB 126 and non-coplanar PCB 138 significantly induced luciferase activity (17-fold, p < 0.0001 and fourfold, p = 0.0006, respectively), whereas coplanar PCB 118 did not induce luciferase activity (1.2-fold) compared with DMSO-exposed cells (Fig. 2C). Thus, it appeared that PCB 118 did not activate AhR at the concentration used, unlike PCB 126. Besides, AhR activation was not restricted to only coplanar congeners because PCB 138 modestly activated AhR.
Aroclor 1260 activation of the human AhR. HepG2 cells were transiently transfected with the reporter plasmid pXRE-SV40-Luc. Benz[a]anthracene (10μM, BA) was used as a positive control. (A) Cells were exposed to Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml, and luciferase induction was normalized and compared with DMSO-exposed cells (0 μg/ml Aroclor 1260). (B) Cells were exposed to 10μM BA or BA plus Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml. The luciferase induction was normalized to that of cells exposed only to DMSO solvent carrier (as in (A), not shown). Luciferase activity in cells exposed to BA and Aroclor 1260 was compared with that of BA-exposed cells. (C) Cells were exposed to selected PCB congeners (10μM) present in Aroclor 1260 and the induction was compared with DMSO-exposed cells. BA (10μM) was used as positive control. Data were normalized to luciferase activity in cells exposed only to DMSO and are expressed as mean ± SEM, n = 4, *p < 0.05.
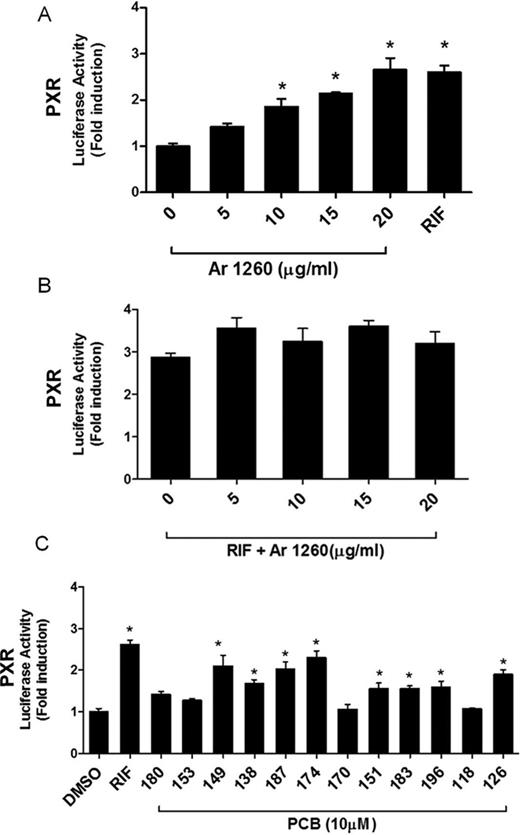
Aroclor 1260 Activation of the Human Pregnane Xenobiotic Receptor
Non-coplanar PCBs, including PCBs 153 and 196, have been predicted to activate the nuclear receptors PXR and CAR, and these PCBs are often referred to as “phenobarbital-like.” Apart from regulating xenobiotic metabolism, PXR activation is implicated in weight gain and obesity (He et al., 2013; Konno et al., 2008). Moreover, because PXR and CAR do share similar ligands to an extent, it is therefore likely that Aroclor 1260, being largely composed of non-coplanar PCBs, will activate PXR. HepG2 cells, cotransfected with pSG5-hPXR and pGL3-DR4-Luc, were exposed to various concentrations of Aroclor 1260 for 24 h. RIF (10μM), a PXR ligand, was used as a positive control. RIF activated PXR-driven luciferase activity (2.6-fold, p < 0.0001) whereas Aroclor 1260 activated the receptor in a concentration-dependent manner with a Km value of 8.75 μg/ml (Fig. 3A). Cells exposed to Aroclor 1260 at 5 μg/ml showed no significant induction in luciferase activity as compared with cells exposed to DMSO only. However, the induction was significant at concentrations of 10 (1.9-fold, p = 0.0024), 15 (2.1-fold, p = 0.0001), and 20 (2.7-fold, p < 0.0001) μg/ml. When transfected cells were exposed to both RIF and Aroclor 1260 (5 μg/ml and above) simultaneously, there was a slightly higher induction (∼18%) compared with RIF-exposed cells alone (Fig. 3B) but this was not significant. These data suggest that Aroclor 1260 activated the PXR reporter system at concentrations of 10 μg/ml and higher and that the mixture did not significantly potentiate or antagonize PXR activation by the receptor ligand, RIF. We then evaluated the ability of selected PCB congeners to activate PXR (Fig. 3C). Of the congeners tested, non-coplanar PCBs that significantly increased luciferase activity at 10μM included PCBs 149 (2.1-fold, p < 0.0001), 138 (1.7-fold, p = 0.0006), 187 (2.0-fold, p < 0.0001), 174 (2.3-fold, p < 0.0001), 151 (1.6-fold, p = 0.0062), 183 (1.6-fold, p = 0.0067), and 196 (1.6-fold, p = 0.0036). Notably, these congeners in total represent more than 30% of the Aroclor 1260 mixture by mass. Interestingly, PCB 126, a well-known AhR ligand and a coplanar congener, also activated human PXR (1.9-fold, p < 0.0001), indicating that a PCB's non-coplanar structure is not solely required for this receptor's activation.
