Summary
The pharmacokinetics of the immunosuppressant mycophenolate mofetil have been investigated in healthy volunteers and mainly in recipients of renal allografts. Following oral administration, mycophenolate mofetil was rapidly and completely absorbed, and underwent extensive presystemic de-esterification. Systemic plasma clearance of intravenous mycophenolate mofetil was around 10 L/min in healthy individuals, and plasma mycophenolate mofetil concentrations fell below the quantitation limit (0.4 mg/L) within 10 minutes of the cessation of infusion. Similar plasma mycophenolate mofetil concentrations were seen after intravenous administration in patients with severe renal or hepatic impairment, implying that the de-esterification process had not been substantially affected.
Mycophenolic acid, the active immunosuppressant species, is glucuronidated to a stable phenolic glucuronide (MPAG) which is not pharmacologically active. Over 90% of the administered dose is eventually excreted in the urine, mostly as MPAG. The magnitude of the MPAG renal clearance indicates that active tubular secretion of MPAG must occur. At clinically relevant concentrations, mycophenolic acid and MPAG are about 97% and 82% bound to albumin, respectively. MPAG at high (but clinically realisable) concentrations reduced the plasma binding of mycophenolic acid. The mean maximum plasma mycophenolic acid concentration (Cmax) after a mycophenolate mofetil 1g dose in healthy individuals was around 25 mg/L, occurred at 0.8 hours postdose, decayed with a mean apparent half-life (t1/2) of around 16 hours, and generated a mean total area under the plasma concentration-time curve (AUC∞) of around 64 mg · h/L. Intra- and interindividual coefficients of variation for the AUC∞ of the drug were estimated to be 25% and 10%, respectively. Intravenous and oral administration of mycophenolate mofetil showed statistically equivalent MPA AUC∞ values in healthy individuals. Compared with mycophenolic acid, MPAG showed a roughly similar Cmax about 1 hour after mycophenolic acid Cmax, with a similar t1/2 and an AUC∞ about 5-fold larger than that for mycophenolic acid.
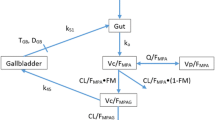
Secondary mycophenolic acid peaks represent a significant enterohepatic cycling process. Since MPAG was the sole material excreted in bile, entrohepatic cycling must involve colonic bacterial deconjugation of MPAG. An oral cholestyramine interaction study showed that the mean contribution of entrohepatic cycling to the AUC∞ of mycophenolic acid was around 40% with a range of 10 to 60%.
The pharmacokinetics of patients with renal transplants (after 3 months or more) compared with those of healthy individuals were similar after oral mycophenolate mofetil. Immediately post-transplant, the mean Cmax and AUC∞ of mycophenolic acid were 30 to 50% of those in the 3-month post-transplant patients. These parameters rose slowly over the 3-month interval. Slow metabolic changes, rather than poor absorption, seem responsible for this nonstationarity, since intravenous and oral administration of mycophenolate mofetil in the immediate post-transplant period generated comparable MPA AUC∞ values. Renal impairment had no major effect on the pharmacokinetic of mycophenolic acid after single doses of mycophenolate mofetil, but there was a progressive decrease in MPAG clearance as glomerular filtration rate (GFR) declined. Compared to individuals with a normal GFR, patients with severe renal impairment (GFR 1.5 L/h/1.73m2) showed 3- to 6-fold higher MPAG AUC values. In renal transplant recipients during acute renal impairment in the early post-transplant period, the plasma MPA concentrations were comparable to those in patients without renal failure, whereas plasma MPAG concentrations were 2- to 3-fold higher. Haemodialysis had no major effect on plasma mycophenolic acid or MPAG. Dosage adjustments appear to not be necessary either in renal impairment or during dialysis. In a single dose study with graded impairment of hepatic oxidative function in patients with cirrhosis, only minor effects on plasma mycophenolic acid or MPAG pharmacokinetics were seen. Other states of hepatic impairment may have different effects, given the central role of the liver in the pharmacokinetics of mycophenolic acid.
No significant pharmacokinetic interactions were seen with cyclosporin, cotrimoxazole, aciclovir, ganciclovir or a combined oral contraceptive. General mechanisms for potential drug interactions are interference with entrohepatic cycling (as seen with cholestyramine), and competition between MPAG and other drugs for excretion at the renal tubule.
Mycophenolic acid acts through specific enzyme inhibition with a consequent concentration-dependent suppression of mitogen-induced lymphocyte transformation. Consistent with this, a logistic correlation between the interdosage interval MPA AUC∞ and the probability of acute rejection was found in renal transplant patients. No correlation could be found for adverse events. Therapeutic drug monitoring seems unnecessary given the low mycophenolic acid variability and the small mycophenolate mofetil clinical dose range.
Similar content being viewed by others
References
European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. Lancet 1995; 345: 1321–5.
