Abstract
-
▴ Dexmedetomidine is a potent α2-adrenoceptor agonist with 8 times higher affinity for the α2-adrenoceptorthan clonidine.
-
▴ Dexmedetomidine has shown sedative, analgesic and anxiolytic effects after intravenous administration to healthy volunteers or postsurgical patients in the intensive care unit.
-
▴ Dexmedetomidine produced a predictable haemo-dynamic decline (dose-dependently decreased arterial blood pressure and heart rate) in postsurgical patients coinciding with reductions in plasma catecholamines.
-
▴ In phase III clinical trials, dexmedetomidine 0.2 to 0.7 µg/kg/h produced clinically effective sedation and significantly reduced the analgesic requirements of postsurgical ventilated intensive care unit patients. There was no clinically apparent respiratory depression after cessation of assisted ventilation.
-
▴ Dexmedetomidine produced rapid and stable sedation in postsurgical ventilated patients while maintaining a high degree of patient reusability and anxiety reduction.
-
▴ Dexmedetomidine was well tolerated in phase III studies. The most frequently observed adverse events were hypotension, bradycardia and nausea.
Similar content being viewed by others


The intensive care unit (ICU) is a stressful environment in which postsurgical mechanically ventilated patients with life-threatening concerns often experience anxiety, pain and sleep deprivation.[1] The primary goals in the treatment of patient comfort are to achieve sedation (while maintaining rousability and cooperation), analgesia and anxiolysis with minimal haemodynamic and respiratory effects.
Current treatments of choice in the ICU include a selection from sedatives (e.g. propofol, midazolam) and analgesics (e.g. morphine), as no single agent is suitable for each individual’s needs. These therapies are associated with limitations including respiratory depression, lack of orientation, severe hypotension and gastrointestinal hypomotility. Numerous studies have highlighted the desirable properties of α2-adrenergic agonists for sedation in ICU patients. α2-Adrenergic agonists produce sedative, analgesic and anxiolytic effects along with haemodynamic stabilisation through α2-adrenoceptor activity in the central nervous system (CNS).[2] Clonidine, the prototypical α2-adrenergic agonist (partial) has been shown to reduce anaesthetic requirements during surgery.[3,4]
1. Pharmacodynamic Profile
Mechanism of Action
• Dexmedetomidine, an imidazole compound, is the pharmacologically active dextroisomer of medetomidine,[5] which displays specific and selective α2-adrenoceptor agonism. Dexmedetomidine is 8 times more specific for α2-adrenoceptors than clonidine.[6] The actions of dexmedetomidine are suggested to be mediated through postsynaptic α2-adrenoceptors which activate pertussis toxin-sensitive G proteins,[7] thereby increasing conductance through potassium ion channels. Studies in transgenic mice have demonstrated that the α2A-adrenoceptor subtype is responsible for relaying the sedative and analgesic properties of dexmedetomidine.[8]
Sedative Effects in Volunteers
• In a phase I, placebo-controlled study, a dose-dependent increase in sedation was seen with a 24-hour maintenance infusion of dexmedetomidine (target plasma concentrations of 0.3, 0.6 and 1.25 µg/L) in 24 healthy individuals.[9] Sedation was assessed using standard sedation scoring scales: the Visual Analogue Scale for Sedation, Ramsay Sedation Score and the Critical Flicker Fusion (CFF) threshold. Target dexmedetomidine concentrations of 0.6 and 1.25 µg/L induced a deeper level and longer duration of sedation than 0.3 µg/L and placebo and produced sedation more rapidly than with 0.3 µg/L (15 and 20 vs 33 minutes). The CFF results were similar between groups, indicating dexmedetomidine-treated individuals were easily roused and cooperative despite having achieved clinically effective sedation, which is unique compared with currently available sedatives.
• The sedative effect of dexmedetomidine was not altered by concurrent administration of esmolol (β-adrenoceptor blocker).[10] Healthy volunteers (n = 36) randomly received a 60-minute infusion of placebo or dexmedetomidine (target plasma concentrations 0.3 or 0.6 µg/L) plus esmolol 100 µg/kg/min at 30 minutes. No clinically significant effects were seen during combined treatment with esmolol and dexmedetomidine compared with esmolol plus placebo administration.[10]
Haemodynamic and Respiratory Effects
• Consistent with the pharmacological effect of other α2-adrenoceptor agonists, dexmedetomidine 1 µg/kg administered as a 2-minute infusion to 6 healthy male volunteers caused significant maximum reductions in heart rate and blood pressure (17 and 23%, respectively, p ≪ 0.05 vs baseline). The haemodynamic declines coincided with reductions in plasma levels of noradrenaline and adrenaline.[11] In 2 phase III trials patients receiving dexmedetomidine 0.2 to 0.7 µg/kg/h consistently had larger mean decreases in blood pressure and heart rate during the infusion than placebo recipients.[12] Return to baseline levels was seen within 6 hours of treatment cessation with no apparent rebound effects.