Aroclor 1260 activation of the human PXR. HepG2 cells were transiently transfected with the expression plasmid pSG5-hPXR and reporter plasmid pGL3-DR4-Luc. Rifampicin (RIF; 10μM) was used as a positive control. (A) Cells were exposed to Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml and luciferase induction was normalized and compared with DMSO-exposed cells (0 μg/ml Aroclor 1260). (B) Cells were exposed to 10μM RIF or RIF plus Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml. The luciferase induction was normalized to that of cells exposed only to DMSO solvent carrier (as in (A), not shown). Luciferase activity in cells exposed to RIF and Aroclor 1260 was compared with that of RIF-exposed cells. (C) Cells were exposed to selected PCB congeners (10μM) present in Aroclor 1260 and the induction was compared with DMSO-exposed cells. RIF (10μM) was used as positive control. Data were normalized to luciferase activity in cells exposed only to DMSO and are expressed as mean ± SEM, n = 4, *p < 0.05.
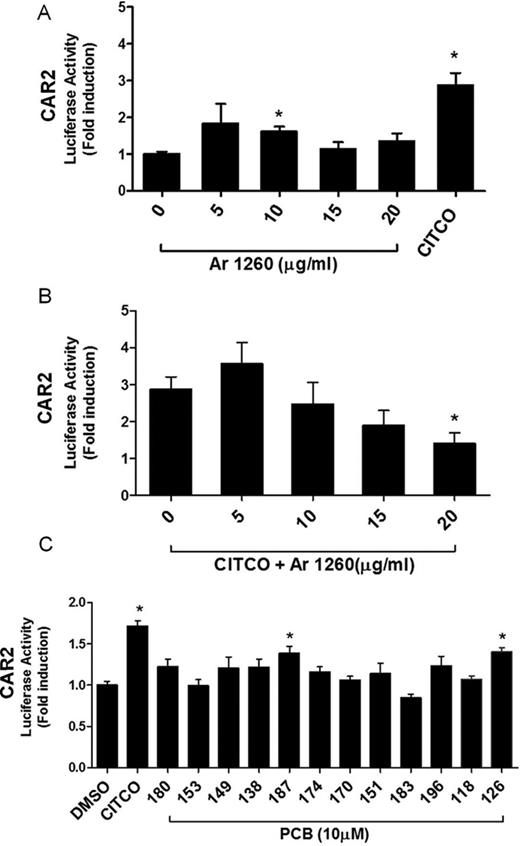
Aroclor 1260 Activation of the Human Constitutive Androstane Receptor (CAR)
In these experiments, HepG2 cells were cotransfected with pCMV6-hCAR (CAR2) and pGL3-DR4-Luc and the ability of Aroclor 1260 to transactivate CAR2 was evaluated. CAR 2 is a human splice variant of the CAR1 reference form of the receptor, but unlike the reference form, CAR2 is not constitutively active; rather, it is a ligand activated receptor (Auerbach et al., 2007). Although somewhat modest, the human CAR agonist CITCO and Aroclor 1260 (10 μg/ml) significantly activated CAR2 (2.8-fold, p < 0.0001 and 1.6-fold, p = 0.0240, respectively) (Fig. 4A). However, increasing the concentration of Aroclor 1260 did not lead to further CAR2 activation (1.1-fold at 15 μg/ml and 1.4-fold at 20 μg/ml, respectively). Rather, this PCB mixture appeared to antagonize CAR2 activation by CITCO (Fig. 4B). Aroclor 1260, at 20 μg/ml, significantly reduced the induction produced by CITCO by 51% (p = 0.0314). These results suggested that Aroclor 1260 may bind to CAR2 and either activate CAR2 or inhibit CAR2 activation by CITCO in a concentration-dependent manner. Interestingly, two of the selected congeners, PCBs 187 (p = 0.0042) and 126 (p = 0.0031), increased luciferase induction significantly at 10μM concentration (Fig. 4C), indicating that some of the PCB congeners in Aroclor 1260 may be CAR2 agonists.
