Summary
Abstract
Cyclosporin is a lipophilic cyclic polypeptide immunosuppressant that interferes with the activity of T cells chiefly via calcineurin inhibition. The original oil-based oral formulation of this drug (Sandimmun®)1 was characterised by high intra- and interpatient pharmacokinetic variability, with poor bioavailability in many patients; a novel microemulsion formulation (Neoral®)1 was therefore developed to circumvent these problems. Studies show increases, attributable chiefly to improved absorption in patients who absorb the drug only poorly from the original formulation, in mean systemic exposure to cyclosporin with the microemulsion, with no clinically significant differences in tolerability or drug interaction profiles.
Cyclosporin microemulsion is at least as effective as the oil-based formulation in renal, liver and heart transplant recipients, with trends towards decreased incidence of acute rejection with the microemulsion formulation in some (statistically significant in a few) trials. Cyclosporin microemulsion and tacrolimus appear to have similar efficacy in preventing acute rejection episodes in most renal, pancreas-kidney, liver and heart transplant recipients. However, there are indications of superior efficacy for tacrolimus in some trials, particularly in the prevention of severe acute rejection and in Black transplant recipients. Current 12-month data also indicate equivalent efficacy of sirolimus in renal transplantation.
Conversion from the oil-based to microemulsion formulation in stable renal, liver and heart transplant recipients is achievable with no change in acute rejection rates. The addition of an anti-interleukin-2 receptor monoclonal antibody and/or mycophenolate mofetil to cyclosporin microemulsion plus corticosteroids decreases rates of acute rejection; corticosteroid withdrawal without increased acute rejection rates was also achieved on the addition of these agents in some trials.
Pharmacoeconomic analyses have shown savings in direct healthcare costs in kidney or liver transplantation when cyclosporin microemulsion is used in preference to the oil-based formulation, although studies incorporating indirect costs or expressing costs in terms of therapeutic outcomes are currently unavailable.
Conclusions: The introduction of cyclosporin microemulsion has consolidated the place of the drug as a mainstay of therapy in all types of solid organ transplantation; research into optimisation of outcomes through more effective therapeutic monitoring in patients receiving this formulation is ongoing. Several novel immunosuppressants have been introduced in recent years: further clinical and pharmacoeconomic research will be needed to clarify the relative positioning of these agents, particularly with respect to specific patient groups. Other new drugs (basiliximab/daclizumab and mycophenolate mofetil) offer particular advantages when used in combination with cyclosporin.
Overview of Pharmacodynamic Properties
Cyclosporin inhibits the activation of the calcium/calmodulin-activated phospha-tase calcineurin via complex formation with cyclophilin, and thereby prevents the translocation of the transcription factor nuclear factor of activated T cells (NF-AT). The drug also inhibits activation of the transcription factor NF-κB, and T cell activation is suppressed by inhibition of interleukin-2 gene expression. In vitro study of porcine aortic endothelial cells has shown complete suppression by cyclosporin of tumour necrosis factor-a-mediated induction of class II major histocompatibility complex expression.
Cyclosporin also has hypertensive effects; potential underlying mechanismsinclude effects on the sympathetic nervous system, upregulation of angiotensin II receptors in vascular smooth muscle cells, increased plasma levels of en-dothelin-1, and effects on whole blood viscosity and plasma fibrinogen levels.
Pharmacokinetic Properties and Monitoring of Therapy
The original oil-based oral formulation of cyclosporin is characterised by widely varying bioavailability. The microemulsion, however, has self-emulsifying properties that enhance bioavailability and reduce pharmacokinetic variability between and within patients.
Assay Methods, Pharmacokinetic Monitoring and Clinical Outcomes. Measurement of cyclosporin concentrations in whole blood by immunoassay is currently used most commonly for monitoring therapy in patients receiving the drug for immunosuppression. Trough cyclosporin concentrations have been most frequently used to direct dosage adjustment, although they have little predictive value with respect to actual systemic exposure in patients receiving the original oil-based formulation.
The introduction of the microemulsion has led to new research into the therapeutic monitoring of cyclosporin therapy, with increasing emphasis on the importance of the 4-hour absorption phase that follows oral administration. Correlation has been shown between areas under curves of cyclosporin concentrations in whole blood versus time (AUCs) taken over 4 hours (abbreviated AUC) and a full 12-hour administration interval in patients undergoing renal transplantation. Other data suggest utility of 2-point sampling (at 2 and 6 hours), and a strong correlation has been shown between freedom from liver graft rejection during the first month after surgery and 6-hour AUCs (AUC6) or peak drug concentrations in blood (Cmax) in patients receiving cyclosporin microemulsion. Close correlations have been reported between drug concentrations measured in blood 2 hours post-dose and 4- or 6-hour AUCs, and there is evidence of improved overall clinical outcome with 2-hour over trough concentration monitoring.
General Pharmacokinetic Properties of Cyclosporin. Cyclosporin undergoes extensive extravascular distribution, with a volume of distribution at steady state of 3 to 5 L/kg after intravenous administration. The drug is 90 to 98% bound to plasma proteins, crosses the placenta, and is distributed into human milk.
Blood concentrations of cyclosporin generally decline in a biphasic manner. The initial elimination half-life is reported to average 1.2 hours, whereas the average terminal elimination half-life is reported to be 8.4 to 27 hours. The drug is metabolised extensively to at least 30 metabolites, chiefly by the hepatic cyto-chrome P450 3A enzyme system. Elimination is primarily biliary; around 6% of each dose is excreted in the urine, with 0.1 % eliminated in the urine as unchanged drug. Clearance is not affected to any significant extent by haemodialysis or renal failure.
Pharmacokinetic Properties of Cyclosporin Microemulsion. In general, significant increases in mean systemic exposure of patients to cyclosporin, with attendant reductions in time to Cmax (tmax), are seen when the microemulsion is used in place of the original oil-based formulation. These overall increases are attributable predominantly to improved absorption in patients who absorb cyclosporin only poorly from the oil-based formulation, with little or no change in good absorbers.
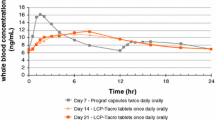
In renal transplantation, increases in AUC of up to 64% have been reported in randomised comparisons and in studies in which patients were converted from the older formulation to the microemulsion, with marked increases in drug exposure in patients previously classified as poor absorbers. Comparisons of variance data in several studies indicate significant reductions in intra- and interpatient pharmacokinetic variability relative to the oil-based formulation in patients receiving the microemulsion. Substantially increased AUCs (median 71% increase in one 6-month study in 25 patients) relative to the oil-based formulation have been reported with the microemulsion in children undergoing renal transplantation.
Significant increases in AUC and Cmax, and decreases in tmax, with cyclosporin microemulsion relative to the oil-based formulation have also been reported in patients undergoing liver transplantation. In one study, no clinically relevant effect of food intake was reported in microemulsion recipients. Most notably, the Canadian NOF-11 trial in 32 children showed exposure to cyclosporin (mean 8-hour AUC) to be increased by over 200% relative to the oil-based formulation in the early post-transplant period in patients treated with the microemulsion.
In general, systemic exposure to cyclosporin given as microemulsion appears greater than with the oil-based formulation when T tubes are open during the first few days after liver transplantation, although data are available to indicate that absorption of cyclosporin from the microemulsion is not fully independent of bile flow.
Enhancement of absorption of cyclosporin from the microemulsion relative to the oil-based formulation has also been reported in patients receiving heart and/or lung allografts. Increases were particularly marked in patients receiving lung transplants, with an increase in mean AUC6 of just over 70% relative to the oil-based formulation after 12 months in a comparative study in 50 recipients of new allografts.
Relative to adults, absorption of and systemic exposure to cyclosporin are substantially reduced in children undergoing bone marrow transplantation who receive the microemulsion. AUCs were increased significantly by GI inflammation in one study. Increased systemic exposure to cyclosporin with the microemulsion has also been reported in renal transplant recipients with diabetes mellitus.
The pharmacokinetic characteristics of cyclosporin are not altered to any clinically significant extent by advanced age.
Effect of Formulation on Cyclosporin Dosage. Cyclosporin dosage reductions were required to maintain required drug concentrations in whole blood after conversion from the oil-based formulation to microemulsion in 12.3 to 87.2% of patients in case series of stable renal transplant recipients. Overall reductions in mean dosage (after initial conversion on a 1: 1 basis) ranged from 4.7 to 14.7% over 8 weeks to 12 months in studies in a total of 1381 patients, and were predominantly statistically significant. Dosage reductions with the microemulsion relative to the original formulation have also been shown in randomised comparative studies, although statistical significance was not attained consistently. Reduced dosage requirements with the microemulsion have also been reported in liver and heart/lung transplant recipients.
Drug Interactions. A wide variety of agents increase (e.g. erythromycin, ketoconazole) or decrease (e.g. phenytoin, phenobarbital) plasma or whole blood concentrations of cyclosporin by competitive hepatic enzyme inhibition or induction, or by other mechanisms (e.g. absorption or binding to P-glycoprotein). Some drugs (e.g. aminoglycosides) are also associated with enhancement of nephrotoxicity of cyclosporin when coadministration takes place.
Recent data indicate possible enhancement by cyclosporin of the potential of HMG-CoA reductase inhibitors to induce rhabdomyolysis. Mycophenolate mofetil may increase systemic exposure to cyclosporin, but the proton pump inhibitor pantoprazole has no apparent pharmacokinetic effect when coad-ministered with the drug.
Therapeutic Efficacy
Comparisons with Cyclosporin Oil-Based Formulation (Sandimmun®). The overall ranges of incidence of biopsy-confirmed acute rejection episodes in the various trials in adult de novo transplant recipients receiving cyclosporin microemulsion or the original oil-based cyclosporin formulation at trough blood concentration-controlled dosages were 25 to 44.2% versus 22 to 60.5% at 3 to 24 months for renal transplantation, 45.9 to 62.7% versus 49.2 to 59.1% at 4 to 24 months for liver transplantation, and (in a single study) 86.2 versus 84.9% at 6 months for heart transplantation. Azathioprine and corticosteroids were given concomitantly in most trials. A trend for improved efficacy in this respect, and for the incidence of more than one acute rejection episode, with the microemulsion formulation was seen in most renal transplantation trials, with a statistically significant difference at 3 months in one for both parameters. There were no significant differences in the end-point incidence of acute rejection between the formulations in adult recipients of liver transplants, but the microemulsion formulation appeared significantly superior in a small trial in children (35 vs 80%; p = 0.01) at 12 months.
