Abstract
Type 2 diabetes mellitus is a complex disease combining defects in insulin secretion and insulin action. New compounds called thiazolidinediones or glitazones have been developed for reducing insulin resistance. After the withdrawal of troglitazone because of liver toxicity, two compounds are currently used in clinical practice, rosiglitazone and pioglitazone. These compounds are generally used in combination with other pharmacological agents. Because they are metabolised via cytochrome P450 (CYP), glitazones are exposed to numerous pharmacokinetic interactions. CYP2C8 and CYP3A4 are the main isoenzymes catalysing biotransformation of pioglitazone (as with troglitazone), whereas rosiglitazone is metabolised by CYP2C9 and CYP2C8. For both rosiglitazone and pioglitazone, the most relevant interactions have been described in healthy volunteers with rifampicin (rifampin), which results in a significant decrease of area under the plasma concentration-time curve [AUC] (54–65% for rosiglitazone, p < 0.001; 54% for pioglitazone, p < 0.001), and with gemfibrozil, which results in a significant increase of AUC (130% for rosiglitazone, p < 0.001; 220–240% for pioglitazone, p < 0.001). The relevance of such drug-drug interactions in patients with type 2 diabetes remains to be evaluated. However, in the absence of clinical data, it is prudent to reduce the dosage of each glitazone by half in patients treated with gemfibrozil. Conversely, rosiglitazone and pioglitazone do not seem to significantly affect the pharmacokinetics of other compounds. Although some food components have also been shown to potentially interfere with drugs metabolised with the CYP system, no published study deals specifically with these possible CYP-mediated food-drug interactions with glitazones.


Similar content being viewed by others
References
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46: 3–19
Scheen AJ. Pathophysiology of type 2 diabetes. Acta Clin Belg 2003; 58: 335–41
Scheen AJ, Lefèbvre PJ. Oral antidiabetic agents: a guide to selection. Drugs 1998; 55: 225–36
Lebovitz HE. Oral therapies for diabetic hyperglycemia. En-docrinol Metab Clin North Am 2001; 30: 909–33
Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002; 287: 360–72
Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006; 29: 1963–72 and Diabetologia 2006; 49: 1711-21
Charpentier G. Oral combination therapy for type 2 diabetes. Diabetes Metab Res Rev 2002; 18 Suppl. 3: S70–6
Van Gaal LF, De Leeuw I. Rationale and options for combination therapy in the treatment of type 2 diabetes. Diabetologia 2003; 46 Suppl. 1: M44–50
Scheen AJ. Current management of coexisting obesity and type 2 diabetes. Drugs 2003; 63: 1165–84
Scheen AJ. Drug treatment of non-insulin-dependent diabetes mellitus in the 1990s: achievements and future developments. Drugs 1997; 54: 355–68
Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65: 385–411
Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–93
Scheen AJ, Lefèbvre PJ. Antihyperglycaemic agents: drug interactions of clinical importance. Drug Saf 1995; 12: 32–45
Scheen AJ. Drug interactions of clinical importance with antihyperglycaemic agents: an update. Drug Saf 2005; 28: 601–31
Martens FM, Visseren FL, Lemay J, et al. Metabolic and additional vascular effects of thiazolidinediones. Drugs 2002; 62: 1463–80
Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351: 1106–18
Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005; 54: 2460–70
Scheen AJ. Hepatotoxicity with thiazolidinediones: is it a class effect? Drug Saf 2001; 24: 873–88
Gillies PS, Dunn CJ. Pioglitazone. Drugs 2000; 60: 333–43
Chilcott J, Tappenden P, Jones ML, et al. A systematic review of the clinical effectiveness of pioglitazone in the treatment of type 2 diabetes mellitus. Clin Ther 2001; 23: 1792–823
Waugh J, Keating GM, Plosker GL, et al. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs 2006; 66: 85–109
Balfour JA, Plosker GL. Rosiglitazone. Drugs 1999; 57: 921–30
Wagstaff AJ, Goa KL. Rosiglitazone: a review of its use in the management of type 2 diabetes mellitus. Drugs 2002; 62: 1805–37
Diamant M, Heine RJ. Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence. Drugs 2003; 63: 1373–405
Meriden T. Progress with thiazolidinediones in the management of type 2 diabetes mellitus. Clin Ther 2004; 26: 177–90
Braunstein S. New developments in type 2 diabetes mellitus: combination therapy with a thiazolidinedione. Clin Ther 2003; 25: 1895–917
Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002; 51: 2796–803
Chiquette E, Ramirez G, DeFronzo R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med 2004; 164: 2097–104
