-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Cecilia Ramirez, Ana Maria Ornstein, Guillermina Maria Luque, Maria Ines Perez Millan, Isabel Garcia-Tornadu, Marcelo Rubinstein, Damasia Becu-Villalobos, Pituitary and Brain Dopamine D2 Receptors Regulate Liver Gene Sexual Dimorphism, Endocrinology, Volume 156, Issue 3, 1 March 2015, Pages 1040–1051, https://doi.org/10.1210/en.2014-1714
Close - Share Icon Share
Abstract
Liver sexual gene dimorphism, which depends mainly on specific patterns of GH secretion, may underlie differential susceptibility to some liver diseases. Because GH and prolactin secretion are regulated by dopaminergic pathways, we studied the participation of brain and lactotrope dopamine 2 receptors (D2Rs) on liver gene sexual dimorphism, to explore a link between the brain and liver gene expression. We used global D2R knockout mice (Drd2−/−) and conducted a functional dissection strategy based on cell-specific Drd2 inactivation in neurons (neuroDrd2KO) or pituitary lactotropes. Disruption of neuronal D2Rs (which impaired the GH axis) decreased most of male or female-predominant class I liver genes and increased female–predominant class II genes in males, consistent with the positive (class I) or negative (class II) regulation of these genes by GH. Notably, sexual dimorphism was lost for class I and II genes in neuroDrd2KO mice. Disruption of lactotrope D2Rs did not modify class I or II genes in either sex, because GH axis was preserved. But surprisingly, 1 class II gene (Prlr) and female-predominant class I genes were markedly up-regulated in lacDrd2KO females, pointing to direct or indirect effects of prolactin in the regulation of selected female-predominant liver genes. This suggestion was strengthened in the hyperprolactinemic Drd2−/− female mouse, in which increased expression of the same 4 liver genes was observed, despite a decreased GH axis. We hereby demonstrate endocrine-mediated D2R actions on sexual dimorphic liver gene expression, which may be relevant during chronic dopaminergic medications in psychiatric disease.
The temporal pattern of pituitary GH secretion, which is sex specific in many species (episodic in males and more stable in females), represents a major component in establishing and maintaining the sexual dimorphism of hepatic gene transcription (1, 2). The mammalian liver, a sexually dimorphic organ, exhibits major differences in the profile of steroid, lipid, and foreign compound metabolism (3, 4), and sexual dimorphic gene expression is a characteristic for more than 1000 liver genes. These genes regulate a wide range of biological processes (1–3), and their sexual dimorphic expression reflects the sex-dependent physiological requirements for steroid metabolism. Accordingly, many enzymes, such as steroid hydroxylases belonging to the cytochrome P450 (CYP) superfamily, are expressed in the liver in unique, sexually biased patterns (5, 6). Such differences are most dramatic in rodents but occur in humans as well (5). Two distinct classes of liver sex-specific genes have been identified related to their dependence of an intact pituitary: class I sex-specific genes, whose expression decreases after hypophysectomy, indicating that pituitary hormones are required for full expression, and class II sex-specific genes, whose expression increases after hypophysectomy, indicating repression by pituitary hormones, mainly GH (7).
Sexual gene dimorphism of the liver may underlie differential susceptibility to some liver diseases, for example, chronic hepatitis, primary sclerosing cholangitis, and hepatocellular carcinoma are predominant in males, whereas primary biliary cirrhosis, autoimmune hepatitis, or alcoholic liver disease are predominant in females (8, 9). Furthermore, sexually dimorphic CYP-catalyzed drug and xenobiotic metabolism and pharmacokinetics have been demonstrated in humans (2, 10). In this context, sex should be considered in preclinical, clinical, or toxicological studies, in order to prevent biased results.
Even though brain dopamine 2 receptors (D2R) regulate pituitary function and are required for full development of the body growth plan (11), the role of dopamine medications on liver dimorphic gene expression has received little attention. Central D2Rs participate not only in control of locomotor activity, executive planning, motor coordination, pain perception, food intake, and aggressive and reward-seeking behaviors but also in the regulation of the GHRH-GH axis (12). Furthermore, lactotrope D2Rs mediate the tonic inhibition that dopamine exerts on prolactin synthesis and release (13), whereas in the periphery, dopamine regulates pancreatic endocrine function, including insulin release from pancreas, and its action on adipocytes (14, 15). A link has been established between obesity, growth, and dopaminergic systems located both within the central nervous system (CNS) or in other tissues (11, 16, 17). Therefore, the complex and integral analysis of the dopaminergic function indicates that the D2R plays key roles in the multifaceted repertoire of adaptive functions that improve fitness, reproductive success, and survival. There is a convergence of central and peripheral actions of dopamine on pathways mediating and reinforcing metabolic homeostasis and endocrine or reproductive programs, and, in this scenario, the role of dopaminergic control of liver gene expression merits special attention.
A broad spectrum of drugs prescribed to treat a variety of disease states, including Parkinson's disease, bipolar disorder, schizophrenia, and depression, exert their effects mainly through the D2Rs and their signaling pathways (18). Because the D2Rs regulate GH, prolactin, and insulin regulation, it is conceivable that these drugs may ultimately alter the liver expression of various drug-metabolizing CYP enzymes, as well as other liver genes, thus affecting the pharmacodynamic characteristics and toxicity of drugs and xenobiotics.
In the present study, we sought to determine the participation of brain and lactotrope D2Rs on sexual dimorphism of liver gene expression using global D2R knockout (KO) mice, and conducting a functional dissection strategy based on cell-specific Drd2 inactivation in neurons or pituitary lactotropes, in order to unravel the contribution of D2Rs at different sites, as well as the endocrine status associated in each mutant model, in maintaining sexually dimorphic liver gene expression. We used a strain of transgenic mice expressing Cre from a mouse prolactin gene promoter, Tg(Prl-cre)1Mrub, and transgenic mice expressing Cre from a rat nestin promoter, Tg(Nes-cre)1Kln/J (19, 20), in order to eliminate D2Rs selectively from pituitary lactotropes or from cells of neural origin, respectively, and compared results with global Drd2 knockout (Drd2−/−) and wild-type mice.
Materials and Methods
Mouse models
D2R knockout mice
Drd2−/− B6.129S2-Drd2tm1low, generated by targeted mutagenesis of the Drd2 gene in embryonic stem cells (21, 22), were used. The original F2 hybrid strain (129S2/Sv x C57BL/6J) containing the mutated Drd2 allele was backcrossed for at least 10 generations to wild-type C57BL/6J mice. Wild-type C57BL/6J mice were used as controls. Assessment of locomotor behavior of Drd2−/− mice revealed a motor impairment, even though these mice did not show a compelling parkinsonian locomotor phenotype (23). However, as previously reported, Drd2−/− mice were hyperprolactinemic, growth retarded, and had altered glucose metabolism (15, 24, 25). Fertility was reduced in female Drd2−/− mice probably due to the high prolactin titers.