The incidence of severe (corticosteroid-resistant in most studies) acute rejection tended to be lower in adult patients receiving the microemulsion formulation than in those receiving the oil-based formulation (0 to 18.5% vs 10.8 to 20.0% at 4 to 24 months) in liver transplantation but not in heart transplantation (46.3 vs 45.8% at 2 years). The difference between the formulations was more apparent in children receiving liver transplants in this respect (6 vs 53% at 12 months; p = 0.004).
There was a trend for fewer recipients of the microemulsion than the oil-based formulation to require antilymphocyte antibody treatment for acute rejection over the first 3 months after renal transplantation. This difference was more marked in heart transplant recipients (6.9 vs 17.7%; p = 0.002 at 24 months).
Graft survival rates for the microemulsion and oil-based formulations were 91 to 96% versus 89 to 98% at 3 to 24 months in renal transplant recipients and 90 to 94.1% versus 86 to 93.8% at 4 to 24 months in liver transplant recipients. Patient survival rates for the microemulsion and oil-based formulations were 98 to 100% versus 99 to 100% in renal transplant recipients and 84.2 to 100% versus 85.9 to 94% in liver transplant recipients. Graft/patient survival rates were 88.3 versus 85.4% at 2 years in heart transplant recipients.
Comparisons with Other Modified Formulations. There are preliminary indications of clinical equivalence between cyclosporin Neoral® and cyclosporins SangCya®, Consupren® and Neoplanta® in de novo renal transplant recipients. Equivalence has also been demonstrated between Neoral® and Consupren® or SangCya® in two small studies in patients with stable existing transplants who were transferred from therapy with the original oil-based formulation of cyclosporin. Available comparisons, however, are predominantly nonblind and are based on small numbers of patients only.
Comparisons with Tacrolimus. The incidence of acute rejection in de novo cyclosporin microemulsion (initially 8 to 15 mg/kg/day) and tacrolimus (initially 0.1 to 0.2 mg/kg/day; both dosages concentration-controlled) recipients was 10 to 39% versus 9 to 40% at 3 to 24 months in renal transplantation, 11 versus 11% at 3 months in simultaneous pancreas-kidney transplantation, 23 to 82.5% versus 17 to 66% at 1 to 30 months in liver transplantation (p < 0.01 favouring tacrolimus in one of seven trials) and 30 versus 24% at 12 months in heart transplantation (in one trial).
The incidence of severe acute rejection in cyclosporin microemulsion and tacrolimus recipients was 0 to 14% versus 0 to 7% at 3 to 24 months in most trials of renal transplantation, 6 to 25% versus 0 to 19% at 1 to 30 months in liver transplantation (p < 0.01 favouring tacrolimus in one of seven trials) and 30 versus 21% at 12 months in heart transplantation. Tacrolimus 0.3 mg/kg/day was associated with significantly lower incidences of acute (20 vs 37%; p < 0.001) and severe acute rejection (9 vs 21%; p < 0.001) than cyclosporin microemulsion 8 to 10 mg/kg/day in the largest trial in renal transplant recipients, a nonblind comparative 6-month study in 577 patients in 50 European centres.
Graft survival rates in cyclosporin and tacrolimus recipients were 78 to 97% versus 83 to 100% at 3 to 24 months in renal transplantation (p < 0.05 favouring tacrolimus in one of nine trials) and 62 to 92% versus 68 to 95% at 1 to 30 months in liver transplantation (p < 0.05 favouring tacrolimus in one of seven trials). Patient survival rates in cyclosporin and tacrolimus recipients were 86 to 100% versus 90 to 100% at 3 to 24 months in renal transplantation, 67 to 98% versus 72 to 98% at 1 to 30 months in liver transplantation and 85 versus 85% at 12 months in heart transplantation.
Interim 6-month data from 425 of 606 liver transplant recipients taking part in a randomised, nonblind study in the UK and Ireland indicate a lower incidence of death, retransplantation or treatment failure for immunological reasons with tacrolimus than with cyclosporin microemulsion (17 vs 28%; p = 0.01).
Black recipients of renal transplants tended to do better on tacrolimus than on cyclosporin microemulsion (acute rejection 14 vs 38%, respectively; severe acute rejection 7 vs 14%, respectively). Similarly, Black recipients of heart transplants did significantly better on tacrolimus at 12 months (acute rejection episodes requiring treatment, p = 0.01; patient/graft survival, p = 0.04).
Comparisons with Sirolimus. The efficacy of cyclosporin microemulsion appears similar to that of sirolimus on the basis of results from two 12-month, randomised, nonblind studies in a total of 161 patients undergoing de novo renal transplantation. Graft and patient survival rates were similar between treatments in both trials; rates of biopsy-confirmed acute rejection were also not statistically significantly different, although there was a trend in favour of cyclosporin in one study (18 vs 27.5%).
Conversion to Cyclosporin Microemulsion. Conversion of stable renal, liver and heart transplantation patients from the oil-based cyclosporin formulation (Sandimmun®) to the microemulsion formulation, at an initial 1: 1 dosage ratio, appears not to affect the rate of acute rejection.
Preliminary evidence suggests that conversion from tacrolimus to cyclosporin microemulsion because of adverse effects or lack of efficacy is comparatively successful in renal and liver transplant recipients.
Use of Other Agents with Cyclosporin Microemulsion-Based Immuno-suppression. Incidences of presumed or biopsy-proven acute rejection were significantly decreased on the addition of mycophenolate mofetil 2 g/day to cyclosporin microemulsion plus corticosteroids in nonblind studies in 173 renal transplant recipients. The addition of mycophenolate mofetil 2 or 3 g/day to cyclosporin microemulsion (initial daily dosage 5 to 15 mg/kg/day) plus corticosteroid-based immunosuppression significantly reduced the incidence of biopsy-proven rejection or treatment failure over 1 year in a randomised, multicentre, double-blind, placebo-controlled study in 491 recipients of first or second renal allografts. A significantly lower incidence of acute rejection was reported with the addition of mycophenolate mofetil to cyclosporin microemulsion and corticosteroids than with the addition of azathioprine in a nonblind study in 57 liver transplant recipients (21.4 vs 44.8%; p < 0.05).
In a double-blind study (n = 376), rates of acute and severe acute rejection at 6 months were significantly reduced in patients receiving concomitant basiliximab 20mg on days 0 and 4 of renal transplantation compared with those receiving cyclosporin microemulsion and corticosteroids alone. A similar study in 346 renal transplant recipients showed statistically significant reductions in 12-month incidences of first acute rejection, second rejection, biopsy-confirmed rejection, and rejection episodes requiring treatment with augmented immuno-suppression (other than corticosteroids) with the addition of basiliximab to cyclosporin microemulsion plus corticosteroid-based immunosuppression. Significantly reduced incidence relative to placebo of biopsy-proven acute rejection has also been noted with addition of daclizumab to cyclosporin microemulsion and corticosteroid therapy.
Two multicentre placebo-controlled, double-blind trials in a total of 1295 patients undergoing renal transplantation showed statistically significant reductions relative to placebo or azathioprine in a composite end-point of acute rejection, graft loss and death when sirolimus 2 or 5 mg/day was added to immunosuppression with cyclosporin microemulsion and corticosteroids.
The addition of basiliximab and/or mycophenolate mofetil also allowed the elimination of corticosteroids from the immunosuppressive regimen without affecting the rate of acute rejection in a number of small studies in patients undergoing renal transplantation. However, a larger (n = 266), placebo-controlled, double-blind study has indicated an increase in risk of acute rejection (particularly among Black patients) upon withdrawal of corticosteroids from renal transplant recipients also receiving cyclosporin microemulsion and mycophenolate mofetil. Similar findings were reported in a further double-blind study in 500 renal transplant recipients, 447 whom received cyclosporin microemulsion in addition to mycophenolate mofetil, although the authors stated that the increase in frequency of serious rejection episodes when corticosteroids were withdrawn was acceptable. Results of corticosteroid withdrawal studies in liver allograft recipients receiving cyclosporin microemulsion or tacrolimus, either as monotherapy or in combination with mycophenolate mofetil, are inconclusive.
Pharmacoeconomic Considerations
Various cost analyses have been carried out from a healthcare provider’s or third party payer’s perspective to assess potential pharmacoeconomic advantages of the use of cyclosporin microemulsion in place of the older oil-based formulation.
Details from a study reported as an abstract have suggested a monthly cost saving of $US52 per patient after conversion from the oil-based formulation to microemulsion in 181 French individuals with stable renal allografts. Costs accounted for and year of costing were not given, however, for this 6-month analysis, which appeared to have been carried out from a healthcare provider’s perspective.
Prospectively gathered resource utilisation data from the MILTON study in 390 de novo liver transplant recipients showed savings (relative to treatment with the oil-based formulation) from a healthcare system perspective of 8 to 10% over the 4-month post-transplant period in patients receiving cyclosporin microemulsion. This was attributed partly to a more rapid discontinuation of intravenous cyclosporin therapy in patients receiving the microemulsion.
Examination of healthcare utilisation based on time in hospital and treatment of acute rejection indicated a cost saving of 2162 Canadian dollars per patient (year of costing and statistical significance not stated) relative to the oil-based formulation in a 3-month retrospective case-control study in 20 de novo liver transplant recipients. Three other analyses in patients undergoing liver transplantation have indicated reductions in direct healthcare costs when patients receive cyclosporin microemulsion rather than the original formulation.
