Published online Oct 14, 2013. doi: 10.3748/wjg.v19.i38.6398
Revised: July 18, 2013
Accepted: August 8, 2013
Published online: October 14, 2013
Bilirubin, a major end product of heme breakdown, is an important constituent of bile, responsible for its characteristic colour. Over recent decades, our understanding of bilirubin metabolism has expanded along with the processes of elimination of other endogenous and exogenous anionic substrates, mediated by the action of multiple transport systems at the sinusoidal and canalicular membrane of hepatocytes. Several inherited disorders characterised by impaired bilirubin conjugation (Crigler-Najjar syndrome type I and type II, Gilbert syndrome) or transport (Dubin-Johnson and Rotor syndrome) result in various degrees of hyperbilirubinemia of either the predominantly unconjugated or predominantly conjugated type. Moreover, disrupted regulation of hepatobiliary transport systems can explain jaundice in many acquired liver disorders. In this review, we discuss the recent data on liver bilirubin handling based on the discovery of the molecular basis of Rotor syndrome. The data show that a substantial fraction of bilirubin conjugates is primarily secreted by MRP3 at the sinusoidal membrane into the blood, from where they are subsequently reuptaken by sinusoidal membrane-bound organic anion transporting polypeptides OATP1B1 and OATP1B3. OATP1B proteins are also responsible for liver clearance of bilirubin conjugated in splanchnic organs, such as the intestine and kidney, and for a number of endogenous compounds, xenobiotics and drugs. Absence of one or both OATP1B proteins thus may have serious impact on toxicity of commonly used drugs cleared by this system such as statins, sartans, methotrexate or rifampicin. The liver-blood cycling of conjugated bilirubin is impaired in cholestatic and parenchymal liver diseases and this impairment most likely contributes to jaundice accompanying these disorders.
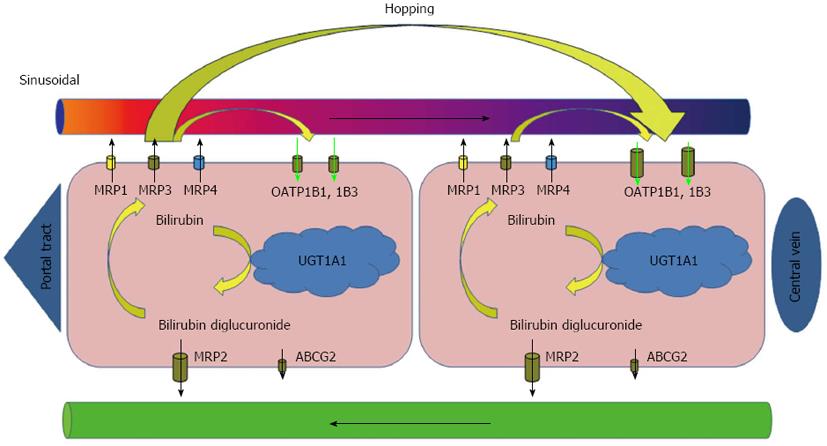
Core tip: Experiments with Oatp1a/1b-null mice and Oatp1a/1b; Abcc3 combination knockout mice plainly demonstrated that even under physiologic conditions a substantial portion of bilirubin glucuronides is not excreted directly into bile but is transported back to the blood by Abcc3. Oatp1a/1b activity accentuated in downstream (centrizonal) hepatocytes allows efficient reuptake of bilirubin conjugates, with a subsequent possibility being safely eliminated by excretion into bile. This and molecular findings in Rotor syndrome suggest that human transporters MRP3 and OATP1Bs form a sinusoidal liver-to-blood cycle which mediates shifting (hopping) of bilirubin and other substrates from periportal to centrizonal hepatocytes (References 18, 19, 22, 125).
