Abstract
Background: Induction of cytochrome P450 (CYP) 3A4 potentially reduces the blood concentrations of substrate drugs to less than one-tenth, which results in ineffective pharmacotherapy. Although the prediction of drug-drug interactions (DDIs) that are mediated by induction of CYP3A4 has been performed mainly on the basis of in vitro information, such methods have met with limited success in terms of their accuracy and applicability. Therefore, a realistic method for the prediction of CYP3A4-mediated inductive DDIs is of major clinical importance.
Objective: The objective of the present study was to construct a robust and accurate method for the prediction of CYP3A4-mediated inductive DDIs. Such a method was developed on the basis of the principle applied for prediction of inhibitory DDIs in a previous report. A unique character of this principle is that the extent of alterations in the area under the plasma concentration-time curve (AUC) is predicted on the basis of in vivo information from minimal clinical studies without using in vitro data.
Methods: The analysis is based on 42 DDI studies in humans reported in 37 published articles over the period 1983–2007. Kinetic analysis revealed that the reduction in the AUC of a substrate of CYP3A4 produced by consecutive administration of an inducer of CYP3A4 could be approximated by the equation 1/(1 + CRCYP3A4 · ICCYP3A4), where CRCYP3A4 is the ratio of the apparent contribution of CYP3A4 to the oral clearance of a substrate and ICCYP3A4 is the apparent increase in clearance of a substrate produced by induction of CYP3A4. Using this equation, the ICCYP3A4 was calculated for seven inducers (bosentan, carbamazepine, efavirenz, phenytoin, pioglitazone, rifampicin [rifampin], and St John’s wort [hypericum]) on the basis of the reduction in the AUC of a coadministered standard substrate of CYP3A4, such as simvastatin, in ten DDI studies. The CRCYP3A4 was calculated for 22 substrates on the basis of the previously reported method from inhibitory DDI studies using a potent CYP3A4 inhibitor such as itraconazole or ketoconazole.
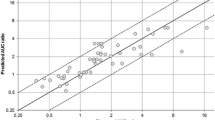
Results: The proposed method enabled the prediction of AUC reduction by CYP3A4 induction with any combination of these substrates and inducers (total 154 matches). To assess the accuracy of the prediction, the AUC reductions in 32 studies were analysed. We found that the magnitude of the deviation between the mean values of the observed and predicted AUCs of all substrate drugs was <20% of the AUCs of the respective substrate drugs before administration of the inducers. In addition, rifampicin was found to be the most potent inducer among the compounds analysed in the present study, with an ICCYP3A4 value of 7.7, followed by phenytoin and carbamazepine, with values of 4.7 and 3.0, respectively. The ICCYP3A4 values of the other CYP3A4 inducers analysed in the present study were approximately 1 or less, which suggests that the AUCs of coadministered drugs may not be reduced to less than approximately half, even if the drug is metabolized solely by CYP3A4.
Conclusion: By using the method reported in the present study, the susceptibilities of a substrate drug of CYP3A4 to inductive DDIs can be predicted quantitatively. It was indicated that coadministration of rifampicin, Phenytoin and carbamazepine may reduce plasma AUCs to less than half for a broad range of CYP3A4 substrate drugs, with CRCYP3A4 values greater than 0.13, 0.21 and 0.33, respectively.