Data from studies in patients receiving de novo kidney or liver transplants have suggested that the direct cost of using cyclosporin microemulsion is similar to or lower than that with tacrolimus. In one study, 6-month direct healthcare costs in 89 renal transplant recipients were £13 216 with cyclosporin microemulsion and £12 982 with tacrolimus (year of costing not stated). In 86 patients receiving liver transplants, the mean cost of cyclosporin microemulsion was 22% lower than that of tacrolimus (on the basis of dosages used over 1 year), although few details were available for this analysis (abstract published only).
Tolerability
The tolerability profile of cyclosporin is characterised by a number of potentially serious adverse effects that are related to exposure, including acute or chronic nephrotoxicity, hypertension and neurotoxicity. The main dose-limiting adverse effect of cyclosporin is nephrotoxicity, which usually presents as a reversible decrease in glomerular filtration rate. Nephrotoxicity is reported to affect 25 to 37% of kidney, heart or liver transplant recipients being treated with cyclosporin and may progress to permanent renal dysfunction in up to 15% of patients. Glomerular capillary thrombosis, progressing to graft failure in some patients, may also occur in transplant patients receiving cyclosporin.
In comparative trials conducted in recipients of renal transplants, hypertension was reported in fewer than 25% of patients treated with either cyclosporin microemulsion or the oil-based formulation. Hypertension was also reported in recipients of liver or heart transplants treated with either cyclosporin formulation.
Neurological symptoms, such as headaches, tremor, paraesthesia and convulsions, are also common adverse effects of cyclosporin in patients who have received transplants (1 source notes tremor in 12 to 21, 31 and 55% of patients receiving kidney, heart or liver transplants, respectively). Factors contributing to the development of convulsions in patients receiving cyclosporin therapy include hypomagnesaemia, hypertension, high-dose methylprednisolone therapy, nephrotoxicity and hypocholesterolaemia.
Numerous comparative double-blind or nonblind clinical trials have shown that the increased bioavailability of cyclosporin and greater systemic exposure achieved with the microemulsion formulation does not result in an increase in incidence or severity of adverse events compared with the original oil-based formulation in stable renal, liver or heart transplant recipients (provided that the dose of the microemulsion formulation is adjusted on the basis of target trough cyclosporin concentrations in whole blood).
Muscle weakness, oedema, epigastric pain, headache and hypertension were the most common events in stable renal transplant patients receiving treatment with cyclosporin microemulsion in a large comparative trial. About 40% of patients treated with either the cyclosporin microemulsion or the oil-based formulation experienced adverse events that were described as ‘serious’ in this study.
In patients who had received primary orthotopic liver transplants, the most common adverse events reported during therapy with cyclosporin microemulsion or the oil-based formulation were infections, cardiovascular effects, hypertension, nervous system effects and renal failure. Clinical diabetes mellitus, hir-sutism and gum hyperplasia developed in small numbers of patients in each treatment group.
Overall, both formulations of cyclosporin were equally well tolerated in a randomised double-blind trial in 380 de novo heart transplant recipients. However, relative to the oil-based formulation, patients treated with the microemul-sion had a lower (not statistically significant) incidence of candidiasis (5.9 vs 10.9%), cytomegalovirus infections (10.1 vs 15.1%) and de novo diabetes mellitus (3.9 vs 8.5%), whereas incidences of gingival hyperplasia and GI symptoms were higher in the microemulsion treatment group than in the comparator group(3.2 vs 2.6% and 81.9 vs 76.5%); these adverse events were transient and mild to moderate in severity.
The tolerability profile of cyclosporin microemulsion was broadly similar to that of tacrolimus (both drugs were given in combination with a corticosteroid and azathioprine) in cadaveric renal transplant recipients in a nonblind randomised study. In contrast, significant differences in the biochemical profiles of patients treated with either cyclosporin microemulsion or tacrolimus were reported in another study in renal transplant recipients. In a study in 577 renal transplant recipients, the incidences of new-onset diabetes mellitus after 6 months’ treatment were 4.5% with tacrolimus group and 2% with cyclosporin microemulsion (statistical significance not stated). Mean serum creatinine levels were similar for both drugs from the end of month 1 to study completion.
Thrombocytopenia and diarrhoea were reported significantly more frequently with sirolimus than with cyclosporin microemulsion in a randomised, nonblind comparison in 78 renal transplant recipients. Increased serum creatinine levels, hyperuricaemia, cytomegalovirus infection and tremor were more frequent with cyclosporin.
At present, there are no published well designed and controlled studies of the efficacy and tolerability of cyclosporin microemulsion in pregnant transplant recipients and their offspring. However, in a retrospective analysis, no notable malformation trends were evident among the 175 children (mean age 4.4 years) of renal transplant recipients who had been treated with cyclosporin during pregnancy.
Dosage and Administration
Cyclosporin microemulsion is available variously in different countries as 10,25, 50 and l00 mg soft gelatin capsules and as an oral solution containing 100 mg/ml. The oral solution may be made more palatable by diluting with orange or apple juice. Blood cyclosporin concentrations increase when cyclosporin microemulsionis taken with grapefruit/grapefruit juice, which should therefore be avoided by patients taking the drug.
Cyclosporin microemulsion is indicated for the prophylaxis of organ rejection in patients who have undergone allogeneic renal, liver or heart transplantation. Cyclosporin microemulsion should be taken twice daily (in two equal doses). An optimal dosage of the drug will produce trough whole blood concentrations sufficient to achieve immunosuppression while preventing high peak blood concentrations and drug-related toxicity. Importantly, because cyclosporin is more bioavailable from the oral microemulsion than from the oil-based oral formulation, the two formulations cannot be interchanged without careful monitoring of the patient by a physician.
Whole blood concentrations of cyclosporin should be measured frequently (three to four times weekly or daily in the early post-transplantation period) in patients receiving treatment with cyclosporin microemulsion, as lower than recommended therapeutic concentrations may result in rejection of the transplanted organ and higher concentrations are likely to produce drug-related toxicity. Renal function, liver function and blood pressure should be monitored closely in patients receiving treatment with cyclosporin microemulsion. In addition, levels of serum lipids, potassium and magnesium should be checked regularly during treatment with the drug. In randomised controlled trials in transplant recipients, most patients received an initial dosage of 10 mg/kg/day. Dosages were adjusted thereafter to achieve target therapeutic trough concentrations in whole blood and then further titrated according to assessments of transplant rejection and tolerability.
Stable transplant recipients receiving the original oil-based formulation of cyclosporin may have their therapy changed to the microemulsion formulation with careful monitoring. In these patients, it is recommended that the initial dosage of cyclosporin microemulsion is the same as that of the previously administered cyclosporin formulation. Thereafter, the dose of cyclosporin microemulsion should be adjusted to obtain a whole blood trough cyclosporin concentration the same as that achieved previously with the original formulation.




















Similar content being viewed by others
References
Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet 1999 Mar 27; 353: 1083–91
Perico N, Remuzzi G. Prevention of transplant rejection: current treatment guidelines and future developments. Drugs 1997 Oct; 54: 533–70
Colvin R. Cellular and molecular mechanisms of allograft rejection. Annu Rev Med 1990; 41: 361–75
Krensky AM, Weiss A, Crabtree G, et al. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med 1990; 322: 510–7
Sayegh M, Turka L. The role of T cell costimulatory activation pathways in transplant rejection. N Engl J Med 1998; 338: 1813–21
Steinman R, Young J. Signals arising from antigen-presenting cells. Curr Opin Immunol 1991; 3: 361–72
Gimmi CD, Freeman GJ, Gribben JG, et al. Human T-cell clonal anergy is induced by antigen presentation in the absence ofB7 costimulation. ProcNatl Acad Sci USA 1993; 90: 6586–90
Noel P, Boise L, Green J, et al. CD28 costimulation prevents cell death during primary T cell activation. J Immunol 1996; 157: 636–42
Linsley P, Ledbetter J. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol 1993; 11:191–212
Lee JI, Canafax DM. Cyclosporine pharmacology. Transplant Proc 1996; 28(4): 2156–8
Noble S, Markham A. Cyclosporin: a review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (Neoral). Drugs 1995 Nov; 50: 924–41