• Dexmedetomidine 2.0 µg/kg, administered as a 2-minute infusion to 37 healthy males, produced a slight increase in carbon dioxide partial pressure (pCO2) and a decrease in minute ventilation with minimal change in ventilatory frequency.[13] In this double-blind placebo-controlled study, the pCO2 increased from 41.9 to a maximum of 46.1mm Hg within 10 minutes (p ≪ 0.05 vs baseline) and gradually subsided thereafter. A mild decrease in minute ventilation occurred after 60 minutes (8.7 to 6.3 L/min, p ≪ 0.05).[13]
• In a phase I study, intravenous dexmedetomidine did not cause respiratory depression in volunteers who received a 24-hour maintenance infusion (target plasma concentrations ranging from 0.3 to 1.25 µg/L).[14] Oxygen saturation (SpO2) remained ≥90% in all individuals. Similarly, dexmedetomidine and placebo recipients had SpO2 values within the normal range in 2 phase III studies.[12] In addition, there was no statistically significant difference between the dexmedetomidine and the placebo group in respiratory rate after extubation.
Other Effects
• Intravenous infusion of dexmedetomidine (target plasma concentration 0.3 or 0.6 µg/L) reduced the vasoconstriction threshold by up to 1.4 °C · µg/L and the shivering threshold by up to 2.0 °C · µg/L compared with placebo, but had no appreciable effect on the sweating threshold in 9 male volunteers.[15] In this randomised, double-blind, crossover study, dexmedetomidine linearly decreased the concentration-response curves for both vasoconstriction and shivering.
• In a double-blind, placebo-controlled trial, the anaesthetic requirements of dexmedetomidine-treated patients undergoing surgery was significantly reduced compared with placebo.[16] Patients (n = 20) were randomised to receive placebo or dexmedetomidine as a 2-stage intravenous infusion (10-minute loading infusion of 1.7 µg/kg followed by a maintenance infusion of 0.6 µg/kg/h). Anaesthesia was induced with thiopental (4.0 mg/kg) and maintained with isoflurane in 70% nitrous oxide and oxygen. Dexmedetomidine reduced the median expired concentration of isoflurane by >90% compared with placebo (p = 0.01) during anaesthetic maintenance. In addition, dexmedetomidine-treated patients who required supplemental isoflurane had a reduced period of requirement compared with placebo-recipients (4 vs 55 min, p = 0.03).
2. Pharmacokinetic Profile
• Various studies have reported an approximately linear relationship between dexmedetomidine dose, the plasma concentration and area under the plasma concentration-time curve (AUC).[17,18] Continuous infusion of dexmedetomidine (for 4 hours) to maintain target plasma concentrations of 0.3 or 0.6 µg/L resulted in AUC values of 2.4 and 5.1 µg/L · h, respectively in 9 volunteers.[18]
• Pharmacokinetic variables were obtained from a phase I, multiple dose study in which dexmedetomidine (target plasma concentrations of 0.3 and 0.6 µg/L) was administered intravenously to 9 individuals.[18] Data obtained after administration of the higher dose, gave the half-life (t1/2) for the distribution (α) and elimination (β) phases as 9 minutes and 2 hours, respectively. Total body clearance (CL) was 0.495 L/h · kg, and the volume of distribution at steady state (Vss) was 1.33 L/kg. No dose-dependent effect on the t1/2, CL or Vss was observed.[18] Dexmedetomidine plasma concentrations for both doses decreased to pretreatment levels within 10 hours of infusion cessation. Values of 6 minutes and 2 hours are cited for t1/2α and t1/2β in the dexmedetomidine prescribing information.[19]
• Dexmedetomidine is markedly protein bound (94%) to serum albumin and α1-glycoprotein.[20] After extensive metabolism in the liver dexmedetomidine is eliminated as methyl and glucuronide conjugates, mainly (95%) via renal excretion.[20]
• The pharmacokinetics of dexmedetomidine (0.6 µg/kg infused over a 10-minute period) were markedly affected by hepatic insufficiency.[21] Patients with severe hepatic failure (n = 5) given dexmedetomidine showed significantly increased Vss (3.2 vs 2.2 L/kg) and t1/2β (7.5 vs 2.6 hours) and decreased CL (0.32 vs 0.64 L/h/kg) compared with 5 age-matched controls (all p ≪ 0.05).[21]
• Dexmedetomidine has been reported to inhibit cytochrome P450 enzyme systems in vitro.[22] Dexmedetomidine (0.002 to 0.8 µg/L) inhibited CYP2D6- dependent dextromethorphan O-demethylase (DEXTROase) activity in human liver microsomes. However, dexmedetomidine displayed reversible mixed (competitive/noncompetitive) inhibitor activity in this system and was less potent than quinidine, a clinically relevant standard CYP2D6 inhibitor (concentration of drug required for 50% inhibition, IC50 = 1.8 vs 0.22 µmol/L). Thus there appears to be little potential for dexmedetomidine to interact with other drugs which are metabolised by cytochrome P450 enzymes.