Similar content being viewed by others
References
Rogers JF, Nafziger AN, Bertino Jr JS. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. Am J Med 2002 Dec 15; 113(9): 746–50
Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev 1997 Feb-May; 29(1–2): 413–580
Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet 1998 Nov; 35(5): 361–90
Kanamitsu SI, Ito K, Okuda H, et al. Prediction of in vivo drug-drug interactions based on mechanism-based inhibition from in vitro data: inhibition of 5-fluoro-uracil metabolism by (E)-5-(2-Bromovinyl)uracil. Drug Metab Dispos 2000 Apr; 28(4): 467–74
Yamano K, Yamamoto K, Katashima M, et al. Prediction of midazolam-CYPSA inhibitors interaction in the human liver from in vivo/in vitro absorption, distribution, and metabolism data. Drug Metab Dispos 2001 Apr; 29 (4 Pt 1): 443–52
Galetin A, Burt H, Gibbons L, et al. Prediction of time-dependent CYP3A4 drug-drug interactions: impact of enzyme degradation, parallel elimination pathways, and intestinal inhibition. Drug Metab Dispos 2006 Jan; 34(1): 166–75
Bjornsson TD, Callaghan JT, Einolf HJ, et al. The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos 2003 Jul; 31(7): 815–32
Lehmann JM, McKee DD, Watson MA, et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 1998 Sep 1; 102(5): 1016–23
Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol 1999 Dec; 56(6): 1329–39
Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998 Jan-Feb; 18(1):84–112
Ripp SL, Mills JB, Fahmi OA, et al. Use of immortalized human hepatocytes to predict the magnitude of clinical drug-drug interactions caused by CYP3A4 induction. Drug Metab Dispos 2006 Oct; 34(10): 1742–8
Kato M, Chiba K, Horikawa M, et al. The quantitative prediction of in vivo enzyme-induction caused by drug exposure from in vitro information on human hepatocytes. Drug Metab Pharmacokinet 2005 Aug; 20(4): 236–43
Smith DA. Induction and drug development. Eur J Pharm Sci 2000 Sep; 11(3): 185–9
Lin JH. CYP induction-mediated drug interactions: in vitro assessment and clinical implications. Pharm Res 2006 Jun; 23(6): 1089–116
Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 2003; 42(9): 819–50
Aventis Pharmaceuticals Inc. Rifadin® (rifampin capsules USP) and Rifadin® IV (rifampin for injection USP) [product label]. Bridgewater (NJ): Aventis Pharmaceuticals Inc, 2004 Jan [online]. Available from URL: http://www.fda.gov/cder/foi/label/2004/50420s072,50627s008lbl.pdf [Accessed 2008 Jul 14]
Bristol-Myers Squibb Company. Sustiva® (efavirenz) capsules and tablets [product label]. Princeton (NJ): Bristol-Myers Squibb Company, 2007 Jan [online]. Available from URL: http://www.fda.gov/cder/foi/label/2007/020972-s029,021360s016lbl.pdf [Accessed 2008 Jul 14]
Ohno Y, Hisaka A, Suzuki H. General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet 2007; 46(8): 681–96
Hewitt NJ, Lecluyse EL, Ferguson SS. Induction of hepatic cytochrome P450 enzymes: methods, mechanisms, recommendations, and in vitro-in vivo correlations. Xenobiotica 2007 Oct–Nov; 37(10–11): 1196–224
Mikus G, Schowel V, Drzewinska M, et al. Potent cytochrome P450 2C19 genotype-related interaction between voriconazole and the cytochrome P450 3A4 inhibitor ritonavir. Clin Pharmacol Ther 2006 Aug; 80(2): 126–35
Abbott Laboratories. Norvir® (ritonavir capsules) soft gelatin (ritonavir oral solution) [product label]. North Chicago (IL): Abbott Laboratories, 2007 Jul [online]. Available from URL: http://www.fda.gov/cder/foi/label/2007/020659-s040,020945s020lbl.pdf [Accessed 2008 Jul 14]
Liu P, Foster G, Gandelman K, et al. Steady-state pharmacokinetic and safety profiles of voriconazole and ritonavir in healthy male subjects. Antimicrob Agents Chemother 2007 Oct; 51(10): 3617–26
Smith PF, DiCenzo R, Morse GD. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin Pharmacokinet 2001; 40(12): 893–905
Gerber JG, Rosenkranz SL, Fichtenbaum CJ, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr 2005 Jul 1; 39(3): 307–12
Whitten DL, Myers SP, Hawrelak JA, et al. The effect of St John’s wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol 2006 Nov; 62(5): 512–26