Aroclor 1260 activation of the human CAR2 transcript in HepG2 cells. HepG2 cells were transiently transfected with the expression plasmid pCMV6-hCAR (CAR2) and reporter plasmid pGL3-DR4-Luc. CITCO (10μM) was used as a positive control. (A) Cells were exposed to Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml and luciferase induction was normalized and compared with DMSO-exposed cells (0 μg/ml Aroclor 1260). (B) Cells were exposed to 10μM CITCO or CITCO plus Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml. The luciferase induction was normalized to that of cells exposed only to DMSO solvent carrier (as in A, not shown). Luciferase activity in cells exposed to CITCO and Aroclor 1260 was compared with that of CITCO-exposed cells. (C) Cells were exposed to selected PCB congeners (10μM) present in Aroclor 1260 and the induction was compared with DMSO-exposed cells. CITCO (10μM) was used as positive control. Data were normalized to luciferase activity in cells exposed only to DMSO and are expressed as mean ± SEM, n = 4, *p < 0.05.
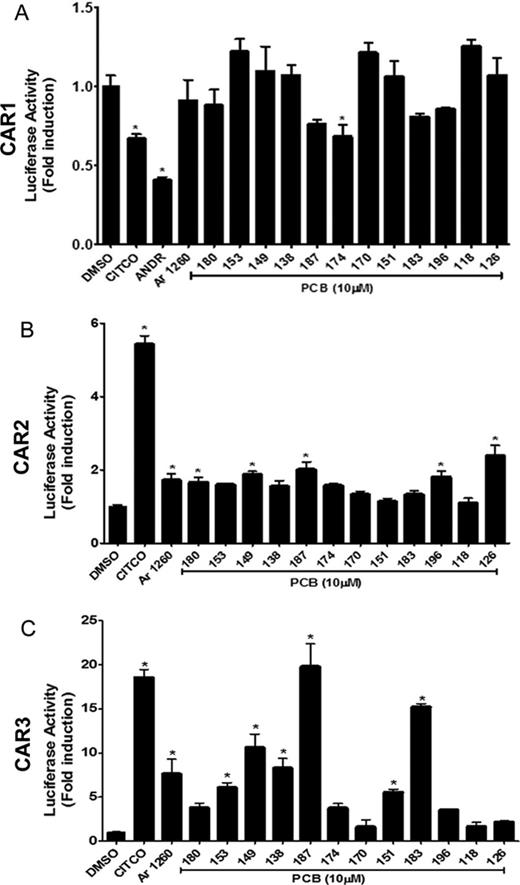
Given the apparent impact of these PCB congeners on human CAR, we designed a complimentary series of assays to further corroborate and better characterize these effects using the primate-derived cell line, COS-1. These results are presented in Figure 5. Due to the high constitutive activity of CAR1, any ligand interactions with the receptor tend to be masked. In these respects, it is notable that PCB 174 significantly inhibited the constitutive activity of CAR1 (p < 0.05), though not as remarkably as ANDR (p < 0.001), a known CAR inverse agonist (Fig. 5A). Although the level of activation of CAR2 did not reach the level of the CITCO positive control (5.4-fold, p < 0.001), Aroclor 1260 as well as several other PCB congeners demonstrated significant CAR2 activation in the COS-1 cell assays (Fig. 5B). Exhibiting a fold change of 2.4 (p < 0.001), PCB 126 elicited the greatest response—even greater than Aroclor 1260 (1.7-fold, p < 0.05). Four additional congeners also activated CAR2: 180 (1.7-fold, p < 0.05), 149 (1.9-fold, p < 0.01), 187 (2.0-fold, p < 0.001), and 196 (1.8-fold, p < 0.01). CAR3, another ligand activated human splice variant, which likely maintains a conserved ligand binding pocket with CAR1, was also tested and exhibited significant activation effects with Aroclor 1260 (7.6-fold, p < 0.001) (Fig. 5C). Several other PCB congeners also demonstrated significant CAR3 activation including PCB 187 (17.1-fold, p < 0.001; comparable to CITCO at 18.5-fold, p < 0.001), PCB 153 (6.0-fold, p < 0.01), PCB 149 (10.6-fold, p < 0.001), PCB 138 (8.3-fold, p < 0.001), PCB 151 (5.5-fold, p < 0.01), and PCB183 (15.1-fold, p < 0.001).