3. Therapeutic Trials
Two randomised, double-blind, placebo-controlled, multicentre, phase III studies[23,24] have assessed the efficacy of dexmedetomidine in postsurgical patients requiring mechanical ventilation and sedation in the ICU. In both studies, the drug was administered as a 2-stage intravenous infusion (10-minute loading infusion of 1 µg/kg followed by an infusion of 0.2 to 0.7 µg/kg/h to maintain a Ramsay sedation score of ≥3 during mechanical ventilation and ≥2 post-extubation). Treatment commenced within 1 hour of admission to the ICU and continued for at least 6 hours after extubation (maximum infusion period of 24 hours). Additional sedative (midazolam or propofol) and analgesic (morphine) agents were added as required and the primary outcome measure for the sedative and analgesic efficacy of dexmedetomidine was patient requirement for these additional medications. Results for the French arm of both these studies were also presented separately.[25]
Sedative Effects
• Dexmedetomidine provided clinically effective sedation in both phase III studies. Dexmedetomidine significantly reduced rescue sedation (midazolam[23] or propofol[24]) requirements compared with placebo in patients requiring postsurgical ventilation and sedation. In one study in 401 patients, the majority of dexmedetomidine recipients (60%) required no rescue sedation whereas the majority of placebo recipients (60%) required >50mg of propofol.[26] Small amounts of propofol (≪50mg) were required by an additional 21% of dexmedetomidine recipients and 15% of placebo recipients.[19] Propofol requirements throughout the study period were 7-fold lower in the dexmedetomidine than in the placebo group (72 vs 513mg, p ≪ 0.0001). In the other study involving 353 patients, most dexmedetomidine recipients (61%) received no rescue sedation; in contrast the majority of placebo recipients (56%) required >4mg of midazolam. An additional 20% of dexmedetomidine recipients and 19% of placebo recipients required only small doses of midazolam (≪4mg).[19] The total dose of midazolam needed during intubation was about 4-fold lower with dexmedetomidine than with placebo (4.83 vs 18.61mg, p = 0.001).[23] Results from the French arm of both multicentre studies also showed that significantly fewer dexmedetomidine than placebo recipients needed midazolam or propofol to maintain a Ramsay score of ≥3 during the period of ventilation, and of ≥2 thereafter (fig. 1).[25] These findings are supported by those from the entire propofol study population, although differences were not significant (fig. 1).
Sedative efficacy of dexmedetomidine (DEX) in postsurgical mechanically ventilated patients in the ICU. In randomised, double-blind placebo-controlled studies,[25,26] IV dexmedetomidine (1 mg/kg for 10 min then 0.2 to 0.7 mg/kg/h to maintain a Ramsay sedation score of ≥3) was started within 1 hour of admission and continued for ô6 hours after extubation. Rescue sedation was provided by midazolam 0.02 mg/kg IV[25] or propofol 0.2 mg/kg IV.[25,26] A total of 401 patients were evaluated in the study that used propofol.[26] Results from 77 patients from the French arm of this study and 48 patients from the French arm of the study using midazolam are also presented.[25] ICU= intensive care unit; IV = intravenous; * p ≪ 0.001 vs placebo.