Faulds D, Goa KL, Benfield P. Cyclosporin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 1993 Jun; 45: 953–1040
Batiuk TD, Kung L, Halloran PF. Evidence that calcineurin is rate-limiting for primary human lymphocyte activation. J Clin Invest 1997; 100(7): 1894–901
Borger P, Kauffman HF, Timmerman JAB, et al. Cyclosporine, FK506, mycophenolate mofetil, and prednisolone differentially modulate cytokine gene expression in human airway-derived epithelial, cells. Transplantation 2000; 69(7): 1408–13
Frantz B, Nordby EC, Bren G, et al. Calcineurin acts in synergy with PMA to inactivate IKB/MAD3, an inhibitor of NF-κB. EMBO J 1994; 13(4): 861–70
Mattila PS, Ullman KS, Fiering S, et al. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J 1990; 9(13): 4425–33
O’Keefe SJ, Tamura J, Kincaid RL, et al. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature 1992; 357(6380): 692–4
Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992; 357(6380): 695–7
Roger T, Out TA, Mukaida N, et al. Enhanced AP-1 andNF-κB activities and stability of interleukin 8 (IL-8) transcripts are implicated in IL-8 mRNA superinduction in lung epithelial H292 cells. Biochem J 1998; 330: 429–35
Borger P, Koeter GH, Timmerman JA, et al. Proteases from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 production in airway epithelial cell lines by transcriptional mechanisms. J Infect Dis 1999; 180(4): 1267–74
Charreau B, Coupel S, Boulday G, et al. Cyclosporine inhibits class II major histocompatibility antigen presentation by xenogeneic endothelial cells to human T lymphocytes by altering expression of the class II transcriptional activator gene. Transplantation 2000; 70(2): 354–61
Zhang W, Li J-L, Hosaka M, et al. Cyclosporine A-induced hypertension involves synapsin in renal sensory nerve endings. Proc Natl Acad Sci USA 2000; 97(17): 9765–70
Gerhardt U, Riedasch M, Hohage H. Cyclosporine A modulates baroreceptor function in kidney transplant recipients. Int J Cardiol 1999; 68(2): 203–8
Ryuzaki M, Stahl LK, Lyson T, et al. sympathoexcitatory response to cyclosporin A and baroreflex resetting. Hypertension 1997; 29(2): 576–82
Ventura HO, Malik FS, Mehra MR, et al. Mechanisms of hypertension in cardiac transplantation and the role of cyclosporine. Curr Opin Cardiol 1997; 12(4): 375–81
Avdonin PV, Cottet-Maire F, Afanasjeva GV, et al. Cyclosporine A up-regulates angiotensin II receptors and calcium responses in human vascular smooth muscle cells. Kidney Int 1999; 55(6): 2407–14
Ishikawa A, Suzuki K, Fujita K. Mechanisms of cyclosporine-induced nephrotoxicity. Transplant Proc 1999; 31(1–2): 1127–8
Åsberg A, Christensen H, Hartmann A, et al. Diltiazem modulates cyclosporin A induced renal hemodynamic effects but not its effect on plasma endothelin-1. Clin Transplant 1998 Oct; 12: 363–70
Linde T, Sandhagen B, Backman U, et al. Altered flow properties of blood and increased plasma fibrinogen in cyclosporin-treated renal allograft recipients. Nephrol Dial Transplant 1999; 14(6): 1525–9
Babarykin D, Amerika D, Adamsone I, et al. Transfer of patients with kidney grafts to Sandimmun Neoral normalizes the calcium level in erythrocyts. Transplant Proc 1996 Dec; 28: 3137
Friman S, Bäckman L. A new microemulsion formulation of cyclosporin: pharmacokinetic and clinical features. Clin Pharmacokinet 1996 Mar; 30: 181–93
Vonderscher J, Meinzer A. Rationale for the development of Sandimmune Neoral. Transplant Proc 1994 Oct; 26: 2925–7
Kahan BD. Individualization of cyclosporine therapy using pharmacokinetic and pharmacodynamic parameters. Transplantation 1985; 40(5): 457–76
Drewe J, Beglinger C, Kissel T. The absorption site of cyclo-sporin in the human gastrointestinal tract. Br J Clin Pharmacol 1992; 33: 39–43
Friman S, Persson H, Karlberg I, et al. The bile acid independent flow is reduced in the transplanted liver. Transplant Int 1992; 5: S163–7
Cyclosporine. In: McEvoy GK, editor. AHFS drug information 2000. Bethesda, MD: American Society of Health-System Pharmacists, 2000: 3374–89
Tredger JM, Roberts N, Sherwood R, et al. Comparison of five cyclosporin immunoassays with HPLC. Clin Chem Lab Med 2000; 38(11): 1205–7
Hamwi A, Veitl M, Manner G, et al. Evaluation of four automated methods for determination of whole blood cyclosporine concentrations. Am J Clin Pathol 1999; 112(3): 358–65
Schutz E, Svinarov D, Shipkova M, et al. Cyclosporin whole blood immunoassays (AxSYM, CEDIA, and Emit): a critical overview of performance characteristics and comparison with HPLC. Clin Chem 1998; 44(10): 2158–64
Steimer W. Performance and specificity of monoclonal immunoassays for cyclosporine monitoring: how specific is specific? Clin Chem 1999; 45(3): 371–81
Lindholm A, Kahan BD. Influence of cyclosporine pharmacokinetics, trough concentrations, and AUC monitoring on outcome after kidney transplantation. Clin Pharmacol Ther 1993; 54: 205–18
Kahan BD, Dunn J, Fitts C, et al. Reduced inter- and intrasubject variability in cyclosporine pharmacokinetics in renal transplant recipients treated with a microemulsion formulation in conjunction with fasting, low-fat meals, or high-fat meals. Transplantation 1995 Feb 27; 59: 505–11
Kahan BD, Welsh M, Schoenberg L, et al. Variable oral absorption of cyclosporine: a biopharmaceutical risk factor for chronic renal allograft rejection. Transplantation 1996; 62(5): 599–606
Dumont RJ, Ensom MHH. Methods for clinical monitoring of cyclosporin in transplant patients. Clin Pharmacokinet 2000 May; 38: 427–47
Nankivell BJ, Hibbins M, Chapman JR. Diagnostic utility of whole blood cyclosporine measurements in renal tranplantation using triple therapy. Transplantation 1994; 58(9): 989–96
Belitsky P, Levy GA, Johnston A. Neoral absorption profiling: an evolution in effectiveness. Transplant Proc 2000 May; 32 Suppl. 3A: 45S–52S
Mahalati K, Belitsky P, Sketris I, et al. Neoral monitoring by simplified sparse sampling area under the concentration-time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation 1999 Jul 15; 68: 55–62
Mahalati K, Belitsky P, Kiberd B, et al. Absorption profiling: a novel method for monitoring Neoral in kidney transplantation that reduces rejection and nephrotoxicity [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: 114
Mahalati K, Belitsky P, West K, et al. Approaching the therapeutic window for cyclosporine in kidney transplantation: a prospective study. J Am Soc Nephrol 2001; 12(4): 828–33
Amante AJ, Kahan BD. Abbreviated AUC strategy for monitoring cyclosporine microemulsion therapy in the immediate posttransplant period. Transplant Proc 1996; 28(4): 2162–3
Barama A, Perner F, Beauregard-Zollinger L, et al. Absorption profiling of cyclosporine therapy for de novo kidney transplantation: a prospective, randomized study comparing sparse sampling to trough monitoring [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: S162–3
Johnston A, David O, Lee M, et al. Predicting patients’ exposure to cyclosporin following Neoral® [abstract]. Ther Drug Monit 1997 Oct; 19: 555
Grant D, Kneteman N, Tchervenkov J, et al. Peak cyclosporine levels (Cmax) correlate with freedom from liver graft rejection: results of a prospective, randomized comparison of Neoral and Sandimmune for liver transplantation (NOF-8). Transplantation 1999 Apr 27; 67: 1133–7
Levy GA, Lake JR, Beauregard-Zollinger L, et al. Improved clinical outcomes for liver transplant recipients using cyclosporine blood level monitoring based on two-hour post-dose levels [abstract]. Transplantation 2000 Apr 27; 69 (8 Suppl.): S387
New method proposed for ciclosporin monitoring in transplant patients. Pharm J 2000; 265(7112): 324
Cantarovich M, Besner J-G, Barkun JS, et al. Two-hour cyclosporine level determination is the appropriate tool to monitor Neoral therapy. Clin Transplant 1998 Jun; 12: 243–9
Cantarovich M, Barkun JS, Tchervenkov JI, et al. Comparison of neoral dose monitoring with cyclosporine trough levels versus 2-hr postdose levels in stable liver transplant patients. Transplantation 1998 Dec 27; 66: 1621–7
Cantarovich M, Elstein E, de Varennes B, et al. Clinical benefit of Neoral dose monitoring with cyclosporine 2-hr post-dose levels compared with trough levels in stable heart transplant patients. Transplantation 1999 Dec 27; 68: 1839–42
Levy GA. C2 monitoring strategy for optimising cyclosporin immunosuppression from the Neoral formulation. Biodrugs 2001; 15(5): 279–90
Cyclosporine. 2001 Mosby’s GenRx [online]. Mosby, Inc; 2001 [26 pages]. Available from URL: http://www.mosbysgenrx [Accessed 2001 Feb 23]
Mueller EA, Kovarik JM, van Bree JB, et al. Improved dose linearity of cyclosporine pharmacokinetics from a microemulsion formulation. Pharm Res 1994 Feb; 11: 301–4
Kovarik JM, Mueller EA, van Bree JB, et al. Reduced inter- and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J Pharm Sci 1994 Mar; 83: 444–6
Mueller EA, Kovarik JM, van Bree JB, et al. Influence of a fat-rich meal on the pharmacokinetics of a new oral formulation of cyclosporine in a crossover comparison with the market formulation. Pharm Res 1994 Jan; 11: 151–5
Keown P, Niese D. Cyclosporine microemulsion increases drug exposure and reduces acute rejection without incremental toxicity in de novo renal transplantation. International Sandimmun Neoral Study Group. Kidney Int 1998 Sep; 54: 938–44
Keown P, Landsberg D, Halloran P, et al. A randomized, prospective multicenter pharmacoepidemiologic study of cyclosporine microemulsion in stable renal graft recipients. Report of the Canadian Neoral Renal Transplantation Study Group. Transplantation 1996 Dec 27; 62: 1744–52