Mice lacking D2Rs in lactotropes (lacDrd2KO)
Mice lacking expression of D2Rs in pituitary lactotropes were generated by crossing mice carrying conditional Drd2loxP/loxP alleles (B6.129S4(FVB)-Drd2tm2.2Mrub) (26), with transgenic mice expressing Cre recombinase driven by the mouse prolactin promoter (Tg(Prl-cre)1Mrub (11) for 2 generations. Because female lac Drd2KO had reduced fertility, breeding pairs of female Drd2loxP/loxP and male Drd2loxP/loxP.Tg(Prl-Cre) mice were used to generate Drd2loxP/loxP (control mice) and Drd2loxP/loxP.Tg(Prl-Cre) (lacDrd2KO) littermates, which were included in each experiment. Tissue specificity of Cre expression in Tg(Prl-cre)1Mrub transgenic mice has been previously validated by us (11, 27); Cre mRNA levels analyzed by real-time PCR in different tissues were highly expressed in the pituitary and very low or absent in the hypothalamus, liver, kidney, ovary, and lung. LacDrd2KO mice were unresponsive to haloperidol-induced prolactin release and responsive to haloperidol-induced catalepsia (11, 27). Mice of mixed genotypes were housed in groups of 4 or 5 in a temperature-controlled room with lights on at 7 am and off at 7 pm and had free access to laboratory chow and tap water.
Mice lacking D2Rs in neurons (neuroDrd2KO)
To ablate D2Rs from cells of neural origin, Drd2loxP/loxP mice (26) were crossed to B6.Cg-Tg(Nes-cre)1Kln/J to obtain cohorts of Drd2loxP/loxP (control mice) and Drd2loxP/loxP.B6.Cg-Tg(Nes-cre)1Kln/J littermates (11). Thereafter, breeding pairs of Drd2loxP/loxP and Drd2loxP/loxP.Tg(Nes-cre)1Kln/J mice were used to generate Drd2loxP/loxP (control) and Drd2loxP/loxP.Tg(Nes-Cre) (neuronDrd2KO) littermates, which were included in each experiment. We previously characterized the neuroDrd2KO obtained in the laboratory by in situ hybridization assays using a Drd2 exon 2 antisense riboprobe and [3H]-nemonapride binding autoradiography, which revealed the absence of Drd2 mRNA and D2Rs, respectively, on coronal brain sections taken from neuroDrd2KO mice. In addition, D2R levels in the pituitary glands of neuroDrd2KO mice were similar to those observed in Drd2loxP/loxP mice (11). No breeding issue were recorded in these mice.
All experimental procedures were performed in 4- to 6-month-old mice, according to guidelines of the institutional animal care and use committee of the Instituto de Biología y Medicina Experimental, Buenos Aires (in accordance with the Division of Animal Welfare, Office for Protection from Research Risks, National Institutes of Health, Animal Welfare Assurance for the Institute of Biology and Experimental Medicine A#5072-01).
Reagents
Unless otherwise specified, all chemicals were purchased from Sigma.
Radioimmunoassay
Aliquots (10 μL) of serum obtained by decapitation of 4-month-old mice were used to assay prolactin by RIA using a kit provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Dr A.F. Parlow, National Hormone and Pituitary Program [NHPP]). Results are expressed in terms of mouse prolactin standard Reference Preparation 3. Intra- and interassay coefficients of variation were 7.2% and 12.8%, respectively. For IGF-I RIA, serum samples (15 μL) and IGF-I standards were subjected to the acid-ethanol cryoprecipitation method as previously described (28). IGF-I was determined using rabbit anti-hIGF-I (UB2-495) provided by Dr L. Underwood and Dr J.J. Van Wyk and distributed by the Hormone Distribution Program of the NIDDK (for antibodies, see Table 1). Recombinant human IGF-I (Chiron Corp) was used as radioligand and unlabeled ligand. The assay sensitivity was 6 pg per tube. Intra- and interassay coefficients of variation were 8.2% and 14.1%, respectively. GH was measured by RIA using kits provided by the NIDDK (Dr A.F. Parlow, NHPP). Results are expressed in terms of mouse GH standard AFP-10783B. Intra- and interassay coefficients of variation were 8.4% and 13.2%, respectively. Pituitaries (1–1.5 mg) were homogenized in ice-cold PBS and centrifuged at 3000 rpm for 5 minutes. Supernatant protein contents were measured with the QUBIT Fluorometer and the QUANT-IT protein Assay kit (Invitrogen). Aliquots of equal quantity of protein were used to assay pituitary GH content.
Antibodies Used
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| IGF-I | Antibody developed using recombinant human IGF-I | Anti-IGF-I | UB2-495 provided by Dr L. Underwood and Dr J.J. Van Wyk, and distributed by the Hormone Distribution Program of the NIDDK. | Rabbit polyclonal | 1:450 |
| GH | — | Anti-GH | NIDDK (Dr A.F. Parlow, NHPP). AFP-10783B | Rabbit polyclonal | 1:1200 |
| Prolactin | — | Antiprolactin | NIDDK (Dr A.F. Parlow, NHPP). AFP-131078 | Rabbit polyclonal | 1:1000 |
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| IGF-I | Antibody developed using recombinant human IGF-I | Anti-IGF-I | UB2-495 provided by Dr L. Underwood and Dr J.J. Van Wyk, and distributed by the Hormone Distribution Program of the NIDDK. | Rabbit polyclonal | 1:450 |
| GH | — | Anti-GH | NIDDK (Dr A.F. Parlow, NHPP). AFP-10783B | Rabbit polyclonal | 1:1200 |
| Prolactin | — | Antiprolactin | NIDDK (Dr A.F. Parlow, NHPP). AFP-131078 | Rabbit polyclonal | 1:1000 |
Antibodies Used
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| IGF-I | Antibody developed using recombinant human IGF-I | Anti-IGF-I | UB2-495 provided by Dr L. Underwood and Dr J.J. Van Wyk, and distributed by the Hormone Distribution Program of the NIDDK. | Rabbit polyclonal | 1:450 |
| GH | — | Anti-GH | NIDDK (Dr A.F. Parlow, NHPP). AFP-10783B | Rabbit polyclonal | 1:1200 |
| Prolactin | — | Antiprolactin | NIDDK (Dr A.F. Parlow, NHPP). AFP-131078 | Rabbit polyclonal | 1:1000 |
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| IGF-I | Antibody developed using recombinant human IGF-I | Anti-IGF-I | UB2-495 provided by Dr L. Underwood and Dr J.J. Van Wyk, and distributed by the Hormone Distribution Program of the NIDDK. | Rabbit polyclonal | 1:450 |
| GH | — | Anti-GH | NIDDK (Dr A.F. Parlow, NHPP). AFP-10783B | Rabbit polyclonal | 1:1200 |
| Prolactin | — | Antiprolactin | NIDDK (Dr A.F. Parlow, NHPP). AFP-131078 | Rabbit polyclonal | 1:1000 |
Immunohistochemistry
Pituitaries from 4-month-old animals fixed in formalin were embedded in paraffin, and immunohistochemistry was performed using fluorescence detection (29). Rabbit polyclonal antibody against mouse GH (dilution 1:750, NHPP, NIDDK-AFP-5672099) (Table 1) and a secondary antibody fluorescein isothiocyanate goat antirabbit IgG (dilution 1:100; Zymed Laboratories, Inc) or donkey antirabbit IgG coupled to Texas Red (dilution 1:100, sc-2784; Santa Cruz Biotechnology, Inc) was used. A total of 4–5 animals per experimental group and 3 pituitary sections per animal were analyzed. Morphometric analysis was performed using a Carl Zeiss transmitted light microscope at a magnification of ×250 and ×400. Image analysis of pituitary sections for performed by ImageJ, version 6.0 software. The number of GH-immunoreactive cells was scored and used to calculate cell percentage (number of GH-positive cells per total nuclei in the sections).