Analgesic Effects
• Dexmedetomidine also significantly reduced rescue analgesic (morphine) requirements compared with placebo in postsurgical patients requiring mechanical ventilation and sedation in the ICU.[23,24] Dexmedetomidine-treated patients required almost 50% less morphine for pain during each study than did placebo recipients (fig. 2), and approximately 43% required no morphine compared with approximately 17% for placebo.[27]
Analgesic efficacy of dexmedetomidine (DEX) in postsurgical mechanically ventilated patients in the ICU. In 2 separate randomised, double-blind, placebo-controlled studies involving 754 patients,[23,24] IV DEX (1 mg/kg for 10 min then 0.2 to 0.7 mg/kg/h to maintain a Ramsay sedation score of ≥3) was started within 1 hour of admission and continued for ≥6 hours after extubation. The mean IV dose of morphine received for pain during each study period is shown. ICU = intensive care unit; IV = intravenous; * p ≪ 0.0001 vs placebo.
In both studies, dexmedetomidine recipients required less morphine for pain during study drug administration, during the first 6.5 hours of study drug administration and from 6.5 hours after study initiation to the end of the study drug administration.
Anxiolytic Effects
• Dexmedetomidine-treated patients experienced less anxiety and were easier to manage than placebo recipients in the 2 phase III studies.[23,24] The percentage of dexmedetomidine-treated patients with a Ramsay score of 1 (patients were anxious, agitated or restless) was significantly less than that seen with placebo (p ≪ 0.0001.[23,24] In addition, dexmedetomidine significantly reduced the Patient Management Index compared with placebo in both studies (p ≪ 0.05,[28] p ≪ 0.001[29]).
4. Tolerability
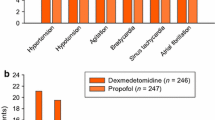
• According to preliminary results from a phase III study (n = 401)[30] the most common treatment-related adverse events associated with dexmedetomidine (mean dose and duration of infusion: 7.1 µg/kg, 15 hours) were hypotension, hypertension, nausea, bradycardia and dry mouth (fig. 3).
Tolerability profile of dexmedetomidine (DEX) in postsurgical mechanically ventilated patients in the ICU. In a randomised, double-blind, placebo controlled study involving 401 patients, DEX was administered intravenously (mean dose: 7.1 mg/kg) to maintain a Ramsay sedation score of ≥3, within 1 hour of admission to ICU and for at least 6 hours after extubation.[30] ICU = intensive care unit.
Placebo recipients showed a reduced incidence of most of these events, except hypertension, compared with the dexmedetomidine recipients.
• In contrast to the above results, tolerability data from patients (n = 353) in another phase III study who received dexmedetomidine (mean dose and duration of infusion: 7 µg/kg, 16 to 17 hours) or placebo revealed a markedly higher incidence of hypertension in the dexmedetomidine (22%) than in the placebo (12%) group.[30] However, the hypertension was generally mild to moderate in severity and easily resolved. The incidence of hypotension and nausea among the dexmedetomidine and placebo recipients was similar between the two studies.
5. Dexmedetomidine: Current Status
Dexmedetomidine is an α2-adrenoceptor agonist that has been approved in the US for use as a sedative for patients in the ICU. It has shown clinical efficacy in providing sedation and analgesia in postsurgical initially intubated and mechanically ventilated patients in an intensive care setting and is well tolerated.
References
Wheeler AP. Sedation, analgesia, and paralysis in the intensive care unit. Chest 1993 Aug; 104: 566–77
Lawrence CJ, Prinzen FW, de LS. The effect of dexmedetomidine on the balance of myocardial energy requirement and oxygen supply and demand. Anesth Analg 1996 Mar; 82: 544–50
Eisenach JC. Alpha-2 agonists and analgesia. Expert Opin Invest Drug 1994 Oct; 3: 1005–10
Hunter JC, Fontana DJ, Hedley LR, et al. Assessment of the role of alpha-2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol 1997 Dec; 122: 1339–44
Savola J-M, Virtanen R. Central alpha2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol 1991 Mar 26; 195: 193–9
Coughlan MG, Lee JG, Bosnjak ZJ, et al. Direct coronary and cerebral vascular responses to dexmedetomidine. Significance of endogenous nitric oxide synthesis. Anesthesiology 1992 Nov; 77: 998–1006
Salonen M, Reid K, Maze M. Synergistic interaction between alpha2-adrenergic agonists and benzodiazepines in rats. Anesthesiology 1992 Jun;76: 1004–11
Guo T-Z, MacMillan LB, Limbird LE, et al. The alpha2A-adrenoceptor mediates anesthesia and analgesia in mice receiving dexmedetomidine [abstr. A702.]. Anesthesiology 1997 Sep; 87 Suppl.
