Abstract
This review aims to provide an extensive overview of the literature on the clinical pharmacokinetics of mycophenolate in solid organ transplantation and a briefer summary of current pharmacodynamic information. Strategies are suggested for further optimisation of mycophenolate therapy and areas where additional research is warranted are highlighted. Mycophenolate has gained widespread acceptance as the antimetabolite immunosuppressant of choice in organ transplant regimens. Mycophenolic acid (MPA) is the active drug moiety.
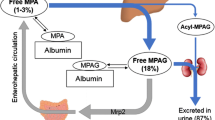
Currently, two mycophenolate compounds are available, mycophenolate mofetil and enteric-coated (EC) mycophenolate sodium. MPA is a potent, selective and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), leading to eventual arrest of T- and B-lymphocyte proliferation. Mycophenolate mofetil and EC-mycophenolate sodium are essentially completely hydrolysed to MPA by esterases in the gut wall, blood, liver and tissue. Oral bioavailability of MPA, subsequent to mycophenolate mofetil administration, ranges from 80.7% to 94%. EC-mycophenolate sodium has an absolute bioavailability of MPA of approximately 72%.
MPA binds 97–99% to serum albumin in patients with normal renal and liver function. It is metabolised in the liver, gastrointestinal tract and kidney by uridine diphosphate gluconosyltransferases (UGTs). 7-O-MPA-glucuronide (MPAG) is the major metabolite of MPA. MPAG is usually present in the plasma at 20- to 100-fold higher concentrations than MPA, but it is not pharmacologically active. At least three minor metabolites are also formed, of which an acyl-glucuronide has pharmacological potency comparable to MPA. MPAG is excreted into the urine via active tubular secretion and into the bile by multi-drug resistance protein 2 (MRP-2). MPAG is de-conjugated back to MPA by gut bacteria and then reabsorbed in the colon.
Mycophenolate mofetil and EC-mycophenolate sodium display linear pharmacokinetics. Following mycophenolate mofetil administration, MPA maximum concentration usually occurs in 1–2 hours. EC-mycophenolate sodium exhibits a median lag time in absorption of MPA from 0.25 to 1.25 hours. A secondary peak in the concentration-time profile of MPA, due to enterohepatic recirculation, often appears 6–12 hours after dosing. This contributes approximately 40% to the area under the plasma concentration-time curve (AUC). The mean elimination half-life of MPA ranges from 9 to 17 hours.
MPA displays large between- and within-subject pharmacokinetic variability. Dose-normalised MPA AUC can vary more than 10-fold. Total MPA concentrations should be interpreted with caution in patients with severe renal impairment, liver disease and hypoalbuminaemia. In such individuals, MPA and MPAG plasma protein binding may be altered, changing the fraction of free MPA available. Apparent oral clearance (CL/F) of total MPA appears to increase in proportion to the increased free fraction, with a reduction in total MPA AUC. However, there may be little change in the MPA free concentration. Ciclosporin inhibits biliary excretion of MPAG by MRP-2, reducing enterohepatic recirculation of MPA. Exposure to MPA when mycophenolate mofetil is given in combination with ciclosporin is approximately 30–40% lower than when given alone or with tacrolimus or sirolimus. High dosages of corticosteroids may induce expression of UGT, reducing exposure to MPA. Other co-medications can interfere with the absorption, enterohepatic recycling and metabolism of mycophenolate. Most pharmacokinetic investigations of MPA have involved mycophenolate mofetil rather than EC-mycophenolate sodium therapy.
In population pharmacokinetic studies, MPA CL/F in adults ranges from 14.1 to 34.9 L/h (ciclosporin co-therapy) and from 11.9 to 25.4 L/h (tacrolimus co-therapy). Patient bodyweight, serum albumin concentration and immunosuppressant co-therapy have a significant influence on CL/F.
The majority of pharmacodynamic data on MPA have been obtained in patients receiving mycophenolate mofetil therapy in the first year after kidney transplantation. Low MPA AUC is associated with increased incidence of biopsy-proven acute rejection. Gastrointestinal adverse events may be dose related. Leukopenia and anaemia have been associated with high MPA AUC, trough concentration and metabolite concentrations in some, but not all, studies. High free MPA exposure has been identified as a risk factor for leukopenia in some investigations. Targeting a total MPA AUC from 0 to 12 hours (AUC12) of 30–60 mg ∙ hr/L is likely to minimise the risk of acute rejection and may reduce toxicity.
IMPDH monitoring is in the early experimental stage. Individualisation of mycophenolate therapy should lead to improved patient outcomes. MPA AUC12 appears to be the most useful exposure measure for such individualisation. Limited sampling strategies and Bayesian forecasting are practical means of estimating MPA AUC12 without full concentration-time profiling. Target concentration intervention may be particularly useful in the first few months post-transplant and prior to major changes in anti-rejection therapy. In patients with impaired renal or hepatic function or hypoalbuminaemia, free drug measurement could be valuable in further interpretation of MPA exposure.





















Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Kaufman DB, Shapiro R, Lucey MR, et al. Immunosuppression: practice and trends. Am J Transplant 2004; 4 Suppl. 9: 38–53
Shaw LM, Nawrocki A, Korecka M, et al. Using established immunosuppressant therapy effectively: lessons from the measurement of mycophenolic acid plasma concentrations. Ther Drug Monit 2004; 26(4): 347–51
Roche Pharmaceuticals. CellCept®: complete product information [online]. Available from URL: http://www.rocheusa.com/products/cellcept [Accessed 2006 Dec 7]
European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet 1995; 345(8961): 1321–5
The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation 1996; 61(7): 1029–37
Sollinger HW, for the US Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 1995; 60(3): 225–32
Novartis Pharmaceuticals Corporation. Myfortic®: prescribing information [online]. Available from URL: http://www.pharma.us.novartis.com/product/pi/pdf/myfortic.pdf [Accessed 2006 Dec 7]
Granger DK. Enteric-coated mycophenolate sodium: results of two pivotal global multicenter trials. Transplant Proc 2001; 33(7–8): 3241–4
Salvadori M, Holzer H, de Mattos A, et al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2004; 4(2): 231–6
Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus 2005; 14 Suppl. 1: s2–8
Halloran P, Mathew T, Tomlanovich S, et al. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation 1997; 63(1): 39–47
Lee WA, Gu L, Miksztal AR, et al. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res 1990; 7(2): 161–6
Bullingham R, Monroe S, Nicholls A, et al. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J Clin Pharmacol 1996; 36(4): 315–24
Armstrong VW, Tenderich G, Shipkova M, et al. Pharmacokinetics and bioavailability of mycophenolic acid after intravenous administration and oral administration of mycophenolate mofetil to heart transplant recipients. Ther Drug Monit 2005; 27(3): 315–21
Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 1998; 34(6): 429–55
Arns W, Breuer S, Choudhury S, et al. Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant 2005; 19(2): 199–206
Pescovitz MD, Conti D, Dunn J, et al. Intravenous mycophenolate mofetil: safety, tolerability, and pharmacokinetics. Clin Transplant 2000; 14(3): 179–88
Braun KP, Glander P, Hambach P, et al. Pharmacokinetics and pharmacodynamics of mycophenolate mofetil under oral and intravenous therapy. Transplant Proc 2002; 34(5): 1745–7
Nowak I, Shaw LM. Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem 1995; 41(7): 1011–7
Langman LJ, LeGatt DF, Yatscoff RW. Blood distribution of mycophenolic acid. Ther Drug Monit 1994; 16(6): 602–7
Bullingham RE, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc 1996; 28(2): 925–9
Weber LT, Shipkova M, Lamersdorf T, et al. Pharmacokinetics of mycophenolic acid (MPA) and determinants of MPA free fraction in pediatric and adult renal transplant recipients. German Study group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. J Am Soc Nephrol 1998; 9(8): 1511–20
Ensom MH, Partovi N, Decarie D, et al. Pharmacokinetics and protein binding of mycophenolic acid in stable lung transplant recipients. Ther Drug Monit 2002; 24(2): 310–4
Nowak I, Shaw LM. Effect of mycophenolic acid glucuronide on inosine monophosphate dehydrogenase activity. Ther Drug Monit 1997; 19(3): 358–60
Atcheson BA, Taylor PJ, Kirkpatrick CM, et al. Free mycophenolic acid should be monitored in renal transplant recipients with hypoalbuminemia. Ther Drug Monit 2004; 26(3): 284–6
Kaplan B, Meier-Kriesche HU, Friedman G, et al. The effect of renal insufficiency on mycophenolic acid protein binding. J Clin Pharmacol 1999; 39(7): 715–20
Shaw LM, Mick R, Nowak I, et al. Pharmacokinetics of mycophenolic acid in renal transplant patients with delayed graft function. J Clin Pharmacol 1998; 38(3): 268–75
Meier-Kriesche HU, Shaw LM, Korecka M, et al. Pharmacokinetics of mycophenolic acid in renal insufficiency. Ther Drug Monit 2000; 22(1): 27–30
Shaw LM, Holt DW, Oellerich M, et al. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit 2001; 23(4): 305–15
Shaw LM, Korecka M, DeNofrio D, et al. Pharmacokinetic, pharmacodynamic, and outcome investigations as the basis for mycophenolic acid therapeutic drug monitoring in renal and heart transplant patients. Clin Biochem 2001; 34(1): 17–22
Venkataramanan R, Ou J, Pisupati J, et al. Does plasma protein binding of mycophenolic acid affect its clearance? Fifth Congress of the International Liver Transplantation Society; 1999 Aug 26-29; Pittsburgh (PA)
Atcheson BA, Taylor PJ, Mudge DW, et al. Mycophenolic acid pharmacokinetics and related outcomes early after renal transplant. Br J Clin Pharmacol 2005; 59(3): 271–80
Picard N, Ratanasavanh D, Premaud A, et al. Identification of the UDP-glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos 2005; 33(1): 139–46
Bowalgaha K, Miners JO. The glucuronidation of mycophenolic acid by human liver, kidney and jejunum microsomes. Br J Clin Pharmacol 2001; 52(5): 605–9
Shipkova M, Armstrong VW, Wieland E, et al. Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Br J Pharmacol 1999; 126(5): 1075–82
Schutz E, Shipkova M, Armstrong VW, et al. Identification of a pharmacologically active metabolite of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Clin Chem 1999; 45(3): 419–22
Shipkova M, Strassburg CP, Braun F, et al. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. Br J Pharmacol 2001; 132(5): 1027–34