Dormandy J, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366: 1279–89
Cox PJ, Ryan DA, Hollis FJ, et al. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metab Dispos 2000; 28: 772–80
Eckland DA, Danhof M. Clinical pharmacokinetics of pioglitazone. Exp Clin Endocrinol Diabetes 2000; 108 Suppl. 2: S234–42
Hanefeld M. Pharmacokinetics and clinical efficacy of pioglitazone. Int J Clin Pract 2001; 121 Suppl.: 19–25
Scheen AJ. Drug-drug and food-drug kinetic interactions with new insulinotropic meglitinide analogues. Clin Pharmacokinet 2007. In press
Yamazaki H, Suzuki M, Tane K, et al. In vitro inhibitory effects of troglitazone and its metabolites on drug oxidation activities of human cytochrome P450 enzymes: comparison with pioglitazone and rosiglitazone. Xenobiotica 2000; 30: 61–70
Sahi J, Black CB, Hamilton GA, et al. Comparative effects of thiazolidinediones on in vitro P450 enzyme induction and inhibition. Drug Metab Dispos 2003; 31: 439–46
Loi C, Young M, Randinitis E, et al. Clinical pharmacokinetics of troglitazone. Clin Pharmacokinet 1999; 37: 91–104
Kirchheiner J, Roots I, Goldammer M, et al. Effect of genetic polymorphisms in cytochrome p450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet 2005; 44(12): 1209–25
Balayssac D, Authier N, Cayre A, et al. Does inhibition of P-glycoprotein lead to drug-drug interactions? Toxicol Lett 2005; 156: 319–29
Scheen AJ. Diabetes, obesity and metabolic syndrome. In: Meckling KA, editor. Nutrient-drug interactions. Boca Reton (FL): Taylor & Francis, 2007: 1–30
Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharma-codynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 2000; 38: 41–57
Holstein A, Egberts EH. Risk of hypoglycaemia with oral antidiabetic agents in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2003; 111: 405–14
Klotz U, Sailer D. Drug interactions: their impact on safe drug therapy in the example of the new thiazolidinedione group (glitazone) [in German]. Arzneimittelforschung 2001; 51: 112–7
Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 1996; 30: 359–71
Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet 2002; 41: 343–70
Martin J, Krum H. Cytochrome P450 drug interactions within the HMG-CoA reductase inhibitor class: are they clinically relevant? Drug Saf 2003; 26: 13–21
Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004; 109 Suppl. III: III50–7
Prueksaritanont T, Vega JM, Zhao J, et al. Interactions between simvastatin and troglitazone or pioglitazone in healthy subjects. J Clin Pharmacol 2001; 41: 573–81
Alsheikh-Ali AA, Abourjaily HM, Karas RH. Risk of adverse events with concomitant use of atorvastatin or simvastatin and glucose-lowering drugs (thiazolidinediones, metformin, sulfonylurea, insulin and acarbose). Am J Cardiol 2002; 89: 1308–10
Alsheikh-Ali AA, Karas RH. Adverse events with concomitant use of simvastatin or atorvastatin and thiazolidinediones. Am J Cardiol 2004; 93: 1417–8
Baldwin SJ, Clarke SE, Chenery RJ. Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. Br J Clin Pharmacol 1999; 48: 424–32
Lim H-K, Duczak Jr N, Brougham L, et al. Automated screening with confirmation of mechanism-based inactivation of CY3A4, CYP2C9, CYP2C19, CYP2D6, and CYP1A2 in pooled human liver microsomes. Drug Metab Dispos 2005; 33: 1211–9
Ogino M, Nagata K, Yamazoe Y. Selective suppression of human CYP3A forms, CYP3A5 and CYP3A7, by troglitazone in HepG2 cells. Drug Metab Pharmacokinet 2002; 17: 42–6
Park JY, Kim KA, Kang MH, et al. Effect of rifampicin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin Pharmacol Ther 2004; 75: 157–62
Niemi M, Backman JT, Neuvonen PJ. Effects of trimethoprim and rifampin on the pharmacokinetics of the cytochrome P450 2C8 substrate rosiglitazone. Clin Pharmacol Ther 2004; 76: 239–49
Niemi M, Backman JT, Granfors M, et al. Gemfibrozil considerably increases the plasma concentrations of rosiglitazone. Diabetologia 2003; 46: 1319–23
Hruska MW, Amico JA, Langaee TY, et al. The effect of trimethoprim on CYP2C8 mediated rosiglitazone metabolism in human liver microsomes and healthy subjects. Br J Clin Pharmacol 2005; 59: 70–9
Pedersen RS, Damkier P, Brosen K. The effects of human CYP2C8 genotype and fluvoxamine on the pharmacokinetics of rosiglitazone in healthy subjects. Br J Clin Pharmacol 2006 Dec; 62(6): 682–9
Miller AK, Inglis AM, Thompson Culkin K, et al. The effect of acarbose on the pharmacokinetics of rosiglitazone. Eur J Clin Pharmacol 2001; 57: 105–9
Rao MN, Mullangi R, Katneni K, et al. Lack of effect of sucralfate on the absorption and pharmacokinetics of rosiglitazone. J Clin Pharmacol 2002; 42: 670–5