Aroclor 1260 activation of the human CAR variant transcripts in COS-1 cells. COS-1 cells were transiently transfected with expression plasmid pTracerCMV2 containing either (A) CAR1, (B) CAR2, or (C) CAR3 transcripts, together with the reporter plasmid, pGL3–2B6XREM/PBREM-TKLuc. Cells were exposed to selected PCB congeners (10μM) present in Aroclor 1260 and the induction was compared with DMSO-exposed cells. CITCO (5μM) was used as a positive control. ANDR (10μM), a CAR inverse agonist, was used as a negative control for CAR1. Data were normalized to luciferase activity in cells exposed only to DMSO and are expressed as mean ± SEM, n = 4, *p < 0.05.
Effect of Aroclor 1260 on the Human Liver-X-Receptor Alpha (LXRα)
The human LXRα is a subtype of LXR that is expressed in the liver and its under- or overactivation eventually leads to steatosis (Ducheix et al., 2013). We therefore hypothesized that Aroclor 1260 may activate LXRα and this activation may subsequently promote hepatic steatosis and NAFLD. HepG2 cells, cotransfected with pSG5-hLXRα and pTK-LXRE-Luc, were exposed to various concentrations of Aroclor 1260 for 24 h and the normalized luciferase activity measured. T0901317, a synthetic LXRα ligand, was used as a positive control. Compared with the fold induction by T0901317 (100nM), Aroclor 1260 did not activate LXRα nor antagonize LXRα activation by T0901317 at any concentration tested (Supplementary Data), thus indicating that this PCB mixture is neither an agonist nor antagonist of the nuclear receptor LXRα.
Effect of Aroclor 1260 on the Human Farnesoid-X-Receptor (FXR)
The ability of Aroclor 1260 to activate FXR, a key regulator of bile acid and energy metabolism, was evaluated. HepG2 cells, cotransfected with pSG5-hFXR and pGL3-IR1-Luc, were exposed to various concentrations of Aroclor 1260 for 24 h. GW4064 (0.5μM), a synthetic FXR agonist, was used as a positive control. GW4064 activated FXR whereas Aroclor 1260 did not induce a response at any of the concentrations tested. In studies to test antagonism, all concentrations tested were without effect on the activation of human FXR (Supplementary Data). Thus, this PCB mixture is neither an agonist nor antagonist for the human FXR.
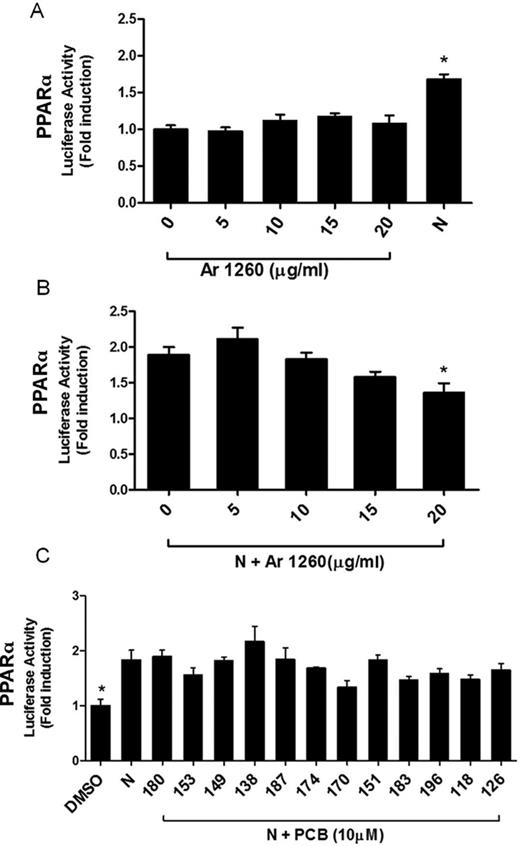
Effect of Aroclor 1260 on the Human Peroxisome-Proliferator Activated Receptors (PPARs)
PPARs are a group of nuclear receptors that mediate peroxisomal proliferation with subtype alpha (α) playing a distinct transcriptional role in lipid metabolism in the liver and the subtype gamma (γ) in the adipose tissue. It was therefore important to evaluate the effect of Aroclor 1260 on PPARs α and γ activation. HepG2 cells were cotransfected with pCMV6-hPPARα and pGL3-DR1-Luc. Nafenopin was used as a positive control. Aroclor 1260 did not activate PPARα at any of the concentrations (Fig. 6A). In studies to test antagonism, Aroclor 1260 at the highest concentration (20 μg/ml) significantly antagonized PPARα activation induced by nafenopin (27%, reduction, p = 0.0222; Fig. 6B). Possible antagonism of PPARα by individual PCB congeners was also tested at 10μM concentration (Fig. 6C). However, none of the selected congeners reduced the induction by nafenopin significantly, indicating that a combination of the congeners is required to produce a marked effect or that other congeners present in this mixture may contribute to this effect. For PPARγ activation, HepG2 cells were cotransfected with pCMV6-hPPARγ and pGL3-DR1-Luc. Pioglitazone was used as a positive control. Aroclor 1260 did not activate PPARγ nor antagonize PPARγ activation by pioglitazone at any of the concentrations tested (Supplemnetal fig. 3).