Shipkova M, Schutz E, Armstrong VW, et al. Determination of the acyl glucuronide metabolite of mycophenolic acid in human plasma by HPLC and Emit. Clin Chem 2000; 46(3): 365–72
Shipkova M, Armstrong VW, Oellerich M, et al. Acyl glucuronide drug metabolites: toxicological and analytical implications. Ther Drug Monit 2003; 25(1): 1–16
Wieland E, Shipkora M, Schutz E, et al. The acyl gluronide of the immunosuppressant mycophenolic acid induces release of proinflammatory cytockines and TNF-alpha mRNA expression [abstract]. Clin Clem 1999; 45 Suppl. 6: A127
Wieland E, Shipkova M, Schellhaas U, et al. Induction of cytokine release by the acyl glucuronide of mycophenolic acid: a link to side effects? Clin Biochem 2000; 33(2): 107–13
Maes BD, Dalle I, Geboes K, et al. Erosive enterocolitis in mycophenolate mofetil-treated renal-transplant recipients with persistent afebrile diarrhea. Transplantation 2003; 75(5): 665–72
Basu NK, Kole L, Kubota S, et al. Human UDP-glucuronosyl-transferases show atypical metabolism of mycophenolic acid and inhibition by curcumin. Drug Metab Dispos 2004; 32(7): 768–73
Bernard O, Guillemette C. The main role of UGT1A9 in the hepatic metabolism of mycophenolic acid and the effects of naturally occurring variants. Drug Metab Dispos 2004; 32(8): 775–8
Picard N, Cresteil T, Premaud A, et al. Characterization of a phase 1 metabolite of mycophenolic acid produced by CYP3A4/5. Ther Drug Monit 2004; 26(6): 600–8
Kuypers DR, Naesens M, Vermeire S, et al. The impact of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther 2005; 78(4): 351–61
Hesselink DA, van Gelder T. Genetic and nongenetic determinants of between-patient variability in the pharmacokinetics of mycophenolic acid. Clin Pharmacol Ther 2005; 78(4): 317–21
Kobayashi M, Saitoh H, Tadano K, et al. Cyclosporin A, but not tacrolimus, inhibits the biliary excretion of mycophenolic acid glucuronide possibly mediated by multidrug resistance-associated protein 2 in rats. J Pharmacol Exp Ther 2004; 309(3): 1029–35
Naderer OJ, Dupuis RE, Heinzen EL, et al. The influence of norfloxacin and metronidazole on the disposition of mycophenolate mofetil. J Clin Pharmacol 2005; 45(2): 219–26
Wollenberg K, Krumme B, Pisarski P, et al. Pharmacokinetics of mycophenolic acid in the early period after kidney transplantation. Transplant Proc 1998; 30(8): 4090–1
Wollenberg K, Krumme B, Schollmeyer P, et al. Pharmacokinetics of mycophenolic acid after renal transplantation. Transplant Proc 1998; 30(5): 2237–9
Liang MZ, Lu YP, Nan F, et al. Pharmacokinetics of mycophenolic acid after a single and multiple oral doses of mycophenolate mofetil in Chinese renal transplant recipients. Transplant Proc 2004; 36(7): 2065–7
Jirasiritham S, Sumethkul V, Mavichak V, et al. The pharmacokinetics of mycophenolate mofetil in Thai kidney transplant recipients. Transplant Proc 2004; 36(7): 2076–8
Undre NA, van Hooff J, Christiaans M, et al. Pharmacokinetics of FK 506 and mycophenolic acid after the administration of a FK 506-based regimen in combination with mycophenolate mofetil in kidney transplantation. Transplant Proc 1998; 30(4): 1299–302
Yeung S, Tong KL, Tsang WK, et al. Pharmacokinetic study of mycophenolate mofetil in Asian renal transplant recipients. Transplant Proc 2000; 32(7): 1753–4
Cho EK, Han DJ, Kim SC, et al. Pharmacokinetic study of mycophenolic acid in Korean kidney transplant patients. J Clin Pharmacol 2004; 44(7): 743–50
Engelbertink R, Smak Gregoor P, Hesse C, et al. High mycophenolic acid area under-the-curve values in renal transplant recipients on long-term mycophenolate mofetil treatment. Transplant Proc 2002; 34(7): 2983–4
Brunet M, Martorell J, Oppenheimer F, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid in stable renal transplant recipients treated with low doses of mycophenolate mofetil. Transpl Int 2000; 13 Suppl. 1: S301–5
Neumann I, Haidinger M, Jager H, et al. Pharmacokinetics of mycophenolate mofetil in patients with autoimmune diseases compared renal transplant recipients. J Am Soc Nephrol 2003; 14(3): 721–7
Mourad M, Malaise J, Chaib Eddour D, et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem 2001; 47(1): 88–94
Kuypers DR, Vanrenterghem Y, Squifflet JP, et al. Twelvemonth evaluation of the clinical pharmacokinetics of total and free mycophenolic acid and its glucuronide metabolites in renal allograft recipients on low dose tacrolimus in combination with mycophenolate mofetil. Ther Drug Monit 2003; 25(5): 609–22
Satoh S, Tada H, Murakami M, et al. The influence of mycophenolate mofetil versus azathioprine and mycophenolic acid pharmacokinetics on the incidence of acute rejection and infectious complications after renal transplantation. Transplant Proc 2005; 37(4): 1751–3
Johnson AG, Rigby RJ, Taylor PJ, et al. The kinetics of mycophenolic acid and its glucuronide metabolite in adult kidney transplant recipients. Clin Pharmacol Ther 1999; 66(5): 492–500
Tedesco-Silva H, Bastien MC, Choi L, et al. Mycophenolic acid metabolite profile in renal transplant patients receiving enteric-coated mycophenolate sodium or mycophenolate mofetil. Transplant Proc 2005; 37(2): 852–5
Budde K, Glander P, Hahn U, et al. Pharmacokinetics and pharmacodynamic comparison of mycophenolate mofetil and enteric-coated mycophenolate sodium in maintenance renal transplant patients [abstract no. 1036]. Am J Transplant 2002; 2002 (2 Suppl. 3): 399