Urine dosage of 20-kDa major urinary protein (MUP)
Urine was collected from female and male 4-month old mice of the different genotypes fractionated by SDS-PAGE and subsequently stained with Coomassie blue. The 20-kDa MUP band was quantified by standard densitometry and results are expressed in arbitrary units, relative to control males.
Tissue RNA extraction and total cDNA preparation for Cyp2d9, Cyp7b1, Mup1/2/6/8, Mup1, Cyp3a16, Cyp3a41, Cyp3a44, Cyp2a4, Cyp2b9, alcohol dehydrogenase-1 (Adh1), prolactin receptor (Prlr), Igfi, glucocorticoid receptor (Glucor), IGF binding protein 3 (Igfbp3), and GH receptor (Ghr) expression by real-time PCR
Common names and category or function of genes are described in Table 2. After euthanasia, liver samples (≈50 mg) were immediately homogenized in TRIzol reagent (Invitrogen) and stored at −70°C until used. Total RNA was isolated from tissue homogenates by use of the TRIzol reagent method. The RNA concentration was determined on the basis of absorbance at 260 nm, its purity was evaluated by the ratio of absorbance at 260/280 nm (>1.8), and its integrity by agarose gel electrophoresis. RNAs were kept frozen at −70°C until analyzed. First-strand cDNA was synthesized from 3 μg of total RNA in the presence of 10 mmol L−1 MgCl2, 50 mmol L−1 Tris · HCl (pH 8.6), 75 mol L−1 KCl, 0.5mM deoxy-nucleoside triphosphate, 1 mol L−1 dithiotheitol, 1-U/μL RnaseOUT (Invitrogen), 0.5-μg oligo(dT)15 primer (Biodynamics), and 20 U of Moloney murine leukemia virus reverse transcriptase (Epicenter). To validate successful deoxyribonuclease I treatment, the reverse transcriptase was omitted in control reactions. The absence of PCR-amplified DNA fragments in these samples indicated the isolation of RNA free of genomic DNA.
Description of Genes
| Gene Name . | Common Name . | Category/Function . | Class . |
|---|---|---|---|
| Male-predominant genes | |||
| Cyp2d9 | Cytochrome P450, family 2, subfamily d, polypeptide9 (testosterone16α-hydroxylase) | Steroid/foreign compound metabolism | IA |
| Cyp7b1 | Cytochrome P450, family 7, subfamily b, polypeptide1 (oxysterol 7-α-hydroxylase) | Steroid/foreign compound metabolism | IB |
| Mup1/2/6/8a | Major urinary proteins (types 1,2 6 and 8) | Scent communication and sexual behavior | IB |
| Mup1a | Major urinary proteins (type 1) | Scent communication and sexual behavior/metabolism | IB |
| Female-predominant genes | |||
| Cyp3a16 | Cytochrome P450, family 3, subfamily a, polypeptide 16 | Steroid/foreign compound metabolism | IA |
| Cyp3a41 | Cytochrome P450, family 3, subfamily a, polypeptide 41 | Steroid/foreign compound metabolism | IA |
| Cyp3a44 | Cytochrome P450, family 3, subfamily a, polypeptide 44 | Steroid/foreign compound metabolism | IA |
| Cyp2a4 | Cytochrome P450, family 2, subfamily a, polypeptide4 (testosterone 15-α-hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Cyp2b9 | Cytochrome P450, family 2, subfamily b, polypeptide9 (testosterone 16-α hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Adh1 | Alcohol dehydrogenase 1 | Alcohol metabolism | IIA |
| Prlr | Prolactin receptor | Receptor, hormone | IIA |
| Sex-independent genes | |||
| Ghr | Growth hormone receptor | Receptor, hormone | |
| Igf1 | Insulin growth factor 1 | Growth factor | |
| Igfbp3 | Igf-binding protein 3 | Circulating protein, binds IGF I/II | |
| Glucor | Glococorticoid receptor | Receptor |
| Gene Name . | Common Name . | Category/Function . | Class . |
|---|---|---|---|
| Male-predominant genes | |||
| Cyp2d9 | Cytochrome P450, family 2, subfamily d, polypeptide9 (testosterone16α-hydroxylase) | Steroid/foreign compound metabolism | IA |
| Cyp7b1 | Cytochrome P450, family 7, subfamily b, polypeptide1 (oxysterol 7-α-hydroxylase) | Steroid/foreign compound metabolism | IB |
| Mup1/2/6/8a | Major urinary proteins (types 1,2 6 and 8) | Scent communication and sexual behavior | IB |
| Mup1a | Major urinary proteins (type 1) | Scent communication and sexual behavior/metabolism | IB |
| Female-predominant genes | |||
| Cyp3a16 | Cytochrome P450, family 3, subfamily a, polypeptide 16 | Steroid/foreign compound metabolism | IA |
| Cyp3a41 | Cytochrome P450, family 3, subfamily a, polypeptide 41 | Steroid/foreign compound metabolism | IA |
| Cyp3a44 | Cytochrome P450, family 3, subfamily a, polypeptide 44 | Steroid/foreign compound metabolism | IA |
| Cyp2a4 | Cytochrome P450, family 2, subfamily a, polypeptide4 (testosterone 15-α-hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Cyp2b9 | Cytochrome P450, family 2, subfamily b, polypeptide9 (testosterone 16-α hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Adh1 | Alcohol dehydrogenase 1 | Alcohol metabolism | IIA |
| Prlr | Prolactin receptor | Receptor, hormone | IIA |
| Sex-independent genes | |||
| Ghr | Growth hormone receptor | Receptor, hormone | |
| Igf1 | Insulin growth factor 1 | Growth factor | |
| Igfbp3 | Igf-binding protein 3 | Circulating protein, binds IGF I/II | |
| Glucor | Glococorticoid receptor | Receptor |
Also known as α2u-globulins.