Barone G, Chang CT, Choc Jr MG, et al. The pharmacokinetics of a microemulsion formulation of cyclosporine in primary renal allograft recipients. Neoral Study Group. Transplantation 1996 Mar 27; 61: 875–80
Wahlberg J, Wilczek HE, Fauchald P, et al. Consistent absorption of cyclosporine from a microemulsion formulation assessed in stable renal transplant recipients over a one-year study period. Transplantation 1995 Oct 15; 60: 648–52
Kabasakul SC, Clarke M, Kane H, et al. Comparison of Neoral and Sandimmun cyclosporin A pharmacokinetic profiles in young renal transplant recipients. Pediatr Nephrol 1997 Jun; 11:318–21
Keiles A, Herman J, Tjandra-Maga TB, et al. Sandimmun-to-Neoral conversion in stable pediatric kidney transplant recipients. Transplant Proc 1998 Aug; 30: 1995–6
Krmar RT, Wühl E, Ding R, et al. Pharmacokinetics of a new microemulsion formulation of cyclosporin A (Neoral) in young patients after renal transplantation. Transplant Int 1996; 9: 476–80
Burckart GJ, Venkataramanan R, Ptachcinski RJ, et al. Cyclosporin absorption following orthotopic liver transplantation. J Clin Pharmacol 1986; 26(8): 647–51
Freeman D, Grant D, Levy G, et al. Pharmacokinetics of a new oral formulation of cyclosporine in liver transplant recipients. Ther Drug Monit 1995; 17: 213–6
Alvarez F, Atkison PR, Grant DR, et al. NOF-11: a one-year pediatric randomized double-blind comparison of Neoral versus Sandimmune in orthotopic liver transplantation. Transplantation 2000 Jan 15; 69: 87–92
Dunn SP, Cooney GF, Kulinsky A, et al. Absorption characteristics of a microemulsion formulation of cyclosporine in de novo pediatric liver transplant recipients. Transplantation 1995 Dec 27; 60: 1438–42
Hoppu K, Jalanko H, Laine J, et al. Comparison of conventional oral cyclosporine and cyclosporine microemulsion formulations in children with a liver transplant: a pharmacokinetic and clinical study. Transplantation 1996; 62(1): 66–71
Melter M, Rodeck B, Kardorff R, et al. Pharmacokinetics of cyclosporine in pediatric long-term liver transplant recipients converted from Sandimmun to Neoral. Transplant Int 1997; 10: 419–25
van Mourik IDM, Thomson M, Kelly DA. Comparison of pharmacokinetics of Neoral and Sandimmune in stable pediatric liver transplant recipients. Liver Transplant Surg 1999 Mar; 5: 107–11
Trull AK, Tan KKC, Tan L, et al. Absorption of cyclosporin from conventional and new microemulsion oral formulations in liver transplant recipients with external biliary diversion. Br J Clin Pharmacol 1995; 39: 627–31
Winkler M, Ringe B, Oldhafer K, et al. Influence of bile on cyclosporin absorption from microemulsion formulation in primary liver transplant recipients. Transplant Int 1995; 8: 324–6
Trull A, Steel L, Sharpies L, et al. Randomized, trough blood cyclosporine concentration-controlled trial to compare the pharmacodynamics of Sandimmune and Neoral in de novo lung transplant recipients. Ther Drug Monit 1999 Feb; 21: 17–26
Cooney GF, Jeevanandam V, Choudhury S, et al. Comparative bioavailability of Neoral and Sandimmune in cardiac transplant recipients over 1 year. Transplant Proc 1998 Aug; 30: 1892–4
Aziz T, el-Gamel A, Keevil B, et al. Clinical impact of Neoral in thoracic organ transplantation. Transplant Proc 1998 Aug; 30: 1900–3
White M, Pelletier GB, Tan A, et al. Pharmacokinetic, hemo-dynamic, and metabolic effects of cyclosporine Sandimmune versus the microemulsion Neoral in heart transplant recipients. J Heart Lung Transplant 1997 Aug; 16: 787–94
Kesten S, Scavuzzo M, Chaparro C, et al. Pharmacokinetic profile and variability of cyclosporine versus Neoral in patients with cystic fibrosis after lung transplantation. Pharmacotherapy 1998 Jul–Aug; 18: 847–50
Schultz KR, Nevill TJ, Toze CL, et al. The pharmacokinetics of oral cyclosporin A (Neoral) during the first month after bone marrow transplantation. Transplant Proc 1998 Aug; 30: 1668–70
Parquet N, Reigneau O, Humbert H, et al. New oral formulation of cyclosporin A (Neoral) pharmacokinetics in allogeneic bone marrow transplant recipients. Bone Marrow Transplant 2000 May; 25: 965–8
Schultz KR, Nevill TJ, Baishaw RF, et al. Effect of gastrointestinal inflammation and age on the pharmacokinetics of oral microemulsion cyclosporin A in the first month after bone marrow transplantation. Bone Marrow Transplant 2000; 26(5): 545–51
Chapman JR, O’Connell PJ, Bovington KJ, et al. Reversal of cyclosporine malabsorption in diabetic recipients of simultaneous pancreas and kidney transplants using a microemulsion formulation. Transplantation 1996 Jun 27; 61: 1699–704
Serafinowicz A, Gaciong Z, Baçzkowska T, et al. Cyclosporine pharmacokinetics in renal allograft recipients with diabetes mellitus with Sandimmune and Sandimmune Neoral. Transplant Proc 1996 Dec; 28: 3140–1
Kovarik JM, Koelle EU. Cyclosporin pharmacokinetics in the elderly. Drugs Aging 1999 Sep; 15: 197–205
Chu SH, Pang ST, Chiang YJ, et al. Converting renal transplant patients maintained on Sandimmune to a new microemulsion formulation, Sandimmune Neoral. Transplant Proc 1998 Nov; 30: 3521–3
Curtis JJ, Lynn M, Jones PA. Neoral conversion from Sand-immune in maintenance renal transplant patients: an individualized approach. J Am Soc Nephrol 1998 Jul; 9: 1293–300
Griffin PJA, Moore RH, Jurewicz WA, et al. Conversion from cyclosporine Sandimmune to cyclosporine Neoral in the stable renal transplant population. Transplant Proc 1997 Feb–Mar; 29: 303
Hourmant M, Antoine C, Bayle F, et al. An open multicenter trial of conversion from Sandimmun to Neoral in stable kidney-transplant patients. Transplant Proc 1997 Aug; 29: 2313–4
Hricik DE, Dixit A, Knauss TC, et al. Benefits of pre-emptive dose reduction for Sandimmune to Neoral conversion in stable renal transplant recipients. Clin Transplant 1998 Dec; 12: 575–8
Huraib S, Al Khudair W, Selim H, et al. Mass conversion from Sandimmun to Sandimmun Neoral: 1½-year experience. Transplant Proc 1997 Nov; 29: 2980–2
Løkkegaard H, Asmundsson P, Clausen P, et al. Conversion from conventional Sandimmun to Neoral therapy in stable renal transplant recipients. Transplant Proc 1996 Aug; 28: 2199–201
Neumayer H-H, Färber L, Haller P, et al. Substitution of conventional cyclosporin with a new microemulsion formulation in renal transplant patients: results after 1 year. Nephrol Dial Transplant 1996 Jan; 11: 165–72
Vathsala A, Lee WT, Lu YM, et al. Safety and efficacy of conversion from once daily Sandimmun to twice daily Neoral cyclosporine in renal allograft recipients. Transplant Proc 1998 Aug; 30: 1746–8
Augeraud C, Viau N, Hurault de Ligny B, et al. Therapeutic conversion Sandimmun®/Neoral®: follow-up of 116 renal transplant recipients in clinical practice [in French]. J Pharm Clin 1999; 18(2): 152–5
Pollard SG, Lear PA, Ready AR, et al. Comparison of microemulsion and conventional formulations of cyclosporine A in preventing acute rejection in de novo kidney transplant patients. U.K. Neoral Renal Study Group. Transplantation 1999 Nov 15; 68: 1325–31
Gracida C, Melchor JL. Renal recipients of haploidentical living donors treated with Neoral or conventional cyclosporine: a comparative follow-up of 3 years. Transplant Proc 1998 Aug; 30: 1742–3
Frei UA, Neumayer H-H, Buchholz B, et al. Randomized, double-blind, one-year study of the safety and tolerability of cyclosporine microemulsion compared with conventional cyclosporine in renal transplant patients. International Sandimmun Neoral Study Group. Transplantation 1998 Jun 15; 65: 1455–60
Park K, Koh YB, Kwak JY, et al. An open randomized parallel group study to compare Sandimmune Neoral with Sand-immune soft gelatin capsule in stable renal transplant patients. Transplant Proc 1996 Jun; 28: 1202–3
Jain A, Gadomski M, Fung J. A prospective study on conversion from Sandimmune to Neoral in stable adult liver transplant recipients. Dig Dis Sci 1999 Apr; 44: 775–7
Pollard SG, Lodge JP. Conversion from Sandimmune to Neoral in stable liver graft recipients. Transplant Proc 1996 Aug; 28: 2244–6
Otto M-G, Mayer AD, Clavien P-A, et al. Randomized trial of cyclosporine microemulsion (Neoral) versus conventional cyclosporine in liver transplantation: MILTON study. Multicentre International Study in Liver Transplantation of Neoral Study Group [published erratum appears in Transplantation 1999 May 27; 67 (10): 1386]. Transplantation 1998 Dec 27; 66: 1632–40
Roy A, Grant DR, Kneteman NM, et al. A randomized, multicenter, double-blind study of Neoral vs Sandimmune in patients undergoing liver transplantation [in French]. Ann Chir 1998; 52: 716–21
Graziadei IW, Wiesner RH, Marotta PJ, et al. Neoral compared to Sandimmune is associated with a decrease in histologic severity of rejection in patients undergoing primary liver transplantation. Transplantation 1997 Sep 15; 64: 726–31
Pethig K, Geiger M, Korn A, et al. Follow-up after conversion to Neoral in stable heart transplant recipients. Transplant Proc 1996 Aug; 28: 2282–4
Svendsen U, Larsen K, Allermand H, et al. Neoral conversion study: shift from Sandimmune classic formulation to Sandimmune Neoral in heart and lung transplant patients. Transplant Proc 1995 Dec; 27: 3477
Zaldonis DB, Keenan RJ, Pham SM, et al. Neoral conversion in stable thoracic transplant patients leads to dose reduction. Transplant Proc 1998 Aug; 30: 1898–9
Dorent R, Albat B, Baladier V, et al. French multicenter study of Neoral conversion in heart transplant patients. Transplant Proc 1997 Aug; 29: 2326–7