- Citation: Sticova E, Jirsa M. New insights in bilirubin metabolism and their clinical implications. World J Gastroenterol 2013; 19(38): 6398-6407
- URL: https://www.wjgnet.com/1007-9327/full/v19/i38/6398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i38.6398
Bilirubin is the end product of heme breakdown. About 80% of bilirubin originates from degradation of erythrocyte haemoglobin in the reticuloendothelial system; the remaining 20% comes from inefficient erythropoiesis in bone marrow and degradation of other heme proteins[1-4]. Water insoluble, unconjugated bilirubin (UCB) bound to albumin is transported to the liver where it is removed from the plasma. The exact mechanism of UCB uptake is unknown; however, passive transmembrane diffusion seems to be combined with active transport mediated by several sinusoidal transporters (see below). Within the cytoplasm of hepatocytes, bilirubin is bound to ligandin and transported to endoplasmic reticulum where conjugation with glucuronic acid takes place. Conjugation is catalysed by the enzyme uridine diphosphate glycosyltransferase 1A1 (UGT1A1; EC2.4.1.17), a member of an enzyme family in the endoplasmic reticulum and nuclear envelope of hepatocytes[5-8]. In addition to the liver, UGT activity has also been detected in the small intestine and kidney[9,10]. UGT1A1 gene (ID: 54658) is a part of a complex locus encoding 13 UDP-glucuronosyltransferases[11]. The locus contains a series of thirteen unique alternate promoters and first exons, followed by four common exons No. 2-5. Theoretically, each of the unique first exons is spliced to the first of the four shared exons. The unique first exons encode different substrate binding domains whereas the other functional domains encoded by the shared exons 2-5 are the same[11-15]. In reality, only 9 of the 13 predicted UGT1As are active genes encoding functional enzymes; four are nonfunctional pseudogenes.
The excretion of conjugated bilirubin into bile is mediated by an ATP-dependent transporter identified as the multidrug resistance-associated protein MRP2/cMOAT and, to a lesser extent, also by ATP-binding cassette (ABC) efflux transporter ABCG2. MRP2 is encoded by ABCC2 and expressed under physiologic conditions at the apical (canalicular) membrane of hepatocytes and, to a much lesser extent, in the kidney, duodenum, ileum, brain and placenta[16]. Since the MRP2 mediated export represents an important step in detoxification of many endogenous and exogenous substrates, the absence of functionally active MRP2 prevents the secretion of these conjugates into bile. Absence of MRP2 mediated transport is followed by upregulation of the basolateral MRP2 homologues at the sinusoidal membrane of hepatocytes and conjugated bilirubin flow is redirected into sinusoidal blood[17]. Aside from MRP2 mediated transport of conjugated bilirubin into bile, recent studies have shown that a significant fraction of the bilirubin conjugated in the liver is, under physiologic conditions, secreted into sinusoidal blood and subsequently reuptaken by hepatocytes for final biliary excretion[18,19]. The process is mediated by sinusoidal transporters MRP3 and organic anion-transporting polypeptides OATP1B1 and OATP1B3. OATP1B transporters facilitate sodium-independent uptake of numerous endogenous and exogenous substrates[20,21]. Since expression of OATP1Bs is higher in centrilobular hepatocytes, the MRP3-OATP1B1/3 loop is likely responsible for shifting (hopping) of conjugated bilirubin and other substrates from the periportal to the centrilobular zone of the liver lobule (Figure 1). Such intralobular substrate transfer may protect periportal hepatocytes against elevated concentrations of various xenobiotics[22]. In addition, the OATP1B proteins mediate hepatic clearance of bilirubin conjugated in splanchnic organs and may represent an important alternative pathway in enterohepatic circulation[18].
OATP1Bs may also contribute to liver uptake of UCB since complete absence of both OATP1Bs in Rotor syndrome (RS, see below) is associated with elevated levels of UCB and single nucleotide polymorphisms in genes encoding OATP1B proteins have been shown to influence serum bilirubin level[23,24]. Furthermore, results of functional studies demonstrate that OATP1B3, but not OATP1B1, may play an important role in the carrier-mediated uptake of foetal UCB by the placental trophoblast and contribute to elimination of UCB across the placental barrier[25,26].
Mild or moderately elevated serum bilirubin seems to be beneficial: Bilirubin is known as a strong antioxidant[27,28] and the protective effects of bilirubin on atherogenesis and cancerogenesis have been demonstrated in both in vitro and in vivo studies[29-33]. On the other hand, patients with profound unconjugated hyperbilirubinemia are at risk for bilirubin encephalopathy (kernicterus)[34,35]. The toxic effects of bilirubin are explained by inhibition of DNA synthesis[36]. Bilirubin may also uncouple oxidative phosphorylation and inhibit adenosine triphosphatase (ATPase) activity of brain mitochondria[37,38]. Bilirubin mediated inhibition of various enzyme systems, RNA synthesis and protein synthesis in the brain and liver, and/or alteration of carbohydrate metabolism in the brain can also contribute to its toxicity[39-43]. The accumulation of bilirubin in plasma and tissues results in characteristic yellow discoloration of tissues known as icterus or jaundice.