Sumethkul V, Na-Bangchang K, Kantachuvesiri S, et al. Standard dose enteric-coated mycophenolate sodium (myfortic) delivers rapid therapeutic mycophenolic acid exposure in kidney transplant recipients. Transplant Proc 2005; 37(2): 861–3
Gabardi S, Tran JL, Clarkson MR. Enteric-coated mycophenolate sodium. Ann Pharmacother 2003; 37(11): 1685–93
Pisupati J, Jain A, Burckart G, et al. Intraindividual and inter-individual variations in the pharmacokinetics of mycophenolic acid in liver transplant patients. J Clin Pharmacol 2005; 45(1): 34–41
Jain A, Venkataramanan R, Hamad IS, et al. Pharmacokinetics of mycophenolic acid after mycophenolate mofetil administration in liver transplant patients treated with tacrolimus. J Clin Pharmacol 2001; 41(3): 268–76
DeNofrio D, Loh E, Kao A, et al. Mycophenolic acid concentrations are associated with cardiac allograft rejection. J Heart Lung Transplant 2000; 19(11): 1071–6
Ensom MH, Partovi N, Decarie D, et al. Mycophenolate pharmacokinetics in early period following lung or heart transplantation. Ann Pharmacother 2003; 37(12): 1761–7
Jacqz-Aigrain E, Khan Shaghaghi E, Baudouin V, et al. Pharmacokinetics and tolerance of mycophenolate mofetil in renal transplant children. Pediatr Nephrol 2000; 14(2): 95–9
Sherbotie J, Bunchman T, Navarra P. Mycophenolate mofetil (MMF) oral suspension in pediatric renal transplantation [abstract; online]. American Society of Transplantation 18th Annual Meeting; 1999 May 15-19; Chicago (IL). Available from URL: http://www.a-s-t.org/library/abstracts99/473.htm [Accessed 2006 Dec 14]
Weber LT, Lamersdorf T, Shipkova M, et al. Area under the plasma concentration-time curve for total, but not for free, mycophenolic acid increases in the stable phase after renal transplantation: a longitudinal study in pediatric patients. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit 1999; 21(5): 498–506
Filler G, Lampe D, Mai I, et al. Dosing of MMF in combination with tacrolimus for steroid-resistant vascular rejection in pediatric renal allografts. Transpl Int 1998; 11 Suppl. 1: S82–5
Weber LT, Schutz E, Lamersdorf T, et al. Pharmacokinetics of mycophenolic acid (MPA) and free MPA in paediatric renal transplant recipients: a multicentre study. The German Study Group on Mycophenolate Mofetil (MMF) Therapy. Nephrol Dial Transplant 1999; 14 Suppl. 4: 33–4
Filler G, Lepage N, Delisle B, et al. Effect of cyclosporine on mycophenolic acid area under the concentration-time curve in pediatric kidney transplant recipients. Ther Drug Monit 2001; 23(5): 514–9
Armstrong VW, Shipkova M, Schutz E, et al. Monitoring of mycophenolic acid in pediatric renal transplant recipients. Transplant Proc 2001; 33(1-2): 1040–3
Ghio L, Ferraresso M, Vigano SM, et al. Mycophenolate mofetil pharmacokinetic monitoring in pediatric kidney transplant recipients. Transplant Proc 2005; 37(2): 856–8
Weber LT, Shipkova M, Armstrong VW, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: a report of the German Study Group on Mycophenolate Mofetil Therapy. J Am Soc Nephrol 2002; 13(3): 759–68
Aigrain EJ, Shaghaghi EK, Baudouin V, et al. Pharmacokinetics of mycophenolate mofetil in eight pediatric renal transplant patients. Transplant Proc 2000; 32(2): 388–90
Shipkova M, Armstrong VW, Weber L, et al. Pharmacokinetics and protein adduct formation of the pharmacologically active acyl glucuronide metabolite of mycophenolic acid in pediatric renal transplant recipients. Ther Drug Monit 2002; 24(3): 390–9
Bunchman T, Navarro M, Broyer M, et al. The use of mycophenolate mofetil suspension in paediatric renal allograft recipients. Pediatric Nephrol 2001; 16: 978–84
Pescovitz MD, Bumgardner G, Gaston RS, et al. Pharmacokinetics of daclizumab and mycophenolate mofetil with cyclosporine and steroids in renal transplantation. Clin Transplant 2003; 17(6): 511–7
Brown NW, Aw MM, Mieli-Vergani G, et al. Mycophenolic acid and mycophenolic acid glucuronide pharmacokinetics in pediatric liver transplant recipients: effect of cyclosporine and tacrolimus comedication. Ther Drug Monit 2002; 24(5): 598–606
Sollinger HW, Deierhoi MH, Belzer FO, et al. RS-61443: a phase I clinical trial and pilot rescue study. Transplantation 1992; 53(2): 428–32
Behrend M, Braun F. Enteric-coated mycophenolate sodium: tolerability profile compared with mycophenolate mofetil. Drugs 2005; 65(8): 1037–50
Ettenger R, Bartosh S, Choi L, et al. Pharmacokinetics of enteric-coated mycophenolate sodium in stable pediatric renal transplant recipients. Pediatr Transplant 2005; 9(6): 780–7
Holt DW. Monitoring mycophenolic acid. Ann Clin Biochem 2002; 39(Pt 3): 173–83
Shum B, Duffull SB, Taylor PJ, et al. Population pharmacokinetic analysis of mycophenolic acid in renal transplant recipients following oral administration of mycophenolate mofetil. Br J Clin Pharmacol 2003; 56(2): 188–97
Shaw LM, Korecka M, van Breeman R, et al. Analysis, pharmacokinetics and therapeutic drug monitoring of mycophenolic acid. Clin Biochem 1998; 31(5): 323–8
Shaw LM, Kaplan B, DeNofrio D, et al. Pharmacokinetics and concentration-control investigations of mycophenolic acid in adults after transplantation. Ther Drug Monit 2000; 22(1): 14–9
Staatz CE, Duffull SB, Kiberd B, et al. Population pharmacokinetics of mycophenolic acid during the first week after renal transplantation. Eur J Clin Pharmacol 2005; 61(7): 507–16
Johnson HJ, Swan SK, Heim-Duthoy KL, et al. The pharmacokinetics of a single oral dose of mycophenolate mofetil in patients with varying degrees of renal function. Clin Pharmacol Ther 1998; 63(5): 512–8