Morrison P, Etropolski M, Bachand RT. Dose-ranging study to evaluate the effects of dexmedetomidine on sedation [abstract no A298]. Anesthesiology 1999; 91: 3A Abstracts of Scientific Papers 1999 Annual Meeting American Society of Anesthesiologists, 1999 Oct 9-13, Dallas (TX)
Gravel N, Richardson C, Searle N, et al. Hemodynamic, cardiac and neurohormonal interactions of esmolol and dexmedetomidine in 36 healthy volunteers [abstract]. Anesth Analg 1999 Apr; 88 Suppl.: 25
Bloor BC, Ward DS, Belleville JP, et al. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 1992 Dec; 77: 1134–42
Grounds M. Dexmedetomidine: phase HI results. Proceedings from the 19th International Symposium on Intensive Care and Emergency Medicine; 1999 Mar 16–19; Brussels: 15-8
Belleville JP, Ward DS, Bloor BC, et al. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology 1992 Dec; 77: 1125–33
Singer M. Dexmedetomidine: phase I/II results. Proceedings from the 19th International Symposium on Intensive Care and Emergency Medicine; 1999 Mar 16–19; Brussels: 11-3
Talke P, Tayefeh F, Sessler DI, et al. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology 1997 Oct; 87: 835–41
Aho M, Erkola O, Kallio A, et al. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg 1992 Dec; 75: 940–6
Scheinin H, Karhuvaara S, Olkkola KT, et al. Pharmacodynam-ics and pharmacokinetics of intramuscular dexmedetomidine. Clin Pharmacol Ther 1992 Nov; 52: 537–46
Khan ZP, Munday IT, Jones RM, et al. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 1: pharmacodynamic and pharmacokinetic interactions. Br J Anaesth 1999 Sep; 83: 372–80
Abbott Laboratories. Precedex™. Dexmedetomidine hydro-chloride injection prescribing Information. Abbott Laboratories, USA. 2000
Mantz J. Dexmedetomidine. Drugs Today 1999 Mar; 35(3): 151–7
Cunningham FE, Baughman VL, Tonkovich L, et al. Pharmacokinetics of dexmedetomidine in patients with hepatic failure [abstract]. Clin Pharmacol Ther 1999 Feb; 65: 128
Rodrigues AD, Roberts EM. The in vitro interaction of dexmedetomidine with human liver microsomal cytochrome P4502D6 (CYP2D6). Drug Metab Dispos 1997 May; 25: 651–5
Bachand R, Scholz J, Pinaud M, et al. The effects of dexmedetomidine in patients in the intensive care setting [abstract no. 622]. Intensive Care Med 1999; 25 Suppl. 1: S160
Martin E, Lehot JJ, Manikis P, et al. Dexmedetomidine: a novel agent for patients in the intensive care setting [abstract no. 623]. Intensive Care Med 1999; 25 Suppl. 1
Mantz J, Goldfarb G, Lehot J-J, et al. Dexmedetomidine efficacy for ICU postoperative sedation [abstract no. 197]. Anesthesiology 1999; 91, no 3A (Sep M9126) Abstracts of Scientific Papers 1999 Annual Meeting American Society Of Anesthiologists, 1999 Oct 9–13, Dallas (TX)
Bachand RT, Werner L, Etropolski M, et al. A phase III study evaluating dexmedetomidine for sedation in postoperative patients [abstract no. 296]. Anesthesiology 1999; 91, no. 3A (Sep M9126) Abstracts of Scientific Papers 1999 Annual Meeting American Society of Anesthesiologists, 1999 Oct 9–13, Dallas (TX)
Abbott/Orion Precedex to be launched in 2000 for ICU market. FDC Rep Pink Sheet 2000 Jan 3; 62(1): 9
Jokinen K, Etropolski M, Paluselli M, et al. Dexmedetomidine and patient management: midazolam study. Proceedings of the XXV Congreso Latinoamericano de Anestesiologia 1999 Nov 3–6: poster 509
Jokinen J, Etropolski M, Paluselli M, et al. Dexmedetomidine and patient management: propofol study. Proceedings of the XXV Congreso Latinoamericano de Anestesiologia 1999 Nov 3–6 Nov: poster 510
Abbott Laboratories. Dexmedetomidine hydrochloride integrated summary of safety. Abbott Laboratories, 1998
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhana, N., Goa, K.L. & McClellan, K.J. Dexmedetomidine. Drugs 59, 263–268 (2000). https://doi.org/10.2165/00003495-200059020-00012
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200059020-00012