Sollinger HW, US Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 1995; 60: 225–32.
Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 1996; 61: 1029–37.
Lee WA, Gu L, Mikstal AR, et al. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res 1990; 7: 161–6.
Brewin TB, Cole MP, Jones CTA, et al. Mycophenolic acid (NSC-129185): preliminary clinical trials. Cancer Chemother Rep 1972; 56: 83–7.
Eugui EM, Almquist SJ, Muller CD, et al. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand J Immunol 1991; 33: 161–73.
Sintchak MD, Fleming MA, Futer O, et al. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell 1996; 85: 921–30.
Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit 1995; 17: 681–4.
Tsina I, Kaloostian M, Lee R, et al. High-performance liquid Chromatographic method for the determination of mycophenolate mofetil in human plasma. J Chromatog B 1996; 681: 347–53.
Tsina I, Chu F, Hama K, et al. Manual and automated (robotic) high-performance liquid chromatography methods for the determination of mycophenolic acid and its glucuronide conjugate in human plasma. J Chromatog B 1996; 675: 119–29.
Bullingham R, Monroe S, Nicholls A, et al. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J Clin Pharmacol 1996; 36: 315–24.
Hofmann-La Roche, Basle, Switzerland. Data on file.
Nowak R, Shaw LM. Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem 1995; 41: 1011–7.
MacKichan JJ. Pharmacokinetic consequences of drug displacement from blood and tissue proteins. Clin Pharmacokinet 1984; 9 (Suppl 1): 32–41.
Parker G, Bullingham R, Kamm B, et al. Pharmacokinetics of oral mycophenolate mofetil in volunteer subjects with varying degrees of hepatic oxidative impairment. J Clin Pharmacol 1996; 36: 332–44.
Langman LJ, LeGatt DF, Yatscoff RW. Blood distribution of mycophenolic acid. Ther Drug Monit 1994; 16: 602–7.
Bullingham R, Shah J, Goldblum R, et al. Effects of food and antacid on the pharmacokinetics of single doses of mycophenolate mofetil in rheumatoid arthritis patients. Br J Clin Pharmacol 1996; 41: 513–6.
Peris-Ribera J-E, Torres-Molina F, Garcia-Carbonell MC, et al. General treatment of the enterohepatic recirculation of drugs and its influence on the area under the plasma level curves, bioavailability, and clearance. Pharm Res 1992; 9: 1306–13.
Sollinger HW, Deierhoi MH, Beizer FO, et al. RS-61443 — a phase I clinical trial and pilot rescue study. Transplantation 1992; 53: 428–32.
Wolfe EJ, Mathur V, Tomlanovich S, et al. Pharmacokinetics of mycophenolate mofetil and intravenous ganciclovir alone and in combination in renal transplant patients. Pharmacotherapy 1997; 17: 591–8.
RS-61443 Investigation Committee. Pilot study of mycophenolate mofetil (RS-61443) in the prevention of acute rejection following renal transplantation in Japanese patients. Transplant Proc 1995; 27: 1421–4.
Bullingham RES, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc 1996; 28: 925–9.
Shah J, Bullingham R, Rice P, et al. Pharmacokinetics of oral mycophenolate mofetil (MMF) and metabolites in renally impaired patients [abstract]. Clin Pharmacol Ther 1995; 57: 149.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31.
Burchell B, Coughtrie MWH., UDP-Glucuronosyltransferases. In: Kalow W, ed. Pharmacogenetics of drug metabolism. New York: Pergamon Press, 1992: 195–225.
Hebert MF, Benet LZ, Gomez D, et al. Pharmacokinetics (PK) of oral mycophenolate mofetil (MMF) in liver transplant patients (abstract). Clin Pharmacol Ther 1994; 55: 164.
Hebert MF, Chang T, Linna TJ, et al. Pharmacokinetics of intravenous mycophenolate mofetil (MMF) in liver transplant patients (abstract). Clin Pharmacol Ther 1996; 59: 184.
Shaw LM, Sollinger HW, Halloran P, et al. Mycophenolate mofetil: a report of the consensus panel. Ther Drug Monitor 1995; 17: 690–6.
Shaw LM. Mycophenolate mofetil: pharmacokinetic strategies for optimizing immunosuppression. Drug Metab Drug Interactions 1997; 14: 33–40.
Langman LJ, LeGatt DF, Yatscoff RW. Pharmacodynamic assessment of mycophenolic acid-induced immunosuppression by measuring IMP dehydrogenase activity. Clin Chem 1995; 41: 295–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bullingham, R.E.S., Nicholls, A.J. & Kamm, B.R. Clinical Pharmacokinetics of Mycophenolate Mofetil. Clin Pharmacokinet 34, 429–455 (1998). https://doi.org/10.2165/00003088-199834060-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199834060-00002