Aroclor 1260 activation of the human PPARα. HepG2 cells were transiently transfected with the expression plasmid pCMV6-hPPARα and reporter plasmid pGL3-DR1-Luc. Nafenopin (N; 50μM) was used as a positive control. (A) Cells were exposed to Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml and luciferase induction was normalized and compared with DMSO-exposed cells (0 μg/ml Aroclor 1260). (B) Cells were exposed to 50μM N or N plus Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml. The luciferase induction was normalized to that of cells exposed only to DMSO solvent carrier (as in (A), not shown). Luciferase activity in cells exposed to N and Aroclor 1260 was compared with that of N-exposed cells. (C) Cells were exposed to selected PCB congeners (10μM) present in Aroclor 1260 and the induction was compared with DMSO-exposed cells. N (50μM) was used as positive control. Data were normalized to luciferase activity in cells exposed only to DMSO and are expressed as mean ± SEM, n = 4, *p < 0.05.
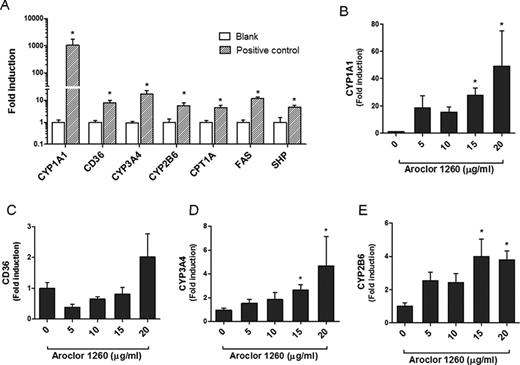
Aroclor 1260 Exposure and Gene Expression in Human Hepatocytes
To confirm our findings, we tested the effects of Aroclor 1260 exposure on target gene expression profiles in human hepatocytes. Primary hepatocytes were exposed to Aroclor 1260 at 5, 10, 15, and 20 μg/ml and gene expression was measured by RT-PCR. The mRNA expression of target genes namely CYP1A1, CD36, CYP3A4, CYP2B6, CPT1A, FAS, and SHP were significantly induced by the respective receptor agonists in primary human hepatocytes (Fig. 7A). Expression levels of CYP1A1, an AhR target gene, were significantly increased when hepatocytes were exposed to Aroclor 1260 (15 μg/ml, p = 0.0018, and 20 μg/ml, p = 0.0066, versus unexposed cells) (Fig. 7B). Contrary to CYP1A1, CD36, another AhR target gene, was not induced by Aroclor 1260 exposure, suggesting that CD36 is not as inducible as CYP1A1 (Fig. 7C). CD36 expression, however, is regulated by other transcription factors in addition to AhR (Zhou et al., 2008). Furthermore, CYP3A4 (PXR target gene) mRNA expression was upregulated by Aroclor 1260 exposure at 15 μg/ml (p = 0.0083) and 20 μg/ml (p = 0.0303) (Fig. 7D). Irrespective of the CAR2 antagonism observed in transfection assays, Aroclor 1260 induced CYP2B6 (CAR target gene) at 15 μg/ml (p = 0.0114) and 20 μg/ml (p = 0.0036) (Fig. 7E). Expression levels of other genes including FAS (LXR target gene), SHP (FXR target gene), and CPT1A (PPARα target gene) were not affected by Aroclor 1260 exposure (Supplementary Data), thus confirming our results obtained from the HepG2 and COS-1 series of experiments.
Effects of Aroclor 1260 on target genes. Primary human hepatocytes were exposed either to receptor ligands or different concentrations of Aroclor 1260. After a 24 h incubation, RNA was isolated and RT-PCR was performed. (A) mRNA levels of selected receptor target genes were upregulated by receptor ligands (positive control) namely BA (10μM) for CYP1A1 and CD36, RIF (10μM) for CYP3A4, CITCO (10μM) for CYP2B6, T0901317 (100nM) for FAS, nafenopin (50μM) for CPT1A, and GW4065 (0.5μM) for SHP. Relative mRNA levels of target genes were also measured in Aroclor 1260-exposed cells namely (B) CYP1A1, (C) CD36, (D) CYP3A4, and (E) CYP2B6.
