Abstract
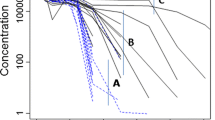
The administration of human biotherapeutics is often associated with a higher incidence of immunogenicity in preclinical species. The presence of anti-drug antibodies (ADAs) in the test samples can affect the accurate measurement of therapeutic protein (TP) in bioanalytical methods designed to support pharmacokinetic (PK) and toxicokinetic (TK) assessments. The impact can vary depending on the bioanalytical method platform and study dosing design. The goal of this study is to evaluate the impact of ADA response on the bioanalytical methods in support of PK/TK and the associated study data interpretation. Sprague Dawley rats were administered with four weekly doses of 50 mg/kg TP, a humanized monoclonal antibody. The TP in serum samples was measured using three bioanalytical methods that quantified bound and/or unbound TP to ADA. The ADA response in the animals was classified into negative, low, medium, and high based on the magnitude of the response. The presence of ADA in samples led to discrepant TP measurements between the methods, especially at time points where the TP concentrations were low. This could be due to ADA interference to the accurate measurement of ADA-bound TP concentrations. The TP concentration at last time point (C last) was reduced by 82.8%, 98.6%, and 99.8%, respectively, for samples containing low, medium, and high levels of ADA. The interfering effects of the ADA on bioanalytical methods and exposure were evident as early as 2 weeks post-dosing. This modeling approach can provide the better understanding of ADA impact on PK exposure in multiple doses.






Similar content being viewed by others
References
Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14(2):296–302.
Swanson SJ, Bussiere J. Immunogenicity assessment in non-clinical studies. Curr Opin Microbiol. 2012;15(3):337–47.
Lee J et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J. 2011;13(1):99–110.
Lee J, Ma H. Specificity and selectivity evaluations of ligand binding assay of protein therapeutics against concomitant drugs and related endogenous proteins. AAPS J. 2007;9(2):E164–70.
Thway TM et al. Experimental and statistical approaches in method cross-validation to support pharmacokinetic decisions. J Pharm Biomed Anal. 2009;49(3):613–8.
Thway TM et al. Model-based strategy for bioanalytical method comparison: measurement of a soluble ligand as a biomarker. J Pharm Biomed Anal. 2012;58:65–70.
Shih JY et al. Implementation of a universal analytical method in early-stage development of human antibody therapeutics: application to pharmacokinetic assessment for candidate selection. Bioanalysis. 2012;4(19):2357–65.
Bautista AC, Salimi-Moosavi H, Jawa V. Universal immunoassay applied during early development of large molecules to understand impact of immunogenicity on biotherapeutic exposure. AAPS J. 2012;14(4):843–9.
White JT, Golob M, Sailstad J. Understanding and mitigating impact of immunogenicity on pharmacokinetic assays. Bioanalysis. 2011;3(16):1799–803.
Stevenson L et al. Paradigm of combination biologics: analytical challenges related to pharmacokinetic assays and interpretation of pharmacokinetic and immunogenicity results. Bioanalysis. 2011;3(5):487–98.
International Conference on Harmonisation; addendum to International Conference on Harmonisation Guidance on S6 Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals; availability. Notice. Fed Regist. 2012; 77(97):29665–6.
Acknowledgments
Financial support for this review was provided by Amgen Inc. We would like to thank Dr. Peng Luan (Amgen Inc. Thousand Oaks, CA) for his critical review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thway, T.M., Magana, I., Bautista, A. et al. Impact of Anti-Drug Antibodies in Preclinical Pharmacokinetic Assessment. AAPS J 15, 856–863 (2013). https://doi.org/10.1208/s12248-013-9484-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9484-4