Miller AK, Di Cicco RA, Freed MI. The effect of ranitidine on the pharmacokinetics of rosiglitazone in healthy adult male volunteers. Clin Ther 2002; 24: 1062–71
Kim KA, Park PW, Kim HK, et al. Effect of quercetin on the pharmacokinetics of rosiglitazone, a CYP2C8 substrate, in healthy subjects. J Clin Pharmacol 2005; 45: 941–6
Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 2003; 42: 819–50
Scheen AJ. Combined thiazolidinedione-insulin therapy: should we be concerned about safety? Drug Saf 2004; 27: 841–56
Walsky RL, Obach RS, Gaman EA, et al. Selective inhibition of cytochrome P4502C8 by montelukast. Drug Metab Dispos 2005; 33: 413–8
Wang JS, Neuvonen M, Wen X, et al. Gemfibrozil inhibits CYP2C8-mediated ceristatin metabolism in human liver microsomes. Drug Metab Dispos 2002; 30: 1352–6
Backman JT, Kyrklund C, Neuvonen M, et al. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther 2002; 72: 685–91
Ogilvie BW, Zhang D, Li W, et al. Glucuronidation converts gemfibrozil to a potent, metabolism-dependent inhibitor of CYP2C8: implications for drug-drug interactions. Drug Metab Dispos 2006; 34: 191–7
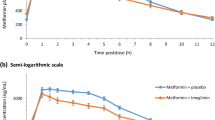
Di Cicco RA, Allen A, Carr A, et al. Rosiglitazone does not alter the pharmacokinetics of metformin. J Clin Pharmacol 2000; 40: 1280–5
Wellington K. Rosiglitazone/metformin. Drugs 2005; 65: 1581–92
Thompson KA, Miller AK, Inglis AML, et al. Rosiglitazone does not markedly alter CYP3A4-mediated drug metabolism [abstract]. Diabetologia 1999; 42 Suppl. 1: A227
Harris RZ, Inglis AML, Miller AK, et al. Rosiglitazone has no clinically significant effect on nifedipine pharmacokinetics. J Clin Pharmacol 1999; 39: 1189–94
Inglis A, Miller A, Culkin K, et al. Lack of effect of rosiglitazone on the pharmacokinetics of oral contraceptives in healthy female volunteers. J Clin Pharmacol 2001; 41: 683–90
Di Cicco RA, Miller AK, Patterson S, et al. Rosiglitazone does not affect the steady-state pharmacokinetics of digoxin. J Clin Pharmacol 2000; 40: 1516–21
Oette M, Kurowski M, Feldt T, et al. Impact of rosiglitazone treatment on the bioavailability of antiretroviral compounds in HIV-positive patients. J Antimicrob Chemother 2005; 56: 416–9
Freed MI, Allen A, Jorkasky DK, et al. Systemic exposure to rosiglitazone is unaltered by food. Eur J Clin Pharmacol 1999; 55: 53–6
Jaakkola T, Laitila J, Neuvonen PJ, et al. Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol 2006; 99: 44–51
Glazer NB, Cheatham WW. Thiazolidinediones for type 2 diabetes: no evidence exists that pioglitazone induces hepatic cytochrome P450 isoform CYP3A4 [letter]. BMJ 2001; 322: 235–6
Nowak SN, Edwards DJ, Clarke A, et al. Pioglitazone: effect on CYP3A4 activity. J Clin Pharmacol 2002; 42: 1299–302
Jaakkola T, Backman JT, Neuvonen M, et al. Effect of rifampicin on the pharmacokinetics of pioglitazone. Br J Clin Pharmacol 2006; 61: 70–8
Deng J-J, Wang F, Li H-D. Effect of gemfibrozil on the pharmacokinetics of pioglitazone. Eur J Clin Pharmacol 2005; 61: 831–6
Jaakkola T, Backman JT, Neuvonen M, et al. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics of pioglitazone. Clin Pharmacol Ther 2005; 77: 404–14
Jaakkola T, Backman JT, Neuvonen M, et al. Montelukast and zafirlukast do not affect the pharmacokinetics of the CYP2C8 substrate pioglitazone. Eur J Clin Pharmacol 2006; 62: 503–9
Kajosaari LI, Jaakkola T, Neuvonen PJ, et al. Pioglitazone, an in vitro inhibitor of CYP2C8 and CYP3A4, does not increase the plasma concentrations of the CYP2C8 and CYP3A4 substrate repaglinide. Eur J Clin Pharmacol 2006; 62: 217–23
Jovanovic L, Hassman DR, Gooch B, et al. Treatment of type 2 diabetes with a combination regimen of repaglinide plus pioglitazone. Diabetes Res Clin Pract 2004; 63: 127–34
Kortboyer JM, Eckland D. Pioglitazone has low potential for drug interactions [abstract]. Diabetologia 1999; 42 Suppl. 1: A228
Carey R, Liu Y. Pioglitazone does not markedly alter oral contraceptive or hormone replacement pharmacokinetics [abstract]. Diabetes 2000; 49 Suppl. 1: A340–1
Geerlof JS, Lebrizzi R, Carey RA. Effect of food on the pharmacokinetics of pioglitazone [abstract]. Diabetes 2000; 49 Suppl. 1: A357
Acknowledgements
No sources of funding were used to assist in the preparation of this review. Authors have no conflicts of interest that are directly relevant to the content of this reveiw.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheen, A.J. Pharmacokinetic Interactions with Thiazolidinediones. Clin Pharmacokinet 46, 1–12 (2007). https://doi.org/10.2165/00003088-200746010-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746010-00001