Shaw LM, Korecka M, Aradhye S, et al. Mycophenolic acid area under the curve values in African American and Caucasian renal transplant patients are comparable. J Clin Pharmacol 2000; 40(6): 624–33
Mudge DW, Atcheson BA, Taylor PJ, et al. Severe toxicity associated with a markedly elevated mycophenolic acid free fraction in a renal transplant recipient. Ther Drug Monit 2004; 26(4): 453–5
Parker G, Bullingham R, Kamm B, et al. Pharmacokinetics of oral mycophenolate mofetil in volunteer subjects with varying degrees of hepatic oxidative impairment. J Clin Pharmacol 1996; 36(4): 332–44
Jain AB, Hamad I, Zuckerman S, et al. Effect of t-tube clamping on the pharmacokinetics of mycophenolic acid in liver transplant patients on oral therapy of mycophenolate mofetil. Liver Transpl Surg 1999; 5(2): 101–6
Bullingham R, Shah J, Goldblum R, et al. Effects of food and antacid on the pharmacokinetics of single doses of mycophenolate mofetil in rheumatoid arthritis patients. Br J Clin Pharmacol 1996; 41(6): 513–6
Morgera S, Budde K, Lampe D, et al. Mycophenolate mofetil pharmacokinetics in renal transplant recipients on peritoneal dialysis. Transpl Int 1998; 11(1): 53–7
Neylan JF. Immunosuppressive therapy in high-risk transplant patients: dose-dependent efficacy of mycophenolate mofetil in African-American renal allograft recipients. US Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1997; 64(9): 1277–82
Schweitzer EJ, Yoon S, Fink J, et al. Mycophenolate mofetil reduces the risk of acute rejection less in African-American than in Caucasian kidney recipients. Transplantation 1998; 65(2): 242–8
Pescovitz MD, Guasch A, Gaston R, et al. Equivalent pharmacokinetics of mycophenolate mofetil in African-American and Caucasian male and female stable renal allograft recipients. Am J Transplant 2003; 3(12): 1581–6
Le Guellec C, Bourgoin H, Buchler M, et al. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in stable renal transplant patients. Clin Pharmacokinet 2004; 43(4): 253–66
van Hest RM, van Gelder T, Vulto AG, et al. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet 2005; 44(10): 1083–96
Morissette P, Albert C, Busque S, et al. In vivo higher glucuronidation of mycophenolic acid in male than in female recipients of a cadaveric kidney allograft and under immunosuppressive therapy with mycophenolate mofetil. Ther Drug Monit 2001; 23(5): 520–5
Gerbase MW, Fathi M, Spiliopoulos A, et al. Pharmacokinetics of mycophenolic acid associated with calcineurin inhibitors: long-term monitoring in stable lung recipients with and without cystic fibrosis. J Heart Lung Transplant 2003; 22(5): 587–90
van Hest RM, Mathot RA, Vulto AG, et al. Mycophenolic acid in diabetic renal transplant recipients: pharmacokinetics and application of a limited sampling strategy. Ther Drug Monit 2004; 26(6): 620–5
Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 1998; 64(6): 672–83
Oellerich M, Shipkova M, Schutz E, et al. Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit 2000; 22(1): 20–6
Kuypers DR, Claes K, Evenepoel P, et al. Long-term changes in mycophenolic acid exposure in combination with tacrolimus and corticosteroids are dose dependent and not reflected by trough plasma concentration: a prospective study in 100 de novo renal allograft recipients. J Clin Pharmacol 2003; 43(8): 866–80
Buchler M, Lebranchu Y, Beneton M, et al. Higher exposure to mycophenolic acid with sirolimus than with cyclosporine co-treatment. Clin Pharmacol Ther 2005; 78(1): 34–42
van Gelder T, Klupp J, Barten MJ, et al. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit 2001; 23(2): 119–28
Pou L, Brunet M, Cantarell C, et al. Mycophenolic acid plasma concentrations: influence of comedication. Ther Drug Monit 2001; 23(1): 35–8
Filler G, Zimmering M, Mai I. Pharmacokinetics of mycophenolate mofetil are influenced by concomitant immunosuppression. Pediatr Nephrol 2000; 14(2): 100–4
Picard N, Premaud A, Rousseau A, et al. A comparison of the effect of cyclosporin and sirolimus on the pharmokinetics of mycophenolate in renal transplant patients. Br J Clin Pharmacol 2006 Oct; 62(4): 477–84
Deters M, Kirchner G, Koal T, et al. Influence of cyclosporine on the serum concentration and biliary excretion of mycophenolic acid and 7-O-mycophenolic acid glucuronide. Ther Drug Monit 2005; 27(2): 132–8
El Haggan W, Ficheux M, Debruyne D, et al. Pharmacokinetics of mycophenolic acid in kidney transplant patients receiving sirolimus versus cyclosporine. Transplant Proc 2005; 37(2): 864–6
Gregoor PJ, de Sevaux RG, Hene RJ, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation 1999; 68(10): 1603–6
Hohage H, Zeh M, Heck M, et al. Differential effects of cyclosporine and tacrolimus on mycophenolate pharmacokinetics in patients with impaired kidney function. Transplant Proc 2005; 37(4): 1748–50
Hubner GI, Eismann R, Sziegoleit W. Drug interaction between mycophenolate mofetil and tacrolimus detectable within therapeutic mycophenolic acid monitoring in renal transplant patients. Ther Drug Monit 1999; 21(5): 536–9
Kaplan B, Meier-Kriesche HU, Minnick P, et al. Randomized calcineurin inhibitor cross over study to measure the pharmacokinetics of co-administered enteric-coated mycophenolate sodium. Clin Transplant 2005; 19(4): 551–8
Shipkova M, Armstrong VW, Kuypers D, et al. Effect of cyclosporine withdrawal on mycophenolic acid pharmacokinetics in kidney transplant recipients with deteriorating renal function: preliminary report. Ther Drug Monit 2001; 23(6): 717–21