Description of Genes
| Gene Name . | Common Name . | Category/Function . | Class . |
|---|---|---|---|
| Male-predominant genes | |||
| Cyp2d9 | Cytochrome P450, family 2, subfamily d, polypeptide9 (testosterone16α-hydroxylase) | Steroid/foreign compound metabolism | IA |
| Cyp7b1 | Cytochrome P450, family 7, subfamily b, polypeptide1 (oxysterol 7-α-hydroxylase) | Steroid/foreign compound metabolism | IB |
| Mup1/2/6/8a | Major urinary proteins (types 1,2 6 and 8) | Scent communication and sexual behavior | IB |
| Mup1a | Major urinary proteins (type 1) | Scent communication and sexual behavior/metabolism | IB |
| Female-predominant genes | |||
| Cyp3a16 | Cytochrome P450, family 3, subfamily a, polypeptide 16 | Steroid/foreign compound metabolism | IA |
| Cyp3a41 | Cytochrome P450, family 3, subfamily a, polypeptide 41 | Steroid/foreign compound metabolism | IA |
| Cyp3a44 | Cytochrome P450, family 3, subfamily a, polypeptide 44 | Steroid/foreign compound metabolism | IA |
| Cyp2a4 | Cytochrome P450, family 2, subfamily a, polypeptide4 (testosterone 15-α-hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Cyp2b9 | Cytochrome P450, family 2, subfamily b, polypeptide9 (testosterone 16-α hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Adh1 | Alcohol dehydrogenase 1 | Alcohol metabolism | IIA |
| Prlr | Prolactin receptor | Receptor, hormone | IIA |
| Sex-independent genes | |||
| Ghr | Growth hormone receptor | Receptor, hormone | |
| Igf1 | Insulin growth factor 1 | Growth factor | |
| Igfbp3 | Igf-binding protein 3 | Circulating protein, binds IGF I/II | |
| Glucor | Glococorticoid receptor | Receptor |
| Gene Name . | Common Name . | Category/Function . | Class . |
|---|---|---|---|
| Male-predominant genes | |||
| Cyp2d9 | Cytochrome P450, family 2, subfamily d, polypeptide9 (testosterone16α-hydroxylase) | Steroid/foreign compound metabolism | IA |
| Cyp7b1 | Cytochrome P450, family 7, subfamily b, polypeptide1 (oxysterol 7-α-hydroxylase) | Steroid/foreign compound metabolism | IB |
| Mup1/2/6/8a | Major urinary proteins (types 1,2 6 and 8) | Scent communication and sexual behavior | IB |
| Mup1a | Major urinary proteins (type 1) | Scent communication and sexual behavior/metabolism | IB |
| Female-predominant genes | |||
| Cyp3a16 | Cytochrome P450, family 3, subfamily a, polypeptide 16 | Steroid/foreign compound metabolism | IA |
| Cyp3a41 | Cytochrome P450, family 3, subfamily a, polypeptide 41 | Steroid/foreign compound metabolism | IA |
| Cyp3a44 | Cytochrome P450, family 3, subfamily a, polypeptide 44 | Steroid/foreign compound metabolism | IA |
| Cyp2a4 | Cytochrome P450, family 2, subfamily a, polypeptide4 (testosterone 15-α-hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Cyp2b9 | Cytochrome P450, family 2, subfamily b, polypeptide9 (testosterone 16-α hydroxylase) | Steroid/foreign compound metabolism | IIA |
| Adh1 | Alcohol dehydrogenase 1 | Alcohol metabolism | IIA |
| Prlr | Prolactin receptor | Receptor, hormone | IIA |
| Sex-independent genes | |||
| Ghr | Growth hormone receptor | Receptor, hormone | |
| Igf1 | Insulin growth factor 1 | Growth factor | |
| Igfbp3 | Igf-binding protein 3 | Circulating protein, binds IGF I/II | |
| Glucor | Glococorticoid receptor | Receptor |
Also known as α2u-globulins.
Quantitative real-time PCR
Sense and antisense oligonucleotide primers were designed on the basis of the published cDNA or by the use of PrimerBlast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Oligonucleotides were obtained from Invitrogen. The sequences are described in Supplemental Table 1.
Quantitative measurements of specific mRNA levels were performed by kinetic PCR using HOT FIREPol EvaGreen qPCR Mix Plus (ROX), Solis BioDyne, 0.4 μmol L−1 primers, and 150-ng cDNA in a final volume of 10.4 μL. After denaturation at 95°C for 15 minutes, the cDNA products were amplified with 40 cycles. Cycle conditions (denaturation, annealing, and extension) for each gene are detailed in Supplemental Table 2, and optical reading stage was performed at 80°C for 33 seconds. The accumulating DNA products were monitored by the ABI 7500 sequence detection system (Applied Biosystems), and data were stored continuously during the reaction. The results were validated on the basis of the quality of dissociation curves generated at the end of the PCR runs by ramping the temperature of the samples from 60°C to 95°C, while continuously collecting fluorescence data. Product purity was confirmed by agarose gel electrophoresis. Each sample was analyzed in duplicate. Relative gene expression levels were calculated according to the comparative cycle threshold (CT) method. Normalized target gene expression relative to cyclophilin was obtained by calculating the difference in CT values, the relative change in target transcripts being computed as 2−ΔCT. To validate the comparative CT method of relative quantification, the efficiencies of each target and housekeeping gene amplification (endogenous cyclophilin) were measured and shown to be approximately equal.
Statistical analysis
Results are expressed as mean ± SEM. The differences between means were analyzed by two-way ANOVA (for the effects of sex and genotype) for independent measures followed by Tukey's honestly significant difference test for unequal n for liver mRNA expression, and ng GH/μg protein, serum prolactin, and IGF-I levels. P < .05 was considered significant.