DISCUSSION
Our laboratory recently identified suspected NAFLD and toxicant-associated steatohepatitis in National Health and Nutrition Examination Survey (NHANES) participants with low-level environmental exposures to POPs, including 20 PCBs (Cave et al., 2010). Epidemiologic studies have shown a positive association between adipose tissue concentrations of PCBs and type 2 diabetes (Arrebola et al., 2013; Silverstone et al., 2012). High serum PCB levels were also associated with elevated serum triglycerides and cholesterol which are major risk factors for cardiovascular diseases (Goncharov et al., 2008). Exposure to PCB mixtures has also been associated with elevated liver enzymes in plasma and hepatomegaly (Maroni et al., 1981). Clearly, chronic exposures to these chlorinated compounds appear to disrupt both lipid and glucose homeostasis and consequently lead to diabetes and associated metabolic disorders such as NAFLD. Therefore, identifying mechanisms by which PCB exposure can lead to such deleterious effects is relevant to public health.
Environmental exposure to POPs such as PCBs is a major health concern, although subtly different from occupational exposure. The health effects from occupational exposures are more related to the smaller, more metabolizable congeners and the subsequent formation of reactive, potentially genotoxic metabolites. In contrast, the effects produced by the non-metabolizable PCBs in the body are expected to be chronic and life-long because they are not eliminated from the system. Aroclor 1260 production represented approximately 10.6% of total PCB production in the United States between 1958 and 1977, which is modest compared with Aroclor 1242 (51.7%) (Mayes et al., 1998). Nonetheless, Aroclor 1260 was selected for this study because of the resemblance in congener composition pattern to that of human fat (McFarland and Clarke, 1989).
Lipid-adjusted serum PCB levels were measured in NHANES participants and the PCB exposed Anniston cohort and reported to range from 75 to 170 ng/g (0.1–0.5μM) (Cave et al., 2010; Goncharov et al., 2011). Additionally, National Toxicology Program (NTP) studies reported the following PCB levels in a 2-year gavage study in rats: lipid-adjusted serum, 4650 ng/g; liver, 64,593 ng/g; adipose, 2,495,994 ng/g (NTP, 2006b). In these studies, PCB liver levels were at least 10-fold higher and PCB adipose levels were at least 200-fold higher than lipid-adjusted serum levels irrespective of the dose administered. In our experiments, we used Aroclor 1260 at concentrations ranging from 5 to 20 μg/ml (∼10–50μM) and selected congeners at 10μM. These concentrations should approximate the range seen in bioaccumulated organs/tissues such as the liver and adipose.
Almost all PCB animal studies have been performed in rodents, where chronic toxicity is generally regarded as a function of the PCBs’ ability to interact with AhR and nuclear receptors. There are clear differences in the ligand affinity of these receptors, in rodents and humans, toward model ligands and xenobiotic compounds, including PCBs. In order to understand more clearly the involvement of PCBs in human NAFLD as demonstrated in human epidemiological studies such as NHANES, it is important to understand specific interactions between PCBs and human receptors. The findings from the current study demonstrate that Aroclor 1260 activates PXR and to a lesser extent AhR, suggesting that congener composition is critical in determining a mixture's mode(s) of action. The effect of Aroclor 1260 on the AhR is likely due to the presence of coplanar congeners such as PCB 126. However, because these coplanar compounds are only a small fraction of this mixture, a higher concentration of Aroclor 1260 would be required to observe the “dioxin-like” effects. PCB 138, a non-coplanar congener, may also be responsible for the Aroclor 1260 effect on the AhR. Parkinson et al. demonstrated that some di-ortho substituted PCBs can induce hepatic microsomal benzo[a]pyrene hydroxylase, consistent with AhR activation. Thus, coplanarity does not necessarily define “dioxin-like” PCBs from “non-dioxin like” ones (Parkinson et al., 1981). The interaction between Aroclor 1260 and the AhR is further confirmed by the CYP1A1 induction at higher exposure levels in primary hepatocytes. The CYP1A1 induction by Aroclor 1260 however was much less than the 1000-fold induction by the AhR ligand, BA. Furthermore, CD36, another AhR target gene, was not induced by Aroclor 1260 versus BA exposure in primary hepatocytes, further suggesting that a higher concentration of Aroclor 1260 may be required for “dioxin-like” effects. In contrast to AhR, Aroclor 1260 appears to be a relatively good human PXR agonist as observed through transient transfection assays and CYP3A4 induction in primary human hepatocytes. PXR is a transcription factor that plays a distinct role in drug metabolism, and recent studies demonstrated its role in maintaining energy homeostasis in the body. PXR activation is associated with decreased expression of genes involved