Holm A, Vicente A, Soberanes A, et al. Immunosuppression (Neoral vs Sandimmune) in pediatric kidney transplantation. Transplant Proc 1997 Feb–Mar; 29: 300–2
Dunn SP, Falkenstein K, Pierson A, et al. Results of conversion from Sandimmune to Neoral in stable pediatric liver transplant recipients after two years. Transplant Proc 1998 Aug; 30: 1962–3
Campana C, Regazzi MB, Buggia I, et al. Clinically significant drug interactions with cyclosporin: an update. Clin Pharmacokinet 1996 Feb; 30: 141–79
Neoral. In: Walker G, editor. ABPI data sheet compendium and summary of product characteristics 1999–2000. London: Datapharm Publications Ltd, 1999: 1005–8
Neoral Soft Gelatin Capsules (cyclosporin capsules for microemulsion. Neoral Oral Solution (cyclosporin oral solution for microemulsion). In: Physicians’ desk reference. 54th ed. Montvale, NJ: Medical Economics Company, Inc., 2000: 2034–44
Neoral Soft Gelatin Capsules (cyclosporin capsules, USP) modified. Neoral Oral Solution (cyclosporin oral solution, USP) modified. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2001 Jan
Mezzano S, Flores C, Ardiles L, et al. Study of Neoral kinetics in adult renal transplantation treated with diltiazem. Transplant Proc 1998 Aug; 30: 1660–2
Åsberg A, Christensen H, Hartmann A, et al. Pharmacokinetic interactions between microemulsion formulated cyclosporine A and diltiazem in renal transplant recipients. Eur J Clin Pharmacol 1999 Jul; 55: 383–7
Sud K, Singh B, Krishna S, et al. Effect of fluconazole on bioavailability of Sandimmun Neoral in renal transplant recipients [abstract]. Nephrology 1997 May; 3 Suppl. 1: S444
Sud K, Singh B, Krishna VS, et al. Unpredictable cyclosporin-fluconazole interaction in renal transplant recipients. Nephrol Dial Transplant 1999; 14(7): 1698–703
Sagedal S, Åsberg A, Hartmann A, et al. Glipizide treatment of post-transplant diabetes does not interfere with cyclosporine pharmacokinetics in renal allograft recipients. Clin Transplant 1998 Dec; 12: 553–6
Chapman JR, Bovington KJ, Eris J, et al. Pharmacokinetic analysis of the effect of two weeks of sirolimus therapy on cyclosporine Neoral blood levels [abstract]. Transplantation 1999 Apr 15; 67: S153
Ku Y-M, Min DI, Flanigan MJ. The effect of grapefruit juice on microemulsion cyclosporine (Neoral®) pharmacokinetics in healthy volunteers [abstract]. Pharmacotherapy 1998 Mar–Apr; 18: 442
Lee M, Min DI, Ku Y-M, et al. Effect of grapefruit juice on pharmacokinetics of microemulsion cyclosporine in African American subjects compared with Caucasian subjects: does ethnic difference matter? J Clin Pharmacol 2001; 41: 317–23
Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoA reductase inhibitors. Drug Saf 2000; 23(3): 197–213
Maltz HC, Balog DL, Cheigh JS. Rhabdomyolysis associated with concomitant use of atorvastatin and cyclosporine. Ann Pharmacother 1999; 33(11): 1176–9
Rial M, Guardia O, Greco G, et al. Area under the curve of Neoral and chronic use of mycophenolate mofetil. Transplant Proc 1998 Jun; 30: 1195–6
Lorf T, Ramadori G, Ringe B, et al. Pantoprazole does not affect cyclosporin A blood concentration in kidney-transplant patients. Eur J Clin Pharmacol 2000; 55(10): 733–5
Lorf T, Ramadori G, Ringe B, et al. The effect of pantoprazole on tacrolimus and cyclosporin A blood concentration in transplant recipients [letter]. Eur J Clin Pharmacol 2000; 56(5): 439–40
Min ZL, Zhao M, Zhu YH, et al. Experience with clinical use of Sandimmun Neoral in renal transplant patients. Transplant Proc 1996 Jun; 28: 1356–7
Abendroth D, Buchholz B, Land W, et al. Comparison of efficacy, safety, and tolerability of Neoral vs Sandimmun in de novo renal transplant patients over 24 months’ treatment. Transplant Proc 1997 Feb–Mar; 29: 275–6
Barone G, Bunke CM, Choc Jr MG, et al. The safety and tolerability of cyclosporine emulsion versus cyclosporine in a randomized, double-blind comparison in primary renal allograft recipients. Neoral Study Group. Transplantation 1996 Mar 27; 61: 968–70
Frei U, Taesch S, Niese D. Use of Sandimmun Neoral in renal transplant patients. Part A. International Sandimmun Neoral Study Group. Transplant Proc 1994 Oct; 26: 2928–31
Hricik DE. Superior renal allograft survival with cyclosporine-based immunosuppression: results of a double-blind, randomized, prospective comparison of Neoral and Sandimmune in cadaveric renal transplant recipients. OLN355 Study Group [abstract]. Transplantation 1999 Apr 15; 67: S150
Niese D. A double-blind randomized study of Sandimmun Neoral versus Sandimmun in new renal transplant recipients: results after 12 months. International Sandimmun Neoral Study Group. Transplant Proc 1995 Apr; 27: 1849–56
Pollak R. The Neoral™ vs Sandimmune® soft-gelatin capsule randomized multicenter three-month double-blind trial (N-103): tolerability and safety profiles. In: Neoral™: the new microemulsion formulation of cyclosporine, special report, May 1995. Cedar Knolls (NJ): World Medical Press, 1995:19–27
Gaston RS, Said M, Ward M, et al. Pharmacokinetic and clinical evaluation of SangCya and Neoral in stable, adult renal transplant patients: preliminary results [abstract]. Transplantation 1999 Apr 15; 67: S161
Barbari A, Stephan A, Kamel G, et al. Experience with new cyclosporine formulations: Consupren and Neoral in renal transplant patients. Transplant Proc 1997 Nov; 29: 2941–4
Kim SC, Han DJ. Neoplanta as a new microemulsion formula of cyclosporine in renal transplantation: comparative study with Neoral for efficacy and safety. Transplant Proc 1998 Nov; 30: 3547–8
McCune T, Light J, Adams P, et al. SangcyaR oral solution compared to NeoralR capsules in de novo adult renal transplant recipients: results of a South-Eastern Organ Procurement Foundation clinical trial [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: S162
Stephan A, Masri MA, Barbari A, et al. A one-year comparative study of Neoral vs Consupren in de novo renal transplant patients. Transplant Proc 1998 Nov; 30: 3533–4
Pinson CW. Neoral (cyclosporine microemulsion) versus Sandimmune (cyclosporine) in U.S. liver transplant recipients: results of the OLN-356 study. Hepatology 1996 Oct; 24 (Pt 2) Program Suppl.: 175A
Donovan J. A randomized, double-blind study of Neoral vs. Sandimmune in primary liver transplant recipients with two year follow up. OLN 354 Study Group [abstract]. Transplantation 1998 Jun 27; 65: S14
Eisen HJ, Hobbs RE, Davis SF, et al. Safety, tolerability and efficacy of cyclosporine microemulsion in heart transplant recipients: a randomized, multicenter, double-blind comparison with the oil based formulation of cyclosporine — results at six months after transplantation. Transplantation 1999 Sep 15; 68: 663–71
Eisen HJ, Mueller EA, Mellein B, et al. Neoral vs Sandimmune in heart transplantation: one year results of a double-blind, international study [abstract]. Transplantation 1999 Apr 15; 67: S7
Eisen HJ, Mueller EA, Mellein B, et al. Two year results of a double-blind, randomized, multicenter, international study of microemulsion vs oil-based cyclosporine in de novo heart transplant patients [abstract]. Circulation 1999 Nov 2; 100 Suppl.: 1–391
Eisen HJ, Mueller EA, Türkin D, et al. Multicenter, randomized, double-blind study on efficacy and safety of microemulsion cyclosporine versus conventional cyclosporine in de novo heart transplant recipients: six month results [abstract]. Transplantation 1998 Jun 27; 65: S189
White SA, Jain S, Williams ST, et al. Randomized trial comparing Neoral and tacrolimus immunosuppression for recipients of renal transplants procured from different donor groups. Transplant Proc 2000 May; 32: 600
Morris-Stiff G, Ostrowski K, Balaji V, et al. Prospective randomised study comparing tacrolimus (Prograf) and cyclosporin (Neoral) as primary immunosuppression in cadaveric renal transplants at a single institution: interim report of the first 80 cases. Transplant Int 1998; 11 Suppl. 1: S334–6
Busque S, Shoker A, Landsberg D, et al. Canadian multicentre trial of tacrolimus/azathioprine/steroids versus tacrolimus/mycophenolate mofetil/steroids versus Neoral/mycophenolate mofetil/steroids in renal transplantation. Transplant Proc 2001; 33: 1266–7
Gonwa TA, Johnson C, Ahsan N, et al. Two year followup of a randomized multicenter kidney transplant study comparing tacrolimus (PG) + azathioprine (AZA) vs cyclosporine (Neoral) + mycophenolate mofetil (MMF) vs tacrolimus + MMF [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: SI 13
Johnson C, Ahsan N, Gonwa T, et al. Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation. Transplantation 2000 Mar 15; 69: 834–41
Morris-Stiff G, Khan A, Quiroga I, et al. Immunosuppression in renal transplantation: meta-analysis should not have included one of the studies [letter]. BMJ 1999; 319: 1136
Jurewicz WA. Immunological and nonimmunological risk factors with tacrolimus and Neoral in renal transplant recipients: an interim report. Transplant Proc 1999 Nov; 31: 64S–6S
Raofi V, Holman DM, Coady N, et al. A prospective randomized trial comparing the efficacy of tacrolimus versus cyclosporine in Black recipients of primary cadaveric renal transplants. Am J Surg 1999; 177(4): 299–302
Sperschneider H. A large, multicentre trial to compare the efficacy and safety of tacrolimus with cyclosporine microemulsion following renal transplantation. European Renal Transplantation Study Group. Transplant Proc 2001; 33: 1279–81