Smak Gregoor PJ, van Gelder T, Hesse CJ, et al. Mycophenolic acid plasma concentrations in kidney allograft recipients with or without cyclosporin: a cross-sectional study. Nephrol Dial Transplant 1999; 14(3): 706–8
Kiberd BA, Puthenparumpil JJ, Fraser A, et al. Impact of mycophenolate mofetil loading on drug exposure in the early posttransplant period. Transplant Proc 2005; 37(5): 2320–3
Kuriata-Kordek M, Boratynska M, Falkiewicz K, et al. The influence of calcineurin inhibitors on mycophenolic acid pharmacokinetics. Transplant Proc 2003; 35(6): 2369–71
Zucker K, Rosen A, Tsaroucha A, et al. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transpl Immunol 1997; 5(3): 225–32
Cremers S, Schoemaker R, Scholten E, et al. Characterizing the role of enterohepatic recycling in the interactions between mycophenolate mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling. Br J Clin Pharmacol 2005; 60(3): 249–56
Cattaneo D, Perico N, Gaspari F, et al. Glucocorticoids interfere with mycophenolate mofetil bioavailability in kidney transplantation. Kidney Int 2002; 62(3): 1060–7
Mudge DW, Atcheson B, Taylor PJ, et al. The effect of oral iron administration on mycophenolate mofetil absorption in renal transplant recipients: a randomized, controlled trial. Transplantation 2004; 77(2): 206–9
Lorenz M, Wolzt M, Weigel G, et al. Ferrous sulfate does not affect mycophenolic acid pharmacokinetics in kidney transplant patients. Am J Kidney Dis 2004; 43(6): 1098–103
Mai I, Stormer E, Bauer S, et al. Impact of St John’s wort treatment on the pharmacokinetics of tacrolimus and mycophenolic acid in renal transplant patients. Nephrol Dial Transplant 2003; 18(4): 819–22
Lu XY, Huang HF, Sheng-Tu JZ, et al. Pharmacokinetics of mycophenolic acid in Chinese kidney transplant patients. J Zhejiang Univ Sci B 2005; 6(9): 885–91
Kuypers DR, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate-glucuronosyltransferase. Clin Pharmacol Ther 2005; 78(1): 81–8
Pieper AK, Buhle F, Bauer S, et al. The effect of sevelamer on the pharmacokinetics of cyclosporin A and mycophenolate mofetil after renal transplantation. Nephrol Dial Transplant 2004; 19(10): 2630–3
Zucker K, Tsaroucha A, Olson L, et al. Evidence that tacrolimus augments the bioavailability of mycophenolate mofetil through the inhibition of mycophenolic acid glucuronidation. Ther Drug Monit 1999; 21(1): 35–43
Hesselink DA, van Hest RM, Mathot RA, et al. Cyclosporine interacts with mycophenolic Acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant 2005; 5(5): 987–94
Vidal E, Cantarell C, Capdevila L, et al. Mycophenolate mofetil pharmacokinetics in transplant patients receiving cyclosporine or tacrolimus in combination therapy. Pharmacol Toxicol 2000; 87(4): 182–4
Meiser BM, Groetzner J, Kaczmarek I, et al. Tacrolimus or cyclosporine: which is the better partner for mycophenolate mofetil in heart transplant recipients? Transplantation 2004; 78(4): 591–8
Smak Gregoor PJ, de Sevaux RG, Hene RJ, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation 1999; 68(10): 1603–6
Kato R, Ooi K, Ikura-Mori M, et al. Impairment of mycophenolate mofetil absorption by calcium polycarbophil. J Clin Pharmacol 2002; 42(11): 1275–80
Morii M, Ueno K, Ogawa A, et al. Impairment of mycophenolate mofetil absorption by iron ion. Clin Pharmacol Ther 2000; 68(6): 613–6
Gimenez F, Foeillet E, Bourdon O, et al. Evaluation of pharmacokinetic interactions after oral administration of mycophenolate mofetil and valaciclovir or aciclovir to healthy subjects. Clin Pharmacokinet 2004; 43(10): 685–92
Wolfe EJ, Mathur V, Tomlanovich S, et al. Pharmacokinetics of mycophenolate mofetil and intravenous ganciclovir alone and in combination in renal transplant recipients. Pharmacotherapy 1997; 17(3): 591–8
Schmidt LE, Rasmussen A, Norrelykke MR, et al. The effect of selective bowel decontamination on the pharmacokinetics of mycophenolate mofetil in liver transplant recipients. Liver Transpl 2001; 7(8): 739–42
Girard H, Court MH, Bernard O, et al. Identification of common polymorphisms in the promoter of the UGT1A9 gene: evidence that UGT1A9 protein and activity levels are strongly genetically controlled in the liver. Pharmacogenetics 2004; 14(8): 501–15
Cattaneo D, Perico N, Remuzzi G. From pharmacokinetics to pharmacogenomics: a new approach to tailor immunosuppressive therapy. Am J Transplant 2004; 4(3): 299–310
Del Tacca M. Prospects for personalized immunosuppression: pharmacologic tools: a review. Transplant Proc 2004; 36(3): 687–9
Fisher MB, Paine MF, Strelevitz TJ, et al. The role of hepatic and extrahepatic UDP-glucuronosyltransferases in human drug metabolism. Drug Metab Rev 2001; 33(3-4): 273–97
Villeneuve L, Girard H, Fortier LC, et al. Novel functional polymorphisms in the UGT1A7 and UGT1A9 glucuronidating enzymes in Caucasian and African-American subjects and their impact on the metabolism of 7-ethyl-10-hydroxy-camptothecin and flavopiridol anticancer drugs. J Pharmacol Exp Ther 2003; 307(1): 117–28
Jinno H, Saeki M, Saito Y, et al. Functional characterization of human UDP-glucuronosyltransferase 1A9 variant, D256N, found in Japanese cancer patients. J Pharmacol Exp Ther 2003; 306(2): 688–93
Beal JL, Jones CE, Taylor PJ, et al. Evaluation of an immunoassay (EMIT) for mycophenolic acid in plasma from renal transplant recipients compared with a high-performance liquid chromatography assay. Ther Drug Monit 1998; 20(6): 685–90