Results
Pituitary somatotropes and GH concentration, liver Igfi mRNA levels, and serum IGF-I and prolactin levels in female and male Drd2−/−, lacDrd2KO, and neuroDrd2KO mice
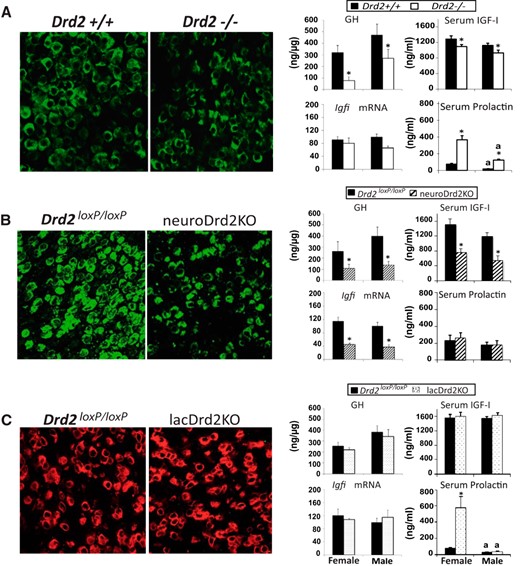
Adult mice (4–6 months old) completely lacking D2Rs (Drd2−/−) showed a reduced population of pituitary somatotropes accompanied by reduced pituitary GH concentration and serum IGF-I levels and an increase in serum prolactin levels (Figure 1A). Liver Igfi mRNA levels were reduced in males but not significantly. Altogether, results point to a disruption of the GH axis and of the inhibitory tone exerted by D2Rs on prolactin secretion that was evident in both sexes.
Pituitary somatotropes, and serum IGF-I and prolactin levels in female and male Drd2−/−, lacDrd2KO, and neuroDrd2KO mice. Left, Representative immunohistochemistry of GH cells in the 3 mouse models in males (40.2 ± 3.7 vs 25.7 ± 0.5%, Drd2+/+ vs Drd2−/−; P < .05; 36.4 ± 2.6 vs 24.6 ± 0.1 for Drd2loxP/loxP vs neuroDrd2KO; P < .05; and 40.9 ± 2.9 vs 36.9 ± 4.0, NS for Drd2loxP/loxP vs lacDrd2KO, NS). Right, ng pituitary GH/μg protein, liver Igfi mRNA levels (normalized to cyclophilin mRNA levels, and in relation to control males; 100%) and serum IGF-I and prolactin levels: n = 20–29 (A), n = 7–10 (B), and n = 6–10 (C). For all panels: a, P < .05 vs genotype-matched females; *, P < .05 vs sex-matched control (Drd2+/+ or Drd2loxP/loxP mice).
When D2Rs were selectively ablated from neurons, prolactin levels were not modified, whereas pituitary somatotrope population and GH concentration, as well as serum IGF-I and liver Igfi mRNA levels, were markedly compromised (Figure 1B), indicating that neuronal D2Rs regulate the GH and not the prolactin axis.
Finally, in lacDrd2KO mice somatotrope population, GH pituitary concentration and serum IGF-I and liver Igfi mRNA levels were unaltered compared with control mice in both sexes, whereas prolactin secretion was manifestly increased in female mice (Figure 1C), highlighting the role of lactotrope D2Rs on prolactin and not GH secretion.
Liver Igfbp3 mRNA levels were not modified in the transgenic models (Supplemental Figure 1).
Expression of male-predominant class I liver genes in female and male Drd2−/−, neuroDrd2KO, and lacDrd2KO mice
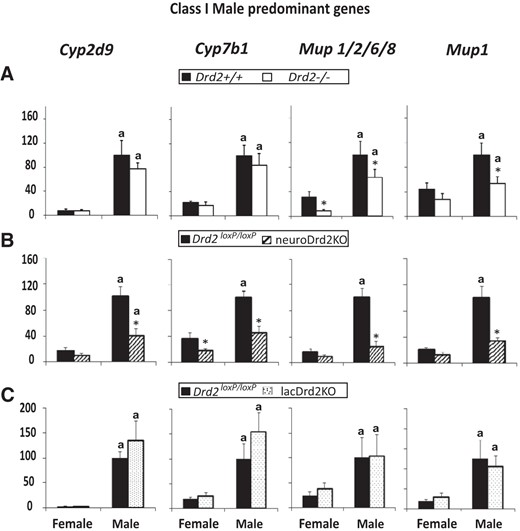
Male-predominant class I liver genes are those that are higher in the liver of male mice and decrease in response to hypophysectomy (7). Class IA male genes (Cyp2d9) are positively regulated by the male but not the female GH secretory pattern; and class IB male genes (Cyp7b1, Mup1/2/6/8, and Mup1) require pituitary hormones for full expression in both sexes (7).
We found that class I male-predominant genes mostly decreased in global Drd2−/− and in neuroDrd2KO mice. In particular, Mup1/2/6/8 and Mup1 mRNA levels were decreased in the liver of Drd2−/− male and female mice and male neuroDrd2KO mice. Cyp2d9 was decreased in male and Cyp7b1 mRNA in both sexes in neuroDrd2KO mice but not in Drd2−/− mice; as a result, sexual dimorphism of the male-predominant liver genes was lost (Cyp7b1, Mup1/2/6/8, and Mup1) or greatly decreased (Cyp2d9) in neuroDrd2KO mice. On the contrary, in lacDrd2KO mice, in which the GH axis is preserved, there were no differences in the mRNA expression levels of these 4 genes, and sexual dimorphism was maintained (Figure 2).
Expression of male-predominant class I liver genes in adult female and male Drd2−/− (A), neuroDrd2KO (B), and lacDrd2KO (C) mice, and their respective controls. Percentage of target mRNA levels normalized to cyclophilin mRNA levels, in relation to control males (100%) are represented in the y-axis. n = 7–12. Two-way ANOVA for the effects of sex and genotype was performed. P of interaction was significant for Cyp2d9, Cyp7b1, Mup1/2/6/8, and Mup1 in neuroDrd2KO mice. For all panels: a, P < .05 vs genotype-matched females; *, P < .05 vs sex-matched control (Drd2+/+ or Drd2loxP/loxP mice).