Yang HC, Holman MJ, Langhoff E, et al. Tacrolimus/‘low-dose’ mycophenolate mofetil versus microemulsion cyclosporine/‘low-dose’ mycophenolate mofetil after kidney transplantation — 1-year follow-up of a prospective, randomized clinical trial. Transplant Proc 1999; 31(1–2): 1121–4
Bruce DS, Woodle ES, Newell KA, et al. Tacrolimus/mycophenolate provides superior immunosuppression relative to Neoral/mycophenolate in synchronous pancreas-kidney transplantation. Transplant Proc 1998 Jun; 30: 1538–40
Bruce DS, Woodle ES, Newell KA, et al. Effects of tacrolimus, mycophenolate mofetil, and cyclosporine microemulsion on rejection incidence in synchronous pancreas-kidney transplantation. Transplant Proc 1998; 30(2): 507–8
Stegall MD, Simon M, Wachs ME, et al. Mycophenolate mofetil decreases rejection in simultaneous pancreas-kidney transplantation when combined with tacrolimus or cyclosporine. Transplantation 1997 Dec 27; 64: 1695–700
Fisher RA, Ham JM, Marcos A, et al. A prospective randomized trial of mycophenolate mofetil with Neoral or tacrolimus after orthotopic liver transplantation. Transplantation 1998 Dec 27; 66: 1616–21
Rolles K, Davidson BR, Burroughs AK. A pilot study of immunosuppressive monotherapy in liver transplantation: tacrolimus versus microemulsified cyclosporin. Transplantation 1999 Oct 27; 68: 1195–8
Canadian Liver Transplant Study Group. The Canadian Prograf in Liver Transplant Trial; the one-year composite outcome [abstract]. Transplantation 1998 Jun 27; 65(12): S14
Stegall MD, Wachs ME, Everson G, et al. Prednisone withdrawal 14 days after liver transplantation with mycophenolate: a prospective trial of cyclosporine and tacrolimus. Transplantation 1997 Dec 27; 64: 1755–60
Zervos XA, Weppler D, Fragulidis GP, et al. Comparison of tacrolimus with microemulsion cyclosporine as primary immunosuppression in hepatitis C patients after liver transplantation. Transplantation 1998 Apr 27; 65: 1044–6
Mühlbacher F. Tacrolimus versus cyclosporin microemulsion in liver transplantation: results of a 3-month study. European Liver Transplantation Tacrolimus vs Cyclosporin Microemulsion Study Group. Transplant Proc 2001; 33: 1339–40
Klupp J, Glanemann M, Bechstein WO, et al. Mycophenolate mofetil in combination with tacrolimus versus Neoral after liver transplantation. Transplant Proc 1999 Feb-Mar; 31: 1113–4
Fisher RA, Wolfe L, Ham JM, et al. 2 Year follow up of a prospective randomized trial of mycophenolate mofetil (MM) with Neoral or tacrolimus following orthotopic liver transplantation [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: S387
O’Grady JG. Tacrolimus vs microemulsified cyclosporine in liver transplantation: preliminary results of the TMC trial [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: 165
Kobashigawa JA, Moriguchi JD, Takemoto S, et al. First year results of a randomized trial of tacrolimus vs Neoral cyclosporine in heart transplant patients [abstract]. J Heart Lung Transplant 2000 Jan; 19: 47
Mehra MR, Uber PA, Park MH, et al. A randomized comparison of an immunosuppressive strategy using tacrolimus and cyclosporine in Black heart transplant patients. Transplant Proc 2001; 33: 1606–7
Groth CG, Bäckman L, Morales J-M, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Transplantation 1999; 67(7): 1036–42
Kreis H, Cisterne JM, Land W, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 2000; 69(7): 1252–60
Higgins RM, Hart P, Lam FT, et al. Conversion from tacrolimus to cyclosporine in stable renal transplant patients: safety, metabolic changes, and pharmacokinetic comparison. Transplantation 2000 Apr 27; 69: 1736–9
Abouljoud M, Kumar MSA, Brayman K, et al. Conversion to Neoral provides effective rescue therapy for liver and kidney transplant patients intolerant of Prograf. Transplant Proc 2001; 33: 1027–8
Jain A, Brody D, Hamad I, et al. Conversion to Neoral for neurotoxicity after primary adult liver transplantation under tacrolimus. Transplantation 2000 Jan 15; 69: 172–6
Yonan NA, Aziz T, El-Gamel A, et al. Long-term safety and efficacy of Neoral in heart transplantation. Transplant Proc 1998 Aug; 30: 1906–9
Birkeland SA. Steroid-free immunosuppression after kidney transplantation with antithymocyte globulin induction and cyclosporine and mycophenolate mofetil maintenance therapy. Transplantation 1998; 66(9): 1207–10
Kim YS, Moon JI, Kim SI, et al. Clear benefit of mycophenolate mofetil-based triple therapy in reducing the incidence of acute rejection after living donor renal transplantations. Transplantation 1999 Aug 27; 68: 578–81
Carmellini M, Vistoli F, Bellini R, et al. Mycophenolate mofetil/Neoral/steroid vs Neoral/steroid therapy for prophylaxis of acute rejection in renal transplant recipients. Transplant Proc 1999 Feb–Mar; 31: 1162–4
Wiesel M, Carl S. A placebo controlled study of mycophenolate mofetil used in combination with cyclosporine and corticoste-roids for the prevention of acute rejection in renal allograft recipients: 1-year results. European Mycophenolate Mofetil Cooperative Study Group. J Urol 1998; 159: 28–33
Herrero JC, Morales E, Dominguez-Gil B, et al. Mycophenolate mofetil, cyclosporine, and steroids after renal transplantation: five-year results at a single center. Transplant Proc 1999; 31: 2263–4
Kahan BD, Rajagopalan PR, Hall ML, et al. Basiliximab (Simulect™) is efficacious in reducing the incidence of acute rejection episodes in renal allograft patients: results at 12 months [abstract]. Transplantation 1998 Jun 27; 65: S189
Nashan B, Moore R, Amlot P, et al. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet 1997 Oct 25; 350: 1193–8
Nashan B, Light S, Hardie IR, et al. Reduction of acute renal allograft rejection by daclizumab. Transplantation 1999; 67(1): 110–5
Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. Rapamune US Study Group. Lancet 2000 Jul 15; 356: 194–202
MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. The Rapamune Global Study Group. Transplantation 2001; 71(2): 271–80
Hueso M, Bover J, Serón D, et al. Low-dose cyclosporine and mycophenolate mofetil in renal, allograft recipients with suboptimal renal function. Transplantation 1998; 66(12): 1727–31
Burke JF, Francos GC, Francos BB, et al. A double-blind, placebo-controlled, three-year study of steroid withdrawal using a Neoral®-based immunosuppressive regimen in primary renal transplant recipients: an interim report [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: S224
Boletis JN, Konstadinidou I, Chelioti H, et al. Successful withdrawal of steroid after renal transplantation. Transplant Proc 2001; 33: 1231–3
Vincenti F, Monaco A, Grinyo J, et al. Rapid steroid withdrawal versus standard steroid treatment in patients treated with Simulect®, Neoral®, and Cellcept®for the prevention of acute rejection in renal transplantation: a multicenter, randomized trial [abstract]. Transplantation 2000 Apr 27; 69 Suppl.: S133
Steroid Withdrawal Study Group. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil: a prospective randomized study. Transplantation 1999; 68(12): 1865–74
Vanrenterghem Y, Lebranchu Y, Hené R, et al. Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 2000; 70(9): 1352–9
Sterneck M, Fischer L, Gahlemann C, et al. Mycophenolate mofetil for prevention of liver allograft rejection: initial results of a controlled clinical trial. Ann Transplant 2000; 5(1): 43–6
Coukell AJ, Plosker GL. Cyclosporin microemulsion (Neoral®): a pharmacoeconomic review of its use compared with standard cyclosporin in renal and hepatic transplantation. Pharma-coeconomics 1998; 14(6): 691–708
Keown P, Lawen JG, Landsberg D, et al. Economic analysis of Sandimmune Neoral in Canada in stable renal transplant patients. Transplant Proc 1995 Apr; 27: 1845–8
Hardens M, Vos WFH, Kobelt-Nguyen G, et al. A retrospective economic analysis of Sandimmun Neoral in de novo and in stable renal transplantation patients in Germany, Austria, Switzerland and Italy [abstract]. European Symposium on Pharmacoeconomics. Gent,Belgium. 1994 May 18–20:21–26
Kingma I, Ludwin D, Dandavino R, et al. Economic analysis of Neoral in de novo renal transplant patients in Canada. Clin Transplant 1997 Feb; 11: 42–8
Baillie GM, Baliga PK, Douzdjian V, et al. Impact of treatment with Neoral versus Sandimmune on outcome variables in liver transplant recipients [abstract]. Hepatology 1997 Oct; 26 (Pt 2): 237a
Arumugam R, Soriano HE, Scheimann AO, et al. Immunosup-pressive therapy with microemulsion cyclosporine A shortens the hospitalization of pediatric liver transplant recipients. Clin Transplant 1998 Dec; 12: 588–92
Karademir S, Sankary H, Fabrega F, et al. Neoral, a cyclosporine microemulsion allows cost savings without adversely affecting clinical or histological variables following orthotopic liver transplantation [abstract]. Hepatology 1997 Oct; 26 (Pt 2 Suppl.): 536
Donovan JP, Sorrell MF, Langnas AN, et al. A comparison of two cyclosporine preparations in long-term liver transplant patients [abstract]. Hepatology 1997; 26 Suppl. (Pt 2): 237A
Peeters P, Kazek M, Abella I, et al. Economic evaluation of Neoral versus Sandimmune maintenance therapy for de novo liver transplant patients: results from an international randomized controlled trial. Milton Study Group. Transplant Proc 1998 Aug; 30: 1838–42
Cogny-Van Weydevelt F, Ngohou C, Bacquaert-Dufour K, et al. Economical interest of Neoral in kidney transplanted recipients [abstract]. Nephrol Dial Transplant 1997 Sep; 12: A204
Hemming AW, Cattral MS, Greig PD, et al. A pharmacoeconomic analysis of Neoral without intravenous cyclosporine in liver transplantation in Canada. Clin Transplant 1998 Oct; 12: 425–9