Brown HS, Ito K, Galetin A, et al. Prediction of in vivo drug-drug interactions from in vitro data: impact of incorporating parallel pathways of drug elimination and inhibitor absorption rate constant. Br J Clin Pharmacol 2005 Nov; 60(5): 508–18
Ito K, Hallifax D, Obach RS, et al. Impact of parallel pathways of drug elimination and multiple cytochrome P450 involvement on drug-drug interactions: CYP2D6 paradigm. Drug Metab Dispos 2005 Jun; 33(6): 837–44
Grimm SW, Richtand NM, Winter HR, et al. Effects of cytochrome P450 3A modulators ketoconazole and carbamazepine on quetiapine pharmacokinetics. Br J Clin Pharmacol 2006 Jan; 61(1): 58–69
Ridtitid W, Wongnawa M, Mahatthanatrakul W, et al. Ketoconazole increases plasma concentrations of antimalarial mefloquine in healthy human volunteers. J Clin Pharm Ther 2005 Jun; 30(3): 285–90
Jalava KM, Olkkola KT, Neuvonen PJ. Effect of itraconazole on the pharmacokinetics and pharmacodynamics of zopiclone. Eur J Clin Pharmacol 1996; 51(3–4): 331–4
Swaisland HC, Ranson M, Smith RP, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet 2005; 44(10): 1067–81
Araki K, Yasui-Furukori N, Fukasawa T, et al. Inhibition of the metabolism of etizolam by itraconazole in humans: evidence for the involvement of CYP3A4 in etizolam metabolism. Eur J Clin Pharmacol 2004 Aug; 60(6): 427–30
Dutreix C, Peng B, Mehring G, et al. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol 2004 Oct; 54(4): 290–4
Venkatakrishnan K, Greenblatt DJ, von Moltke LL, et al. Five distinct human cytochromes mediate amitriptyline N-demethylation in vitro: dominance of CYP 2C19 and 3A4. J Clin Pharmacol 1998 Feb; 38(2): 112–21
Miceli JJ, Anziano RJ, Robarge L, et al. The effect of carbamazepine on the steady-state pharmacokinetics of ziprasidone in healthy volunteers. Br J Clin Pharmacol 2000; 49 Suppl. 1: 65S–70S
Varis T, Kivisto KT, Neuvonen PJ. The effect of itraconazole on the pharmacokinetics and pharmacodynamics of oral prednisolone. Eur J Clin Pharmacol 2000 Apr; 56(1): 57–60
Lebrun-Vignes B, Archer VC, Diquet B, et al. Effect of itraconazole on the pharmacokinetics of prednisolone and methylprednisolone and Cortisol secretion in healthy subjects. Br J Clin Pharmacol 2001 May; 51(5): 443–50
Binet I, Wallnofer A, Weber C, et al. Renal hemodynamics and pharmacokinetics of bosentan with and without cyclosporine A. Kidney Int 2000 Jan; 57(1): 224–31
Dingemanse J, Schaarschmidt D, van Giersbergen PL. Investigation of the mutual pharmacokinetic interactions between bosentan, a dual endothelin receptor antagonist, and simvastatin. Clin Pharmacokinet 2003; 42(3): 293–301
Furukori H, Otani K, Yasui N, et al. Effect of carbamazepine on the single oral dose pharmacokinetics of alprazolam. Neuropsychopharmacology 1998 May; 18(5): 364–9
Cooney GF, Mochon M, Kaiser B, et al. Effects of carbamazepine on cyclosporine metabolism in pediatric renal transplant recipients. Pharmacotherapy 1995 May–Jun; 15(3): 353–6
Kondo S, Fukasawa T, Yasui-Furukori N, et al. Induction of the metabolism of etizolam by carbamazepine in humans. Eur J Clin Pharmacol 2005 May; 61(3): 185–8
Ucar M, Neuvonen M, Luurila H, et al. Carbamazepine markedly reduces serum concentrations of simvastatin and simvastatin acid. Eur J Clin Pharmacol 2004 Feb; 59(12): 879–82
Freeman DJ, Laupacis A, Keown PA, et al. Evaluation of cyclosporin-phenytoin interaction with observations on cyclosporin metabolites. Br J Clin Pharmacol 1984 Dec; 18(6): 887–93
Wong YW, Yeh C, Thyrum PT. The effects of concomitant phenytoin administration on the steady-state pharmacokinetics of quetiapine. J Clin Psychopharmacol 2001 Feb; 21(1): 89–93
Takeda Pharmaceuticals America, Inc. Actos® (pioglitazone hydrochloride) tablets [product label]. Deerfield (IL): Takeda Pharmaceuticals America, Inc., 2007 Feb [online]. Available from URL: http://www.fda.gov/cder/foi/label/2007/021073s026lbl.pdf [Accessed 2008 Jul 14]
Schmider J, Brockmoller J, Arold G, et al. Simultaneous assessment of CYP3A4 and CYP1A2 activity in vivo with alprazolam and caffeine. Pharmacogenetics 1999 Dec; 9(6): 725–34
Backman JT, Luurila H, Neuvonen M, et al. Rifampin markedly decreases and gemfibrozil increases the plasma concentrations of atorvastatin and its metabolites. Clin Pharmacol Ther 2005 Aug; 78(2): 154–67