Premaud A, Debord J, Rousseau A, et al. A double absorptionphase model adequately describes mycophenolic acid plasma profiles in de novo renal transplant recipients given oral mycophenolate mofetil. Clin Pharmacokinet 2005; 44(8): 837–47
Payen S, Zhang D, Maisin A, et al. Population pharmacokinetics of mycophenolic acid in kidney transplant pediatric and adolescent patients. Ther Drug Monit 2005; 27(3): 378–88
Tredger JM, Brown NW, Adams J, et al. Monitoring mycophenolate in liver transplant recipients: toward a therapeutic range. Liver Transpl 2004; 10(4): 492–502
Kuypers DR, Claes K, Evenepoel P, et al. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther 2004; 75(5): 434–47
Pillans PI, Rigby RJ, Kubler P, et al. A retrospective analysis of mycophenolic acid and cyclosporin concentrations with acute rejection in renal transplant recipients. Clin Biochem 2001; 34(1): 77–81
Okamoto M, Wakabayashi Y, Higuchi A, et al. Therapeutic drug monitoring of mycophenolic acid in renal transplant recipients. Transplant Proc 2005; 37(2): 859–60
Lu YP, Lin B, Liang MZ, et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in Chinese kidney transplant recipients treated with mycophenolate mofetil. Transplant Proc 2004; 36(7): 2079–81
Kuriata-Kordek M, Boratynska M, Klinger M, et al. The efficacy of mycophenolate mofetil treatment in the prevention of acute renal rejection is related to plasma level of mycophenolic acid. Transplant Proc 2002; 34(7): 2985–7
van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999; 68(2): 261–6
Takahashi K, Ochiai T, Uchida K, et al. Pilot study of mycophenolate mofetil (RS-61443) in the prevention of acute rejection following renal transplantation in Japanese patients. RS-61443 Investigation Committee — Japan. Transplant Proc 1995; 27(1): 1421–4
Smak Gregoor PJ, van Gelder T, van Besouw NM, et al. Mycophenolic acid trough levels after kidney transplantation in a cyclosporine-free protocol. Transpl Int 2000; 13 Suppl. 1: S333–5
Krumme B, Wollenberg K, Kirste G, et al. Drug monitoring of mycophenolic acid in the early period after renal transplantation. Transplant Proc 1998; 30(5): 1773–4
Hesse CJ, Vantrimpont P, van Riemsdijk-van Overbeeke IC, et al. The value of routine monitoring of mycophenolic acid plasma levels after clinical heart transplantation. Transplant Proc 2001; 33(3): 2163–4
Meiser BM, Pfeiffer M, Schmidt D, et al. Combination therapy with tacrolimus and mycophenolate mofetil following cardiac transplantation: importance of mycophenolic acid therapeutic drug monitoring. J Heart Lung Transplant 1999; 18(2): 143–9
Yamani MH, Starling RC, Goormastic M, et al. The impact of routine mycophenolate mofetil drug monitoring on the treatment of cardiac allograft rejection. Transplantation 2000; 69(11): 2326–30
Gajarski RJ, Crowley DC, Zamberlan MC, et al. Lack of correlation between MMF dose and MPA level in pediatric and young adult cardiac transplant patients: does the MPA level matter? Am J Transplant 2004; 4(9): 1495–500
Weber LT, Schutz E, Lamersdorf T, et al. Therapeutic drug monitoring of total and free mycophenolic acid (MPA) and limited sampling strategy for determination of MPA-AUC in paediatric renal transplant recipients. The German Study Group on Mycophenolate Mofetil (MMF) Therapy. Nephrol Dial Transplant 1999; 14 Suppl. 4: 34–5
Cox VC, Ensom MH. Mycophenolate mofetil for solid organ transplantation: does the evidence support the need for clinical pharmacokinetic monitoring? Ther Drug Monit 2003; 25(2): 137–57
Smak Gregoor PJ, Hesse CJ, van Gelder T, et al. Relation of mycophenolic acid trough levels and adverse events in kidney allograft recipients. Transplant Proc 1998; 30(4): 1192–3
Cattaneo D, Gaspari F, Ferrari S, et al. Pharmacokinetics help optimizing mycophenolate mofetil dosing in kidney transplant patients. Clin Transplant 2001; 15(6): 402–9
Mourad M, Malaise J, Chaib Eddour D, et al. Pharmacokinetic basis for the efficient and safe use of low-dose mycophenolate mofetil in combination with tacrolimus in kidney transplantation. Clin Chem 2001; 47(7): 1241–8
Hubner GI, Eismann R, Sziegoleit W. Relationship between mycophenolate mofetil side effects and mycophenolic acid plasma trough levels in renal transplant patients. Arzneimittel Forschung 2000; 50(10): 936–40
Kaplan B, Gruber SA, Nallamathou R, et al. Decreased protein binding of mycophenolic acid associated with leukopenia in a pancreas transplant recipient with renal failure. Transplantation 1998; 65(8): 1127–9
Behrend M. Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf 2001; 24(9): 645–63
Shaw LM, Sollinger HW, Halloran P, et al. Mycophenolate mofetil: a report of the consensus panel. Ther Drug Monit 1995; 17(6): 690–9
Shaw LM, Nicholls A, Hale M, et al. Therapeutic monitoring of mycophenolic acid: a consensus panel report. Clin Biochem 1998; 31(5): 317–22
Shaw LM, Korecka M, Venkataramanan R, et al. Mycophenolic acid pharmacodynamics and pharmacokinetics provide a basis for rational monitoring strategies. Am J Transplant 2003; 3(5): 534–42
Nicholls AJ. Opportunities for therapeutic monitoring of mycophenolate mofetil dose in renal transplantation suggested by the pharmacokinetic/pharmacodynamic relationship for mycophenolic acid and suppression of rejection. Clin Biochem 1998; 31(5): 329–33