in lipid catabolism namely carnitine palmitoyltransferase-1a and 3-hydroxy-3-methylglutaryl-CoA-synthase and increased expression of the lipogenic gene stearoyl-CoA desaturase1 which could contribute to NAFLD (Nakamura et al., 2007). Exposure to PXR agonists also decreases blood glucose levels in fasting mice and this effect is related to forkhead box O1 (FoxO1) sequestration by PXR (Kodama et al., 2004). A recent study by He et al. reported that ablation of this receptor in mice was protective against diet-induced and genetic obesity and improved insulin resistance (He et al., 2013). PXR activation clearly leads to disruption in the body's energy balance and this may be one of the mechanisms through which PCBs exert their effects on hepatic energy metabolism. Using transient transfection assays, Tabb et al. demonstrated that highly chlorinated PCBs including PCBs 184 and 197 inhibited human PXR (Tabb et al., 2004). However, PCB 184 is not a component of Aroclor 1260 and PCB 197 accounts for only 0.07% of its total congener composition. This concentration is not likely sufficient to inhibit human PXR. In our study, the majority of PCBs in Aroclor 1260 appear to be PXR agonists.
As its name implies, wild type CAR is a constitutively active receptor due to a truncation that prevents internalization of the transactivation domain (AF2) and results in recruitment of co-activators. In vivo, CAR is regulated through its sequestration in the cytosol by chaperone proteins. Upon ligand binding, the receptor translocates to the nucleus and binds to its respective gene response elements. The cytosolic localization process is absent in immortalized cell lines. Consequently, performing transactivation studies in cell lines using in vitro reporter-based systems can be challenging due to the absence of ligand activation effect normally exhibited by CAR. However, in humans, the CAR transcript is spliced into several variants, in particular CAR2 and CAR3 that constitute approximately one-third of the CAR mRNA pool and encode functional CAR proteins (Auerbach et al., 2007; Omiecinski et al., 2011). Interestingly, both CAR2 and CAR3 splice variants have additional amino acids within the receptor's ligand binding/heterodimerization domain that function to configure these variants as ligand-activated receptors, unlike the parent CAR. Aroclor 1260's interaction with human CAR is perhaps crucial, given the fact that non-coplanar PCBs, such as PCB 153, activate murine CAR target genes, including Cyp2b10. Of further interest, CAR has recently been identified as an anti-obesity nuclear receptor whose activation prevents obesity and improves type 2 diabetes through inhibition of lipogenesis and improved insulin sensitivity (Gao et al., 2009). A recent study by Al-Salman and Plant demonstrated that non-coplanar PCBs, namely PCBs 153, 180, and 194, are agonists for the human PXR and CAR at 10μM (Al-Salman and Plant, 2012). In that study, a receptor activation assay was performed in Huh7 cells using Checkmate mammalian two hybrid system where activated PXR/CAR bind to the DNA-binding domain of the NR Gal4 fusion plasmid. In the current investigation, we deployed a series of transactivation assays to evaluate the potential role of various PCB congeners to activate human CAR/PXR directly. In HepG2 cells, Aroclor 1260 activated CAR2 at 10 μg/ml but not at higher concentrations. The mixed agonism/antagonism observed with CAR2 appeared concentration-dependent with antagonism seen at higher concentrations. In additional studies conducted with COS-1 cells, the results obtained with CAR3 were particularly intriguing in that several PCB congeners were identified as human CAR activators, in particular PCBs 187, 183, 149, 138, as well as Aroclor 1260 (ranked in decreasing order of potency). CAR3 is likely a highly sensitive and reasonable surrogate for CAR1, based on previous reports that include modeling of the receptors’ ligand binding pockets (Faucette et al., 2007; Omiecinski et al., 2011), whereas CAR2 appears to possess subtle alterations within its binding pocket that modify its ligand interaction profile compared with CAR1 (DeKeyser et al., 2011).
In other experiments, Aroclor 1260 exposure induced the CAR target gene, CYP2B6 in human hepatocytes, indicating that this agent acts primarily as a CAR activator and not antagonist. This result is consistent with the activation profile exhibited by Aroclor 1260 on human CAR3 in the transactivation assay. However, it should be noted that ligand binding to CAR is not the sole mechanism by which CAR is activated. Recently, Mutoh et al. reported that phenobarbital, a well-known CAR agonist, indirectly activated CAR by inhibiting the epidermal growth factor receptor signaling which subsequently dephosphorylated CAR and mediated its translocation into the nucleus (Mutoh et al., 2013). We anticipate that some of the PCB congeners may not directly interact with CAR, but may act through the same mechanism as phenobarbital. Further studies will be required to test this possibility.