Everson GT, Shrestha R, Trouillot T, et al. Costs of cyclosporine (Neoral®) and tacrolimus (Prograf®) in the first year after liver transplantation (OLT) [abstract]. Transplantation 1998 Jun 27;65:S51
Arumugam R, Soriano HE, Ozaki CF, et al. Microemulsion cyclosporin (Neoral) immunosuppression for orthotopic liver transplantation (OLT) in children reduces hospital stay [abstract no. 191]. Fourth Congress of the ILTS; 1997 Oct 15–17; Seattle (WA), C-78
Morris-Stiff G, Richards T, Singh J, et al. Pharmaco-economic study of FK 506 (Prograf) and cyclosporine A Neoral in cadaveric renal transplantation. Transplant Proc 1998 Jun; 30: 1285–6
Rossi SJ, Schroeder TJ, Hariharan S, et al. Prevention and management of the adverse effects associated with immunosup-pressive therapy. Drug Saf 1993 Aug; 9: 104–31
Hansen JM, Fogh-Andersen N, Christensen NJ, et al. Cyclosporine-induced hypertension and decline in renal function in healthy volunteers. J Hypertens 1997 Mar; 15: 319–26
Brennan DC, Barbeito R, Burke J, et al. Safety of Neoral conversion in maintenance renal transplant patients: a one-year, double-blind study. Novartis OLN-353 Study Group. Kidney Int 1999 Aug; 56: 685–91
Cole E, Keown P, Landsberg D, et al. Safety and tolerability of cyclosporine and cyclosporine microemulsion during 18 months of follow-up in stable renal transplant recipients: a report of the Canadian Neoral Renal Study Group. Transplantation 1998 Feb 27; 65: 505–10
Pescovitz MD, Barone G, Choc Jr MG, et al. Safety and tolerability of cyclosporine microemulsion versus cyclosporine: two-year data in primary renal allograft recipients: a report of the Neoral Study Group. Transplantation 1997 Mar 15; 63: 778–80
Shah MB, Martin JE, Schroeder TJ, et al. Evaluation of the safety and tolerability of Neoral and Sandimmune: a meta-analysis. Transplant Proc 1998 Aug; 30: 1697–700
Grant D, Rochon J, Levy G. Comparison of the long-term tolerability, pharmacodynamics, and safety of Sandimmune and Neoral in liver transplant recipients. Ontario Liver Transplant Study Group. Transplant Proc 1996 Aug; 28: 2232–3
Truwit CL, Denaro CP, Lake JR, et al. MR imaging of reversible cyclosporin A-induced neurotoxicity. Am J Neuroradiol 1991; 12(4): 651–9
de Groen PC, Aksamit AJ, Rakela J, et al. Central nervous system toxicity after liver transplantation: the role of cyclosporine and cholesterol. N Engl J Med 1987; 317(14): 861–6
Fryer JP, Fortier MV, Metrakos P, et al. Central pontine myelinolysis and cyclosporine neurotoxicity following liver transplantation. Transplantation 1996; 61(4): 658–61
Buchholz B, Korn A. Safety and tolerability of Sandimmun Neoral vs Sandimmun in de novo renal transplant patients after 24 months’ treatment. German Neoral Study Group. Transplant Proc 1996 Aug; 28: 2187–8
Barone G, Bunke CM, Choc Jr MG, et al. Safety and tolerability of Neoral vs Sandimmune: 1-year data in primary renal allograft recipients. Neoral Study Group. Transplant Proc 1996 Aug; 28: 2183–6
Offermann G, Korn A. Safety and tolerability of CyA microemulsion formulation (Sandimmun Neoral) in stable renal transplant patients after 24 months of treatment. German Neoral Study Group. Transplant Proc 1996 Aug; 28: 2204–6
Hsieh H, Chien YS, Hsu KT, et al. Conversion to Sandimmun Neoral in stable renal transplant recipients over 1 year: hepatitis, liver dysfunction, dosing intervals, and therapeutic ranges. Transplant Proc 1998 Nov; 30: 3552–4
Gentil Govantes MA, Gómez Ullate P, Errasti P, et al. Improvement of correlation between oral dose of cyclosporine and cyclosporinemia after substitution of cyclosporine standard presentation by cyclosporine microemulsion one, in 1345 patients with kidney transplantation. Spanish Sandimmune Neoral Conversion Group. Transplant Proc 1998 Aug; 30: 1658–9
Moore RH. UK multicentre study to assess the safety and tolerability of Neoral in stable renal transplant patients. U.K. Neoral Study Group. Transplant Proc 1996 Aug; 28: 2202–3
Feutren G, Wong R, Jin J, et al. Safety and tolerability of Neoral in transplant recipients. Transplant Proc 1996 Aug; 28: 2177–82
Gaspari F, Perico N, Pisoni R, et al. How to convert from traditional cyclosporine to the microemulsion formulation in stable renal transplant patients? Clin Transplant 1998 Oct; 12: 379–90
Pescovitz MD. Conversion from Sandimmune to Neoral in stable renal transplant recipients. Sandoz Study Group OLN-353. Transplant Proc 1996 Aug; 28: 2196–8
Baruch Y, Assy N, Kramsky R, et al. Safety of conversion from cyclosporine Sandimmune to cyclosporine Neoral in stable liver transplant patients. Transplant Proc 1998 Aug; 30: 1852–3
Pethig K, Ruhparwar A, Korn A, et al. Conversion from Sandimmune to Neoral in stable heart transplant recipients. Transplant Proc 1996 Aug; 28: 2287–9
Seydoux C, Stumpe F, Hurni M, et al. Renal function one year after switching from Sandimmun to Neoral. Clin Transplant 1999 Dec; 13:461–4
Shah MB, Martin JE, Schroeder TJ, et al. Validity of open labeled versus blinded trials: a meta-analysis comparing Neoral and Sandimmune. Transplant Proc 1999; 31(1–2): 217–9
Shah MB, Martin JE, Schroeder TJ, et al. The evaluation of the safety and tolerability of two formulations of cyclosporine: Neoral and Sandimmune. A meta-analysis. Transplantation 1999 Jun 15; 67: 1411–7
Moore RH. UK multicentre study to assess the safety and tolerability of Neoral in stable renal transplant patients. UK Neoral Study Group. Transplant Int 1996; 9 Suppl. 1: S311–3
Shah MB, Martin JE, Schroeder TJ, et al. A meta-analysis to assess the safety and tolerability of two formulations of cyclosporine: Sandimmune and Neoral. Transplant Proc 1998 Dec; 30: 4048–53
Warshaw A, Supran S, Barefoot L, et al. Neoral is associated with less neurotoxicity after liver transplantation [abstract]. Transplantation 1999 Apr 15; 67: S191
Kobashigawa JA, Moriguchi JD, Chuang JM, et al. A randomized trial of tacrolimus vs Neoral cyclosporine in heart transplant recipients [abstract]. J Am Coll Cardiol 2000 Feb; 35 Suppl. A: 222A
Armenti VT, McGrory CH, Cater JR, et al. Pregnancy outcomes in female renal transplant recipients. Transplant Proc 1998; 30(5): 1732–4
Nyberg G, Haljamäe U, Frisenette-Fich C, et al. Breast-feeding during treatment with cyclosporine. Transplantation 1998; 65(2): 253–5
Food and Drug Administration. FDA Talk Paper. Nationwide recall of SangCya oral solution [online]. FDA; 2000 Jul 10. Available from URL: http://www.fda.gov/bbs/topics/AN-SWERS/ANS01025.html [Accessed 2001 Jul 19]
Abbott Laboratories Online. Gengraf (cyclosporine capsules, USP [modified]). Abbott Laboratories Inc.; 2000 Oct. Available from URL: http://www.rxabbott.com/ot/gen/gen02/htm
Plosker GL, Foster RH. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs 2000; 59(2): 323–89
Bardsley-Elliot A, Noble S, Foster RH. Mycophenolate mofetil: a review of its use in the management of solid organ transplantation. Biodrugs 1999 Nov; 12: 363–410
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: A. Åsberg, Laboratory for Renal Physiology, The National Hospital, Oslo, Norway; P. Atkison, London Health Sciences Centre, London, Ontario, Canada; S.A. Birkeland, Department of Nephrology, Odense University Hospital, Odense, Denmark; A. Chandraker, Laboratory of Immunogenetics and Transplantation, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA; M.H.H. Ensom, Faculty of Pharmaceutical Sciences, Children’s and Women’s Health Centre of British Columbia, Vancouver, British Columbia, Canada; R.A. Fisher, Transplant Division, Medical College of Virginia/Virginia Commonwealth University, Richmond, Virginia, USA; U. Frei, Virchow-Klinikum, Humboldt-Universität, Berlin, Germany; J.M. Grinyó, Department of Nephrology, Hospital Prínceps d’España, Barcelona, Spain; A.B. Jain, Thomas E. Starzl Transplantation Institute, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA; G.V. Mazariegos, Thomas E. Starzl Transplantation Institute, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA; B. Nashan, Klinik für Viszeral- und Transplantation-schirurgie, Medizinische Hochschule Hannover, Hannover, Germany; A.J. Olyaei, Division of Nephrology, Hypertension and Clinical Pharmacology, Oregon Health Sciences University, Portland, Oregon, USA; R. Pollak, Division of Transplantation, Department of Surgery, University of Illinois at Chicago, Chicago, Illinois, USA.
Data Selection
Sources: Medical literature published in any language since November 1995 on cyclosporin microemulsion, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘cyclosporin microemulsion’ or ‘cyclosporin modified’ or ‘microemulsion-based formulation’ or ‘cyclosporin A and microemulsion’. EMBASE search terms were ‘cyclosporin microemulsion’ or ‘microemulsion-based formulation’ or ‘cyclosporin A and microemulsion’. AdisBase search terms were ‘cyclosporin microemulsion’ or ‘cyclosporin-modified’ or ‘microemulsion-based formulation’. Searches were last updated 7 Sep 2001.
Selection: Studies in patients undergoing solid organ transplantation who received cyclosporin microemulsion. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Cyclosporin, pharmacodynamics, pharmacokinetics, therapeutic use, pharmacoeconomics, tolerability, dosage and administration, transplant rejection, review.
Rights and permissions
About this article
Cite this article
Dunn, C.J., Wagstaff, A.J., Perry, C.M. et al. Cyclosporin. Drugs 61, 1957–2016 (2001). https://doi.org/10.2165/00003495-200161130-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161130-00006