Willis C, Taylor PJ, Salm P, et al. Quantification of free mycophenolic acid by high-performance liquid chromatography-atmospheric pressure chemical ionisation tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 2000; 748(1): 151–6
Jones CE, Taylor PJ, Johnson AG. High-performance liquid chromatography determination of mycophenolic acid and its glucuronide metabolite in human plasma. J Chromatogr B Biomed Sci Appl 1998; 708(1-2): 229–34
Teshima D, Otsubo K, Kitagawa N, et al. High-performance liquid chromatographic method for mycophenolic acid and its glucuronide in serum and urine. J Clin Pharm Ther 2003; 28(1): 17–22
Streit F, Shipkova M, Armstrong VW, et al. Validation of a rapid and sensitive liquid chromatography-tandem mass spectrometry method for free and total mycophenolic acid. Clin Chem 2004; 50(1): 152–9
Atcheson B, Taylor PJ, Mudge DW, et al. Quantification of free mycophenolic acid and its glucuronide metabolite in human plasma by liquid-chromatography using mass spectrometric and ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci 2004; 799(1): 157–63
Premaud A, Rousseau A, Le Meur Y, et al. Comparison of liquid chromatography-tandem mass spectrometry with a commercial enzyme-multiplied immunoassay for the determination of plasma MPA in renal transplant recipients and consequences for therapeutic drug monitoring. Ther Drug Monit 2004; 26(6): 609–19
Schutz E, Shipkova M, Armstrong VW, et al. Therapeutic drug monitoring of mycophenolic acid: comparison of HPLC and immunoassay reveals new MPA metabolites. Transplant Proc 1998; 30(4): 1185–7
Shipkova M, Schutz E, Armstrong VW, et al. Overestimation of mycophenolic acid by EMIT correlates with MPA metabolite. Transplant Proc 1999; 31(1-2): 1135–7
Filler G, Mai I. Limited sampling strategy for mycophenolic acid area under the curve. Ther Drug Monit 2000; 22(2): 169–73
Willis C, Taylor PJ, Salm P, et al. Evaluation of limited sampling strategies for estimation of 12-hour mycophenolic acid area under the plasma concentration-time curve in adult renal transplant patients. Ther Drug Monit 2000; 22(5): 549–54
Yeung S, Tong KL, Tsang WK, et al. Determination of mycophenolate area under the curve by limited sampling strategy. Transplant Proc 2001; 33(1–2): 1052–3
Pawinski T, Hale M, Korecka M, et al. Limited sampling strategy for the estimation of mycophenolic acid area under the curve in adult renal transplant patients treated with concomitant tacrolimus. Clin Chem 2002; 48(9): 1497–504
Schutz E, Armstrong VW, Shipkova M, et al. Limited sampling strategy for the determination of mycophenolic acid area under the curve in pediatric kidney recipients. German Study Group on MMF Therapy in Pediatric Renal Transplant Recipients. Transplant Proc 1998; 30(4): 1182–4
Premaud A, Le Meur Y, Debord J, et al. Maximum a posteriori bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit 2005; 27(3): 354–61
Budde K, Glander P, Bauer S, et al. Pharmacodynamic monitoring of mycophenolate mofetil. Clin Chem Lab Med 2000; 38(11): 1213–6
van Gelder T. Mycophenolate mofetil: how to further improve using an already successful drug? Am J Transplant 2005; 5(2): 199–200
Weigel G, Griesmacher A, Karimi A, et al. Effect of mycophenolate mofetil therapy on lymphocyte activation in heart trans plant recipients. J Heart Lung Transplant 2002; 21(10): 1074–9
Sanquer S, Breil M, Baron C, et al. Induction of inosine monophosphate dehydrogenase activity after long-term treatment with mycophenolate mofetil. Clin Pharmacol Ther 1999; 65(6): 640–8
Goldsmith D, Carrey EA, Edbury S, et al. Mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase, causes a paradoxical elevation of GTP in erythrocytes of renal transplant patients. Clin Sci (Lond) 2004; 107(1): 63–8
Glander P, Hambach P, Braun KP, et al. Effect of mycophenolate mofetil on IMP dehydrogenase after the first dose and after long-term treatment in renal transplant recipients. Int J Clin Pharmacol Ther 2003; 41(10): 470–6
Jagodzinski P, Lizakowski S, Smolenski RT, et al. Mycophenolate mofetil treatment following renal transplantation decreases GTP concentrations in mononuclear leucocytes. Clin Sci (Lond) 2004; 107(1): 69–74
Glander P, Braun KP, Hambach P, et al. Non-radioactive determination of inosine 5′-monophosphate dehydro-genase (IMPDH) in peripheral mononuclear cells. Clin Biochem 2001; 34(7): 543–9
Glander P, Hambach P, Braun KP, et al. Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant 2004; 4(12): 2045–51
Vannozzi F, Filipponi F, Di Paolo A, et al. An exploratory study on pharmacogenetics of inosine-monophosphate dehydrogenase II in peripheral mononuclear cells from liver-transplant recipients. Transplant Proc 2004; 36(9): 2787–90
Kuypers DR. Immunosuppressive drug monitoring: what to use in clinical practice today to improve renal graft outcome. Transpl Int 2005; 18(2): 140–50
Acknowledgements
Dr Staatz would like to acknowledge financial support from an Australian National Health and Medical Research Council Neil Hamilton Fairley Fellowship. The authors have no pharmaceutical industry affiliation and have no pecuniary interests (personal or professional), grants or other potential conflicts of interest with any pharmaceutical company that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Staatz, C.E., Tett, S.E. Clinical Pharmacokinetics and Pharmacodynamics of Mycophenolate in Solid Organ Transplant Recipients. Clin Pharmacokinet 46, 13–58 (2007). https://doi.org/10.2165/00003088-200746010-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746010-00002