MUP excretion in female and male Drd2−/−, neuroDrd2KO, and lacDrd2KO mice
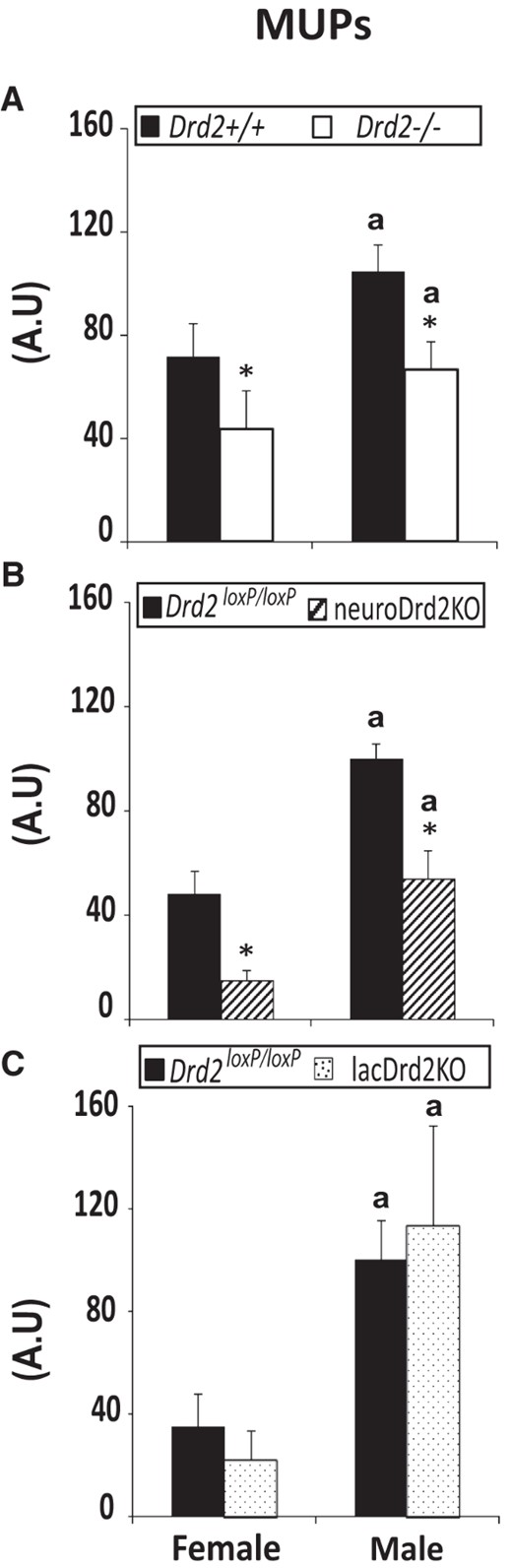
We assessed protein MUP excretion by SDS-PAGE and Coomassie staining in urine samples and found a similar sex and genotype dependent pattern compared with liver Mup1/2/6/8 and Mup1 mRNA expression in the 3 models. MUP levels were always higher in control males compared with control females and were decreased in female and male Drd2−/− compared with Drd2+/+ mice, as well as in female and male neuroDrd2KO compared with Drd2loxP/loxP controls, and were unaltered in lacDrd2KO mice (Figure 3). These data demonstrate that liver gene expression of Mup was paralleled by protein levels. Furthermore, because this protein is an indicator of sex-dependent GH pulsatility (30), results confirm alterations of the GH axis in Drd2−/− and neuroDrd2KO and not in lacDrd2KO mice.
Urine MUP excretion in adult female and male Drd2−/− (A), neuroDrd2KO (B), and lacDrd2KO mice (C), and their respective controls. Urine samples taken from male and female male Drd2−/−, neuroDrd2KO, and lacDrd2KO mice, and their respective controls (n = 9–18) were analyzed by SDS-PAGE electrophoresis. The 20-kDa band was quantified by densitometry for each urine sample and expressed in arbitrary units (A.U.). a, P < .05 vs genotype-matched females; *, P < .05 vs sex-matched control (Drd2+/+ or Drd2loxP/loxP mice).
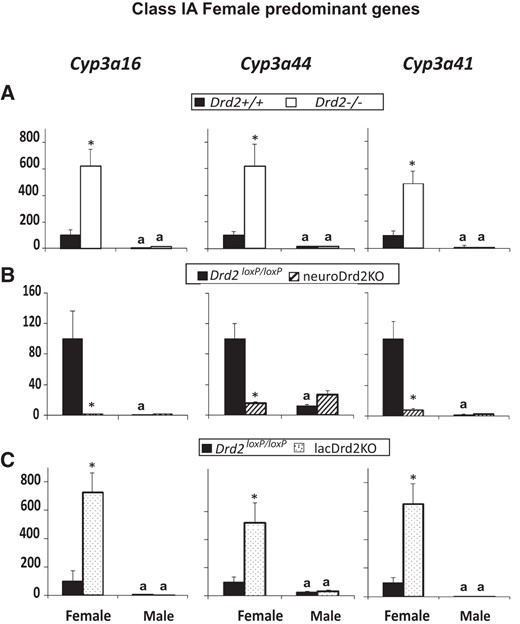
Expression of female-predominant class IA liver genes in female and male Drd2−/−, neuroDrd2KO, and lacDrd2KO mice
Female-predominant class IA liver genes, such as Cyp3a16, Cyp3a44, and Cyp3a41, are defined as genes that are higher in the liver of female mice, decrease after hypophysectomy, and are positively regulated by the female but not male GH secretory profile (7).
Female predominance was observed for Cyp3a16, Cyp3a44, and Cyp3a41 mRNA levels. Unexpectedly, in the 2 mutant mouse models with high prolactin levels (Drd2−/− and lacDrd2KO mice), expression of the 3 genes increased dramatically in female mice and was not altered in male mice, sexual dimorphism being preserved. On the contrary, in neuroDrd2KO mice mRNA expression of the 3 genes decreased in females (in concordance with the definition of class I genes) and was unaltered in males. Consequently, when the Drd2 gene was ablated specifically from neurons, sexual dimorphism was lost for these genes, and when it was ablated specifically from lactotropes, there was a dramatic increase in females, and sexual dimorphism was maintained (Figure 4). We also evaluated liver Glucor mRNA levels, because Cyp3a44 and Cyp3a41 may be regulated by glucocorticoids (31, 32). There was effect of genotype in Glucor mRNA levels in Drd2−/− mice (Supplemental Figure 1), which may be indicative of increased action of glucocorticoids in this hyperprolactinemic female knockout mice. In lacDrd2KO and neuroDrd2KO mice there was no effect of genotype or sex.
Expression of female-predominant class IA liver genes in female and male Drd2−/− (A), neuroDrd2KO (B), and lacDrd2KO (C) mice. Percentage of target mRNA levels normalized to cyclophilin mRNA levels, in relation to control females, is represented in the y-axis. n = 7–12. Two-way ANOVA for the effects of sex and genotype was performed. P of interaction was significant for the 3 genes in all mice models. For all panels: a, P < .05 vs genotype-matched females; *, P < .05 vs sex-matched control (Drd2+/+ or Drd2loxP/loxP mice).
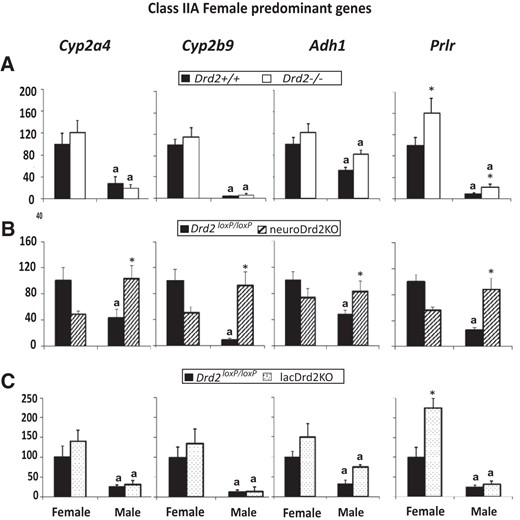
Expression of female-predominant class IIA liver genes in female and male Drd2−/−, neuroDrd2KO, and lacDrd2KO mice
Female-predominant class IIA liver genes are defined as genes that are higher in female mice, whose expression increase after hypophysectomy, and are repressed by the male but not by the female GH secretion pattern (7).