Previous studies showed that co-exposure to PCB 153 and high fat diet worsened NAFLD and obesity in male C57Bl/6 mice through upregulation of fatty acid synthase, an LXRα target gene (Wahlang et al., 2013). However, in the current study, Aroclor 1260 interacted with neither LXRα nor FXR. Although there was no direct effect by PCBs on either LXRα or FXR, we cannot rule out the possibility of an indirect interaction via cross-talk or alterations in nuclear receptor expression. However, nuclear receptor cross-talk is complex and not well established especially in relation to PCBs. Therefore, more studies are required to elaborate on the possibility of cross-talk. Aroclor 1260 did not activate PPARα but it antagonized PPARα activation by nafenopin at higher concentrations. However, none of the congeners tested at 10μM exhibited any antagonism. Robertson et al. reported the suppression of peroxisomal enzyme activities and CYP4A expression in male Sprague Dawley rats treated with coplanar PCBs, including PCBs 77, 122, 126, and 169 (Robertson et al., 2007). Considering the fact that Aroclor 1260 has a lesser percentage of coplanar PCBs than non-coplanar structural components, we may reconcile the possibility that a higher concentration of this PCB mixture may be required to antagonize PPARα whereas PPARγ remained unaffected.
In summary, Aroclor 1260 is an activator of human PXR and CAR 2/3 variants. It also appears to activate human AhR and antagonize human PPARα at higher concentrations. This study is clinically relevant because human receptor activation by a PCB mixture that simulates PCB bioaccumulation in humans was examined at relevant concentrations. Furthermore, the effects of PCBs on human nuclear receptors such as LXRα and FXR and in human hepatocytes have never been assessed before. Additionally, this is the first study to evaluate PCB activation on the CAR variants. Because PCB exposures have been associated with obesity, NAFLD, and metabolic syndrome, and these disorders are intimately involved with nuclear receptor activation, the results clearly provide new insight into potential mode(s) of PCB action in human NAFLD.
FUNDING
National Institute of Environmental Health Sciences (1RO1ES021375 and T35ES14559); National Institute of Health (K23AA018399); National Institute for General Medical Sciences (5RO1GM066411 to C.J.O.).
The authors would like to thank Dr David Hein and Mark Doll for supplying the human hepatocytes and Dr Ming Song for critical review of the manuscript.
REFERENCES



![Aroclor 1260 activation of the human AhR. HepG2 cells were transiently transfected with the reporter plasmid pXRE-SV40-Luc. Benz[a]anthracene (10μM, BA) was used as a positive control. (A) Cells were exposed to Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml, and luciferase induction was normalized and compared with DMSO-exposed cells (0 μg/ml Aroclor 1260). (B) Cells were exposed to 10μM BA or BA plus Aroclor 1260 at 0, 5, 10, 15, and 20 μg/ml. The luciferase induction was normalized to that of cells exposed only to DMSO solvent carrier (as in (A), not shown). Luciferase activity in cells exposed to BA and Aroclor 1260 was compared with that of BA-exposed cells. (C) Cells were exposed to selected PCB congeners (10μM) present in Aroclor 1260 and the induction was compared with DMSO-exposed cells. BA (10μM) was used as positive control. Data were normalized to luciferase activity in cells exposed only to DMSO and are expressed as mean ± SEM, n = 4, *p < 0.05.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/toxsci/140/2/10.1093_toxsci_kfu083/2/m_kfu083fig2.jpeg?Expires=1716215420&Signature=LQ0KRu6zCHtvA2pxY1GQGfXgCyZb0xgU5dbitXBCTVpz10~EHNTS17AdjbJxjew4vPDG91YNdEh4GJmV~r1DmhCc-QUynL3cwrdzvqLOWvDFDgaI7iTwTwnR8GVpkCKsgmNOFa~fxb6HCrBHJKKT8A3daibmmwiJ-kDfpDf-T-yAEc46jvu8uIQFJZyNKNucCkWSqgJOcS0z-7qmA1Koxdrxj4xdH~6pOsFDlzBd0SvxhLQjb5UEAUwMhezSPHWfXNagqfYClWacGelC1UUgIRJNLEa2RoyHXiNI9V3NK1whC7ghZWmmsOiwqchUJPMEJzCtkLmodFnBABKq6BCM-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments