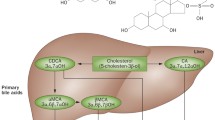
Abstract
Bile acids promote bile formation and facilitate dietary lipid absorption. Animal and human studies showing disturbed bile acid metabolism in diabetes mellitus suggest a link between bile acids and glucose control. Bile acids are activating ligands of the farnesoid X receptor (FXR), a nuclear receptor with an established role in bile acid and lipid metabolism. Evidence suggests a role for FXR also in maintenance of glucose homeostasis. Animal and human studies employing bile acid sequestrants (bile acid binding agents), which interrupt the enterohepatic circulation of bile acids and effectively reduce plasma cholesterol, support a link between bile acid and glucose metabolism. In lipid-lowering trials, bile acid sequestrants, such as colesevelam hydrochloride, colestyramine (cholestyramine) and colestilan (colestimide), have also been shown to lower plasma glucose and glycosylated haemoglobin levels, suggesting the utility of these agents as a potential therapy for type 2 diabetes. In this article, we review the relationship between bile acid metabolism and glucose homeostasis, and present data demonstrating the utility of bile acid sequestrants in the management of diabetes.




Similar content being viewed by others
References
International Diabetes Federation. Diabetes atlas [online]. Available from URL: http://www.eatlas.idf.org [Accessed 2006 Aug 21]
DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care 1992 Mar; 15(3): 318–68
Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65(3): 385–411
Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. Embo J 2006 Apr 5; 25(7): 1419–25
Modica S, Moschetta A. Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett 2006 Oct 9; 580(23): 5492–9
Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol 2005 Oct; 25(10): 2020–30
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006 Jan 26; 439(7075): 484–9
Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus: a short-term, double-blind, crossover trial. Ann Intern Med 1994 Sep 15; 121(6): 416–22
Zieve FJ, Kalin MF, Schwartz SL, et al. Results of the Glucose-Lowering effect of WelChol Study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther 2007; 29(1): 74–83
Weinman SA, Kemmer N. Bile secretion and cholestasis. In: Yamada T, editor. Textbook of gastroenterology. 4th ed. Philadelphia (PA): Lippincott Williams and Wilkins, 2003: 366–88
Insull Jr W. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J 2006 Mar; 99(3): 257–73
Hassan AS, Ravi Subbiah MT, Thiebert P. Specific changes of bile acid metabolism in spontaneously diabetic Wistar rats. Proc Soc Exp Biol Med 1980 Sep; 164(4): 449–52
Hassan AS, Hedeen K, Ravi Subbiah MT. Effect of maternal diabetes on fetal bile acid metabolism in the rat. Biochem Med 1981 Apr; 25(2): 168–73
Nervi FO, Gonzalez A, Valdivieso VD. Studies on cholesterol metabolism in the diabetic rat. Metabolism 1974 Jun; 23(6): 495–503
Wey HE, Yunker RL, Harris P, et al. Effect of streptozotocin-induced diabetes in neonatal rat on bile acid pool changes in adult life: selective sensitivity in females. Biochem Med 1984 Apr; 31(2): 167–73
Nervi FO, Severin CH, Valdivieso VD. Bile acid pool changes and regulation of cholate synthesis in experimental diabetes. Biochim Biophys Acta 1978 May 25; 529(2): 212–23
van Waarde WM, Verkade HJ, Wolters H, et al. Differential effects of streptozotocin-induced diabetes on expression of hepatic ABC-transporters in rats. Gastroenterology 2002 Jun; 122(7): 1842–52
Uchida K, Makino S, Akiyoshi T. Altered bile acid metabolism in nonobese, spontaneously diabetic (NOD) mice. Diabetes 1985 Jan; 34(1): 79–83
Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000 Jul; 6(1): 87–97
Villanueva GR, Herreros M, Perez-Barriocanal F, et al. Enhancement of bile acid-induced biliary lipid secretion by streptozotocin in rats: role of insulin deficiency. J Lab Clin Med 1990 Apr; 115(4): 441–8
Twisk J, Hoekman MF, Lehmann EM, et al. Insulin suppresses bile acid synthesis in cultured rat hepatocytes by down-regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase gene transcription. Hepatology 1995 Feb; 21(2): 501–10
Hyogo H, Roy S, Paigen B, et al. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J Biol Chem 2002 Sep 13; 277(37): 34117–24
Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med 1977 Jun 16; 296(24): 1365–71
Abrams JJ, Ginsberg H, Grundy SM. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes 1982 Oct; 31(10): 903–10
Andersen E, Hellstrom P, Hellstrom K. Cholesterol biosynthesis in nonketotic diabetics before and during insulin therapy. Diabetes Res Clin Pract 1987 Jul–Aug; 3(4): 207–14
Andersen E, Karlaganis G, Sjovall J. Altered bile acid profiles in duodenal bile and urine in diabetic subjects. Eur J Clin Invest 1988 Apr; 18(2): 166–72
Farmer JA, Gotto AM Jr. Choosing the right lipid-regulating agent. A guide to selection. Drugs 1996 Nov; 52(5): 649–61
The Lipid Research Clinics Coronary Primary Prevention Trial results: I. Reduction in incidence of coronary heart disease. JAMA 1984 Jan 20; 251(3): 351–64
Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med 1990 Nov 8; 323(19): 1289–98
Eckel RH. Treating dyslipidemia of the metabolic syndrome: where’s the evidence? Nat Clin Pract Endocrinol Metab 2007 Jun; 3(6): 437
Koike K, Murakami K, Nozaki N, et al. Colestilan, a new bile acid-sequestering resin, reduces bodyweight in postmenopausal women who have dieted unsuccessfully. Drugs R D 2005; 6(5): 273–9
Brand SJ, Morgan RG. Stimulation of pancreatic secretion and growth in the rat after feeding cholestyramine. Gastroenterology 1982 Oct; 83(4): 851–9
Koop I, Lindenthal M, Schade M, et al. Role of cholecystokinin in cholestyramine-induced changes of the exocrine pancreas. Pancreas 1991 Sep; 6(5): 564–70
Kogire M, Gomez G, Uchida T, et al. Chronic effect of oral cholestyramine, a bile salt sequestrant, and exogenous cholecystokinin on insulin release in rats. Pancreas 1992; 7(1): 15–20
Kobayashi M, Ikegami H, Fujisawa T, et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes 2007 Jan; 56(1): 239–47
Bays H, Goldberg R, Truitt K, et al. Addition of colesevelam HCl to patients with type 2 diabetes mellitus inadequately controlled on a metformin-based therapy improves glycemic control [abstract no. 204]. 16th Annual American Association of Clinical Endocrinologists Meeting and Clinical Congress; 2007 Apr 11–15; Seattle (WA), 18
Fonseca V, Rosenstock J, Truitt K, et al. Colesevelam HCl added to sulfonylurea-based therapy in patients with inadequately controlled type 2 diabetes mellitus improves glycemic control [abstract no. 409]. 16th Annual American Association of Clinical Endocrinologists Meeting and Clinical Congress; 2007 Apr 11–15 Seattle (WA), 10
Goldberg R, Truitt K. Colesevelam HCl improves glycemic control in type 2 diabetes mellitus subjects managed with insulin therapy [abstract no. 1581]. American Heart Association Scientific Sessions; 2006 Nov 12–15; Chicago (IL)
Kawabata Y, Ikegami H, Fujisawa T, et al. Bile-acid binding resin ameliorates glycemic control in patients with type 2 diabetes [abstract]. Diabetes 2006; 55 Suppl. 1: A120
Hashim SA, Bergen SS Jr, Van Itallie TB. Experimental steatorrhea induced in man by bile acid sequestrant. Proc Soc Exp Biol Med 1961 Jan; 106: 173–5
Thomson AB, Keelan M. Feeding rats diets containing chenoor ursodeoxycholic acid or cholestyramine modifies intestinal uptake of glucose and lipids. Digestion 1987; 38(3): 160–70
Cariou B, Duran-Sandoval D, Kuipers F, et al. Farnesoid X receptor: a new player in glucose metabolism? Endocrinology 2005 Mar; 146(3): 981–3
De Fabiani E, Mitro N, Gilardi F, et al. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem 2003 Oct 3; 278(40): 39124–32
Duran-Sandoval D, Cariou B, Fruchart JC, et al. Potential regulatory role of the farnesoid X receptor in the metabolic syndrome. Biochimie 2005 Jan; 87(1): 93–8
Duran-Sandoval D, Mautino G, Martin G, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes 2004 Apr; 53(4): 890–8
Stayrook KR, Bramlett KS, Savkur RS, et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 2005 Mar; 146(3): 984–91
Yamagata K, Daitoku H, Shimamoto Y, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxol. J Biol Chem 2004 May 28; 279(22): 23158–65
Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A 2006 Jan 24; 103(4): 1006–11
Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science 1999 May 21; 284(5418): 1362–5
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999 May 21; 284(5418): 1365–8
Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999 May; 3(5): 543–53
Forman BM, Goode E, Chen J, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995 Jun 2; 81(5): 687–93
Kok T, Hulzebos CV, Wolters H, et al. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem 2003 Oct 24; 278(43): 41930–7
Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 2006 Apr 21; 281(16): 11039–49
Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of hepatocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem 2001 Nov 9; 276(45): 41690–9
Inoue Y, Yu AM, Yim SH, et al. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J Lipid Res 2006 Jan; 47(1): 215–27
Cariou B, van Harmelen K, Duran-Sandoval D, et al. Transient impairment of the adaptive response to fasting in FXR-deficient mice. FEBS Lett 2005 Aug 1; 579(19): 4076–80
Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 2006 Apr; 116(4): 1102–9
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000 Aug 12; 321(7258): 405–12
Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003 Jan 30; 348(5): 383–93
Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004 Jan 21; 291(3): 335–42
Acknowledgements
Bart Staels and Folkert Kuipers were supported by the EU Grant Hepadip (N° 018734). Bart Staels received additional support by a grant from the Agence Nationale de la Recherche (N° A05056GS). Editorial support was provided by Karen Stauffer, PhD and funded by Daiichi Sankyo, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Staels, B., Kuipers, F. Bile Acid Sequestrants and the Treatment of Type 2 Diabetes Mellitus. Drugs 67, 1383–1392 (2007). https://doi.org/10.2165/00003495-200767100-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767100-00001