Female predominance was observed for Cyp2a4, Cyp2b9, Adh1, and Prlr mRNA levels. In neuroDrd2KO mice, mRNA expression of the 4 genes increased in males and was unaltered in females; as a consequence, sexual dimorphism was lost. In the 2 mutant mice models with high prolactin levels (Drd2−/− and lacDrd2KO mice), no changes in the expression or sexual dimorphism of Cyp2a4, Cyp2b9, and Adh1 mRNA levels were found, whereas Prlr mRNA expression increased in female and male Drd2−/− mice and in female lacDrd2KO mice, sexual dimorphism being maintained (Figure 5).
Expression of female-predominant class IIA liver genes in female and male Drd2−/− (A), neuroDrd2KO (B), and lacDrd2KO (C) mice. Percentage of target mRNA levels normalized to cyclophilin mRNA levels, in relation to control females, is represented in the y-axis. n = 7–12. Two-way ANOVA for the effects of sex and genotype was performed. P of interaction was significant for Cyp2a4, Cyp2b9, Adh1, and Prlr in neuroDrd2KO mice, and for Prlr in lacDrd2KO mice. For all panels: a, P < .05 vs genotype-matched females; *, P < .05 vs sex-matched control (Drd2+/+ or Drd2loxP/loxP mice).
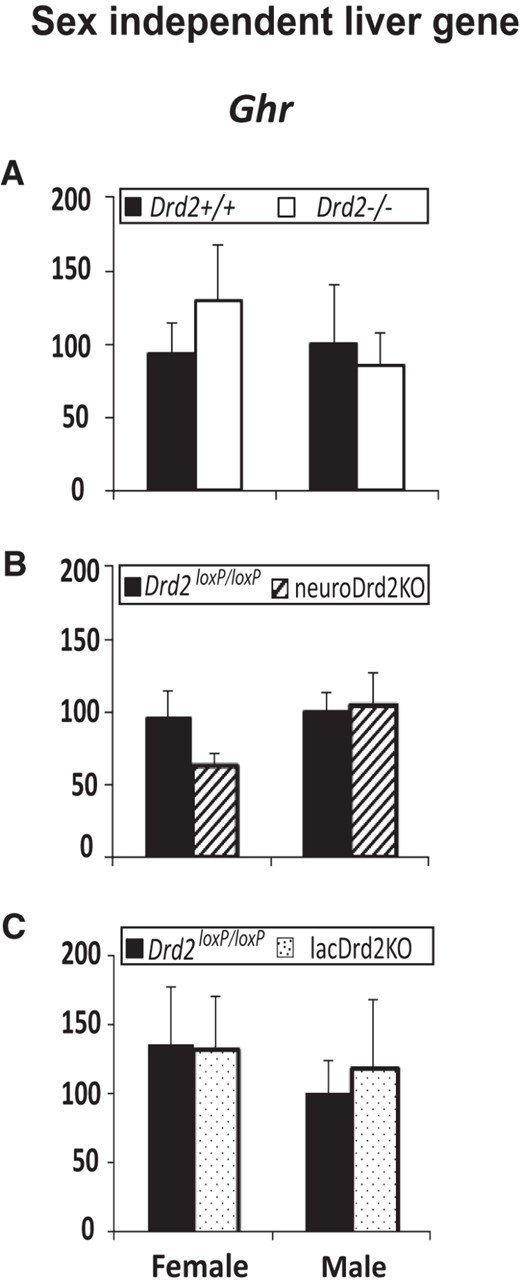
Expression of a sex independent liver gene in female and male Drd2−/−, neuroDrd2KO, and lacDrd2KO mice
Ghr mRNA levels were similar in both sexes in the 3 transgenic models and not modified by total or selective lactotrope or neuron D2R ablation (Figure 6).
Expression of a sex-independent liver gene in female and male Drd2−/− (A), neuroDrd2KO (B), and lacDrd2KO (C) mice, and their respective controls. Percentage of Ghr mRNA levels normalized to cyclophilin mRNA levels, in relation to control males. n = 7–12. Two-way ANOVA for the effects of sex and genotype was performed. No differences were found.
Discussion
Drugs affecting brain D2Rs may modify CYP enzyme activity in the liver, establishing a possible unexplored link between the CNS and liver gene expression. Previously, it has been described that in male rats, after lesioning the tuberoinfundibular dopaminergic pathway, a decrease in the activity and protein levels of liver CYP2B and CYP2C11 and an increase in CYP1A were found, and after lesioning the tuberoinfundibular or mesolimbic dopaminergic pathways, an increase in liver CYP3A was evidenced (33). Furthermore, neuroleptics, such as sulpiride or remoxipride, which are D2R antagonists, down-regulated liver CYP2C11 and CYP3A protein or mRNA expression in male rats (34). Simultaneously, a feminization in androstenedione metabolism was observed. Conversely, stimulation of dopamine receptors increased CYP2B, CYP2C11, and CYP3A (35).
Genes coding for the different CYP isoforms are regulated principally by GH but also by prolactin, glucocorticoids, and thyroid hormones, which are all under brain control (36). Hypothalamic neurons controlling pituitary function receive dopaminergic innervation; therefore, the contribution of brain monoaminergic systems to the regulation of liver CYPs is conceivable and may be indirect, establishing a brain-pituitary-liver connection.
On the other hand, autonomic nervous system (projecting to the liver) and dopamine present in the blood, kidney, or pancreas (14) may also contribute to the physiological regulation of liver function. Accordingly, it has been described that dopamine acting on pancreatic D2Rs inhibits insulin secretion (15) and the decrease in liver CYP23A, CYP2C, and CYP2D mRNA expression (37), and CYP2E1 (38) found after blockade of D2Rs in male rats was ascribed to rising insulin levels.
Most of the above mentioned studies were performed in only 1 sex, but a hallmark of liver gene expression is the sexual dimorphism found in a great number of genes and, furthermore, that almost 90% of sex-specific liver genes are dependent on GH secretory profiles (1, 39). These genes encode signal transduction molecules, various receptors, and enzymes of steroid and foreign compound metabolism, in particular CYPs. Two distinct classes of liver sex-specific genes have been identified: genes whose expression decreases after hypophysectomy, indicating that pituitary hormones (mainly GH) are required for full expression (class I sex-specific genes) and genes whose expression increases after hypophysectomy, indicating repression by pituitary hormones (class II sex-specific genes) (7). A large majority (88%) of the male-specific genes affected by hypophysectomy are class I genes (ie, are induced by the male pituitary GH profile), with only 10% being class II genes. In contrast, most (64%) pituitary-dependent female-specific genes are class II genes (ie, are suppressed by male pituitary GH profile).
Our results propose that the dopaminergic system is paramount in regulating liver Cyps and other sex-biased genes and that this regulation proceeds via D2Rs expressed in the pituitary as well as in the brain. Disruption of neuronal D2Rs altered the GH axis, and consequently, male-predominant class IB genes decreased either in both sexes or only in males, male-predominant class IA decreased in males, and female-predominant class IA genes decreased in females, whereas female-predominant class IIA genes increased in males. These results are consistent with up-regulation of class I and down-regulation of class II liver genes by GH. It should be underscored that sexual dimorphism was lost for class I and II liver genes in neuroDrd2KO mice (with the exception of Cyp2d9). These results highlight the importance of neuronal D2Rs in maintaining sexual differences in liver gene expression, positively regulating class I and negatively regulating class II genes, via their impact on differential patterns of GH secretion.
Loss of sexual dimorphism of liver gene expression may underlie alterations in several metabolic processes. In particular, genome wide analysis of sex-specific effects of the prophet of pituitary-specific transcription factor-1 mutation (prop1df) in male and female dwarf Ames mice described an almost complete loss of sex-specific sexual dimorphism in the liver of genes related to fatty acid, steroid hormone, and xenobiotic metabolism (40). Also, the urine from neuroDrd2KO adult males failed to promote aggression and territorial behavior in control male challengers due to feminization of MUP excretion, in contrast to the urine taken from control adult males (11). Furthermore, exposure to endocrine disruptors (41) or hazardous substances, such as arsenic, has profound effects on the sexual dimorphism of liver gene expression (42). Also, tumorigenic hepatitis was strongly associated with liver-sex disruption, defined as the loss of a sex-identifying hepatic molecular signature in mice (8). Therefore, it is plausible that maintenance of sexual dimorphic liver expression is needed for the normal sex-dependent physiological requirements for steroid metabolism and other liver-mediated actions.
Disruption of lactotrope D2Rs did not modify class I genes in males, or female-predominant class IIA genes in both sexes, in concordance with a preserved GH axis. But surprisingly, 1 class IIA gene (Prlr) and the 3 female-predominant class IA genes were markedly up-regulated in lacDrd2KO females. These results point to prolactin as a direct or indirect strong regulator of selected female genes in the liver. This suggestion was strengthened by results obtained in the hyperprolactinemic Drd2−/− mice, in which the expression of the same 4 genes was increased in female livers despite the GH reduction observed. It has been described that pregnancy up-regulates liver Cyp3a mRNA expression in mice (43) and humans (43–45), authors proposing as a probable cause the increased serum GH and estradiol levels observed during pregnancy. Nevertheless, in the present mouse models, up-regulation of Cyp3a and Prlr genes was found in the absence of high GH or estrogen levels, pointing to an unreported direct or indirect prolactin action at the liver.
To this respect, it has been described that expression of Cyp3a41 and Cyp3a44 genes are under multihormonal regulation, in which GH plays a central role, although cooperative action of glucocorticoids may be important (31, 32). Nevertheless, it has also been described that dexamethasone did not modify liver Cyp3a41 expression in male/female mice (32, 46), that constitutive Cyp3a expression is not modified in glucocorticoid receptor null mice (47), and that responsiveness to glucocorticoids differs between Cyp3a41 and Cyp3a44 gene expression a difference that we did not find in the present results (31, 32). However, we did find reduced levels of liver Glucor mRNA in Drd2−/− mice, indicating a possible action of glucocorticoids, because increased glucocorticoid action may down-regulate the glucocorticoid receptor (48). Therefore, we cannot discard that increased Cyp3a levels found in these females may be related to prolactin and/or glucocorticoid levels or to a cooperative action between the hormones.
In this context, several studies have indicated that in pregnancy, when high levels of prolactin are present, liver enzyme activity is compromised. For example, a considerable reduction in the exposure of HIV-infected pregnant women to protease inhibitors was found compared with men, nonpregnant, or postpartum women (49, 50), and it was suggested that protease inhibitor clearance through liver CYP3A4/5 may be increased during pregnancy (44). The reduction in drug exposure was in the order of 70%–90%, indicating that the metabolism and systemic clearance of the drugs are drastically modified during pregnancy. Therefore, the induction in liver Cyp3a41, Cyp3a44, and Cyp3a16 found in the transgenic models with high serum prolactin may be important when designing appropriate regimens of narrow therapeutic-window drugs cleared by CYP3A enzymes for pregnant women or women under antipsychotic medication.
Liver gene expression changes in global Drd2−/− mice compared with wild-type mice were similar to those obtained in neuroDrd2KO mice with regard to class I male-predominant genes, even though sexual dimorphism was preserved in the first model. In contrast, for female-predominant type IIA genes, no increase in Drd2−/− male livers was evidenced, and sexual dimorphism was preserved. This may indicate that disruption of D2Rs in other nonneuronal tissues may compensate the effect of low GH on some liver genes. To this respect, it has been described that insulin participates in liver gene regulation (37, 51) and that β-cell D2Rs inhibit insulin response to glucose (15).
In conclusion, we propose a critical role for D2Rs in sexually dimorphic liver CYP and gene regulation, acting both indirectly through GH control at the CNS, or prolactin secretion from pituitary lactotropes. Many drugs used chronically in psychiatric diseases exert their effects mainly through the D2R. It is therefore conceivable that these drugs could alter the expression of several drug-metabolizing CYPs, thus affecting the pharmacodynamic characteristics and toxicity of drug substrates during pharmacotherapy. Therefore, the role of dopamine on sexual dimorphic liver gene expression should be considered particularly in the context of side effects and consequences of dopaminergic medications, and furthermore, the present results highlight the importance of studying health risks and endocrine mechanisms through a sex lens (52).
Acknowledgments
We thank the National Institute of Diabetes and Digestive and Kidney Diseases' National Hormone and Pituitary Program and Dr A. F. Parlow for prolactin and IGF- RIA kits and GH and prolactin antibodies.
This work was supported by the Consejo de Investigaciones Cientificas y Tecnicas Grants PIP 640-2009 and 304-2012 (to D.B.-V.), Agencia Nacional de Promoción Científica y Técnica, Buenos Aires, Argentina (D.B.-V. and M.R.), an International Research Scholar grant of The Howard Hughes Medical Institute (M.R.), and the Tourette Syndrome Association (M.R.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- Adh1
alcohol dehydrogenase-1
- CNS
central nervous system
- CT
cycle threshold
- CYP
cytochrome P450
- D2R
dopamine 2 receptor
- Ghr
GH receptor
- Glucor
glucocorticoid receptor
- Igfbp3
IGF binding protein 3
- KO
knockout
- MUP
major urinary protein
- NHPP
National Hormone and Pituitary Program
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- Prlr
prolactin receptor.
References