Kivisto KT, Lamberg TS, Neuvonen PJ. Interactions of buspirone with itraconazole and rifampicin: effects on the pharmacokinetics of the active 1-(2-pyrimidinyl)-piperazine metabolite of buspirone. Pharmacol Toxicol 1999 Feb; 84(2): 94–7
Hebert MF, Roberts JP, Prueksaritanont T, et al. Bioavailability of cyclosporine with concomitant rifampin administration is markedly less than predicted by hepatic enzyme induction. Clin Pharmacol Ther 1992 Nov; 52(5): 453–7
Bolton AE, Peng B, Hubert M, et al. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol 2004 Feb; 53(2): 102–6
Ridtitid W, Wongnawa M, Mahatthanatrakul W, et al. Effect of rifampin on plasma concentrations of mefloquine in healthy volunteers. J Pharm Pharmacol 2000 Oct; 52(10): 1265–9
Backman JT, Olkkola KT, Neuvonen PJ. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther 1996 Jan; 59(1): 7–13
Chung E, Nafziger AN, Kazierad DJ, et al. Comparison of midazolam and simvastatin as cytochrome P450 3A probes. Clin Pharmacol Ther 2006 Apr; 79(4): 350–61
Holtbecker N, Fromm MF, Kroemer HK, et al. The nifedipine-rifampin interaction: evidence for induction of gut wall metabolism. Drug Metab Dispos 1996 Oct; 24(10): 1121–3
McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed) 1983 Mar 19; 286(6369): 923–5
Löfdahl CG, Meilstrand T, Svedmyr N, et al. Increased metabolism of prednisolone and rifampicin after rifampicin treatment [abstract]. Am Rev Respir Dis 1984; 129: A201
Kyrklund C, Backman JT, Kivisto KT, et al. Rifampin greatly reduces plasma simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 2000 Dec; 68(6): 592–7
Shi J, Montay G, Bhargava VO. Clinical pharmacokinetics of telithromycin, the first ketolide antibacterial. Clin Pharmacokinet 2005; 44(9): 915–34
Villikka K, Kivisto KT, Backman JT, et al. Triazolam is ineffective in patients taking rifampin. Clin Pharmacol Ther 1997 Jan; 61(1): 8–14
Villikka K, Kivisto KT, Luurila H, et al. Rifampin reduces plasma concentrations and effects of Zolpidem. Clin Pharmacol Ther 1997 Dec; 62(6): 629–34
Villikka K, Kivisto KT, Lamberg TS, et al. Concentrations and effects of zopiclone are greatly reduced by rifampicin. Br J Clin Pharmacol 1997 May; 43(5): 471–4
Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003 Sep 17; 290(11): 1500–4
Johne A, Schmider J, Brockmoller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol 2002 Feb; 22(1): 46–54
Bauer S, Stornier E, Johne A, et al. Alterations in cyclosporin A pharmacokinetics and metabolism during treatment with St John’s wort in renal transplant patients. Br J Clin Pharmacol 2003 Feb; 55(2): 203–11
Dresser GK, Schwarz UI, Wilkinson GR, et al. Coordinate induction of both cytochrome P4503A and MDR1 by St John’s wort in healthy subjects. Clin Pharmacol Ther 2003 Jan; 73(1): 41–50
Mai I, Bauer S, Perloff ES, et al. Hyperforin content determines the magnitude of the St John’s wort-cyclosporine drug interaction. Clin Pharmacol Ther 2004 Oct; 76(4): 330–40
Smith PF, Bullock JM, Booker BM, et al. Induction of imatinib metabolism by Hypericum perforatum. Blood 2004 Aug 15; 104(4): 1229–30
Wang Z, Gorski JC, Hamman MA, et al. The effects of St John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther 2001 Oct; 70(4): 317–26
Hall SD, Wang Z, Huang SM, et al. The interaction between St John’s wort and an oral contraceptive. Clin Pharmacol Ther 2003 Dec; 74(6): 525–35
Yang J, Tucker GT, Rostami-Hodjegan A. Cytochrome P450 3A expression and activity in the human small intestine. Clin Pharmacol Ther 2004 Oct; 76(4): 391
Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 1997 Dec; 283(3): 1552–62
Suzuki H, Sugiyama Y. Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur J Pharm Sci 2000 Nov; 12(1): 3–12
Wacher VJ, Silverman JA, Zhang Y, et al. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J Pharm Sci 1998 Nov; 87(11): 1322–30
Saitoh H, Aungst BJ. Possible involvement of multiple P-glycoprotein-mediated efflux systems in the transport of verapamil and other organic cations across rat intestine. Pharm Res 1995 Sep; 12(9): 1304–10
Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem 2001 May 4; 276(18): 14581–7
Combalbert J, Fabre I, Fabre G, et al. Metabolism of cyclosporin A: IV. Purification and identification of the rifampicin-inducible human liver cytochrome P-450 (cyclosporin A oxidase) as a product of P450IIIA gene subfamily. Drug Metab Dispos 1989 Mar–Apr; 17(2): 197–207
Ged C, Rouillon JM, Pichard L, et al. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol 1989 Oct; 28(4): 373–87
Glaeser H, Drescher S, Eichelbaum M, et al. Influence of rifampicin on the expression and function of human intestinal cytochrome P450 enzymes. Br J Clin Pharmacol 2005 Feb; 59(2): 199–206
Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest 1999 Jul; 104(2): 147–53
Hamman MA, Bruce MA, Haehner-Daniels BD, et al. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther 2001 Mar; 69(3): 114–21
Westphal K, Weinbrenner A, Zschiesche M, et al. Induction of P-glycoprotein by rifampin increases intestinal secretion of talinolol in human beings: a new type of drug/drug interaction. Clin Pharmacol Ther 2000 Oct; 68(4): 345–55
Zhang H, Cui D, Wang B, et al. Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: a new look at an old drug. Clin Pharmacokinet 2007; 46(2): 133–57
Sinofsky FE, Pasquale SA. The effect of fluconazole on circulating ethinyl estradiol levels in women taking oral contraceptives. Am J Obstet Gynecol 1998 Feb; 178(2): 300–4
Hilbert J, Messig M, Kuye O, et al. Evaluation of interaction between fluconazole and an oral contraceptive in healthy women. Obstet Gynecol 2001 Aug; 98(2): 218–23
Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet 2000 Feb; 38(2): 111–80
Barditch-Crovo P, Trapnell CB, Ette E, et al. The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clin Pharmacol Ther 1999 Apr; 65(4): 428–38
Guengerich FP. Metabolism of 17 alpha-ethynylestradiol in humans. Life Sci 1990; 47(22): 1981–8
Li AP, Hartman NR, Lu C, et al. Effects of cytochrome P450 inducers on 17alpha-ethinyloestradiol (EE2) conjugation by primary human hepatocytes. Br J Clin Pharmacol 1999 Nov; 48(5): 733–42
Shimizu M, Fuse K, Okudaira K, et al. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos 2005 Oct; 33(10): 1477–81
Lau YY, Huang Y, Frassetto L, et al. effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther 2007 Feb; 81(2): 194–204
Hirano M, Maeda K, Shitara Y, et al. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos 2006 Jul; 34(7): 1229–36
Ernest II CS, Hall SD, Jones DR. Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J Pharmacol Exp Ther 2005 Feb; 312(2): 583–91
Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol 1997 Aug; 44(2): 190–4
Perloff ES, Duan SX, Skolnik PR, et al. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos 2005 Jun; 33(6): 764–70
Zhou S, Yung Chan S, Cher Goh B, et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet 2005; 44(3): 279–304
Pichard L, Fabre I, Fabre G, et al. Cyclosporin A drug interactions: screening for inducers and inhibitors of cytochrome P-450 (cyclosporine A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos 1990 Sep–Oct; 18(5): 595–606
Kostrubsky VE, Ramachandran V, Venkataramanan R, et al. The use of human hepatocyte cultures to study the induction of cytochrome P-450. Drug Metab Dispos 1999 Aug; 27(8): 887–94
Li AP, Reith MK, Rasmussen A, et al. Primary human hepatocytes as a tool for the evaluation of structure-activity relationship in cytochrome P450 induction potential of xenobiotics: evaluation of rifampin, rifapentine and rifabutin. Chem Biol Interact 1997 Nov 6; 107(1–2): 17–30
Acknowledgements
This study was supported by Health and Labour Sciences Research Grants for Research on Regulatory Science of Pharmaceuticals and Medical Devices from the Ministry of Health, Labour and Welfare, Japan. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohno, Y., Hisaka, A., Ueno, M. et al. General Framework for the Prediction of Oral Drug Interactions Caused by CYP3A4 Induction from In Vivo Information. Clin Pharmacokinet 47, 669–680 (2008). https://doi.org/10.2165/00003088-200847100-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200847100-00004