Abstract
Background
Influenza is a potentially life-threatening illness that affects approximately 10% of the population annually, with resulting personal misery and societal disruption. Oseltamivir is a novel influenza treatment that has been extensively investigated. We describe a series of retrospective analyses investigating various measures of clinical efficacy across different populations of influenza-infected patients enrolled in studies of oseltamivir (Tamiflu®) that were conducted within the clinical development programme.
Methods
Adolescents and adults (13–97 years, n = 4015) presenting within 36 hours of onset of influenza symptoms were randomised to receive oseltamivir 75mg or placebo twice daily for 5 days during local influenza outbreaks. Of these patients, 2413 had laboratory-confirmed influenza and are included in the analysis. Approximately 30% (n = 739) of patients were ‘high risk’; 20% were healthy elderly subjects (n = 488) and 10% (n = 251) were patients with chronic respiratory and/or cardiac conditions. The primary endpoint was time to alleviation of a seven-symptom cluster in influenza-infected patients. Supplementary analyses were conducted using a variety of illness definitions and symptom clusters to investigate the sensitivity of the assessment of overall efficacy to differing disease definitions and also to explore efficacy in important subpopulations.
Results
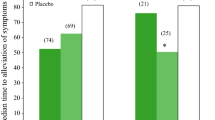
A total of 2413 patients had confirmed influenza infection (placebo: n = 1063; oseltamivir: n = 1350). Across all populations, the time to alleviation of illness was reduced by 19% (median duration 100.6 hours [95% CI 94.8–104.7]) compared with placebo (124.5 hours [95% CI 117.7–132.3], p < 0.0001). Oseltamivir recipients returned to normal health status, regained ability to perform usual activities and regained normal sleep patterns significantly faster than placebo recipients. The median duration of troublesome influenza symptoms was significantly reduced by oseltamivir treatment, e.g. fatigue by 29% and myalgia by 26% (both p < 0.0001). After 48 hours of treatment, 57% more placebo than oseltamivir recipients remained febrile, despite greater use of acetaminophen by placebo recipients. In addition, the median duration of acute febrile illness was significantly shortened by oseltamivir treatment compared with placebo in patients with cardiac disease (44.0 hours vs 64.7 hours, p = 0.026) or chronic obstructive airways disease (37.9 hours vs 53.8 hours, p = 0.004). Efficacy was similar among influenza A- and influenza B-infected patients. Oseltamivir was well tolerated, with transient gastrointestinal effects (observed in one in seven oseltamivir-treated patients compared with one in 12 patients on placebo) that only rarely resulted in study discontinuation.
Conclusions
Oral oseltamivir is a well tolerated and effective treatment for influenza in adolescents and adults, including the elderly and patients with chronic cardiac and/or respiratory disease.







Similar content being viewed by others
Notes
1The use of tradenames is for product identification purposes only and does not imply endorsement
References
Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2000; 49(RR-03): 1–38
Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Ann Rev Med 2000; 51: 407–21
Brown LE, Hampson AW, Webster RG, editors. Options for the control of influenza III. New York: Elsevier Science BV, 1996: 17–25
Kavet J. A perspective on the significance of pandemic influenza. Am J Public Health 1977; 67: 1063–70
Monto AS. Influenza: quantifying morbidity and mortality. Am J Med 1987; 82: 20–5
Air GM, Laver WG. The neuraminidase of influenza virus. Proteins 1989; 6: 341–56
Jackson HC, Roberts NA, Wang ZM, et al. Management of influenza: use of new antivirals and resistance in perspective. Clin Drug Invest 2000; 20: 447–54
KurowskiM, Barrett J, Waalberg E, et al. Oral oseltamivir rapidly delivers active drug levels to middle ear and sinuses in humans [abstract no 509]. International Conference on Antimicrobial Agents and Chemotherapy; 2000 Sept 17–20; Toronto
Nicholson KG, Aoki FY, Osterhaus ADME, et al. on behalf of the Neuraminidase Inhibitor Flu Treatment Investigator Group. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 2000; 355: 1845–50
Robson R, Jennings L, Saiedabadi N, et al. Oral oseltamivir reduces the duration of influenza illness by 2.7 days in previously healthy adults [abstract no. 80.019]. Proceedings of the 9th International Congress of Infectious Diseases; 2000 Apr 10–13; Buenos Aires, 184
Treanor JJ, Hayden FG, Vrooman PS, et al. for the US Oral Neuraminidase Study Group. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized, controlled trial. JAMA 2000; 283: 1016–24
Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons Ltd, 1972
Martin C, Mahoney P, Ward P. Oral oseltamivir reduces febrile illness in patients considered at high risk of influenza complications. World Congress on options for the control of Influenza IV, Crete, Greece. International Congress Series 1219 (2001): 807-11.
Zaug M, Mahoney P, Ward P. Effective treatment of influenza with oral oseltamivir in a vaccinated population of high-risk patients. World Congress on options for the control of Influenza IV, Crete, Greece. International Congress Series 1219 (2001): 813-6.
Roche: Data on file
Hayden FG, Fritz R, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection: relation to symptom formation and host defence. J Clin Invest 1998; 101: 643–9
Hayden FG, Treanor JT, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized, controlled trials for prevention and treatment. JAMA 1999; 282: 1240–6
Aoki F, MacLeod M, Paggiaro P, et al. Early administration of oral oseltamivir maximises the benefits of influenza treatment. J Antimicrob Chemother 2003; 51: 123–9
Acknowledgements
F. Hoffmann-La Roche Ltd funded this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Barghoorn, J., Bagdonas, A. et al. Clinical Benefits with Oseltamivir in Treating Influenza in Adult Populations. Clin. Drug Investig. 23, 561–569 (2003). https://doi.org/10.2165/00044011-200323090-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200323090-00002