Inherited disorders of bilirubin excretory pathway played the key role in understanding the individual steps of the bilirubin excretory pathway. Disrupted regulation of hepatobiliary transport systems explained jaundice in many acquired liver disorders[44-48]. Additional information was obtained from a number of animal models of hereditary jaundice. These include the Gunn rat and Ugt1(-/-) mouse mimicking the Crigler-Najjar syndrome type I[49-51], the Bolivian population of squirrel monkeys mimicking Gilbert syndrome (GS)[52,53] and mutant TR or GY (Groningen yellow) rats with organic anion excretion defect (TR -/-), Eizai hyperbilirubinuria rats (EHBR), mutant Corriedale sheep, and Mrp2(-/-) mice, all modelling the Dubin-Johnson syndrome (DJS)[54-58].
Conjugation of bilirubin in endoplasmic reticulum is catalysed by the enzyme UGT1A1. Mutations in UGT1A1 can lead to decreased expression or partial or even complete inactivation of the enzyme[59]. By contrast, expression of UGT1A1 can be increased by phenobarbital (PB) administration. PB response activity is delineated to a 290-bp distal enhancer module sequence (-3483/-3194) glucuronosyltransferase phenobarbital response enhancing motif (gtBPREM) of the human UGT1A1[59,60]. gtBPREM is activated by the nuclear orphan receptor, human constitutive active receptor (hCAR). CAR is a cytoplasmic receptor which, after treatment with activators such as PB, translocates into the nucleus, forms a heterodimer with the retinoid X receptor and activates the PB response enhancer element.
Three types of inherited, predominantly unconjugated hyperbilirubinemia with different levels of UGT1A1 activity are recognised: Crigler-Najjar syndrome type I (CN1), type II (CN2) and GS.
CN1 (MIM#218800), the most deleterious form, described in 1952 by Crigler and Najjar[61], is characterised by complete or almost complete absence of UGT1A1 enzyme activity with severe jaundice[62]. Icterus occurring shortly after birth is complicated by bilirubin encephalopathy (kernicterus). Until the introduction of phototherapy and plasmapheresis, kernicterus was fatal in almost all cases during the first two years of life or caused serious brain damage with permanent neurologic sequelae. Intermittent phototherapy is lifelong and it results in a thorough elimination of water-soluble photoisomers of unconjugated bilirubin via bile. The effectiveness of phototherapy may decrease gradually with age and patients are at higher risk of sudden brain damage[63].
Although new treatment modalities such as hepatocyte or hepatic progenitor cell transplantation have already been used to treat CN1 patients, liver transplantation is still considered to be the only definitive treatment for CN1[63-67]. Gene therapy seems to be a promising therapeutic possibility for the patients with CN1 in the near future[68,69].
CN2 (Arias syndrome, MIM #606785), described by Arias in 1962[70], is characterised by reduced UGT1A1 enzyme activity with a moderate degree of nonhemolytic jaundice. Bilirubin levels do not exceed 350 μmol/L and CN2 is only rarely complicated by kernicterus[71]. Virtually all the mutations responsible for the syndrome are autosomal recessive, as in CN1, but several observations have also suggested the possibility of autosomal dominant pattern of inheritance[72-74].
An important clinical difference between CN type I and type II is the response to PB treatment, with no effect in type I (complete loss of the UGT1A1 enzyme activity) and a decrease of serum bilirubin levels by more than 30% in CN type II (some residual UGT1A1 activity is preserved). Moreover, bilirubin glucuronides are present in bile in CN2. However, the method of choice for the diagnosis of CN syndrome is mutation analysis of UGT1A1[75].
GS (MIM #143500), described in 1901 by Gilbert and Lereboulet[76], is characterised by fluctuating mild, unconjugated nonhemolytic hyperbilirubinemia < 85 μmol/L without overt haemolysis, usually diagnosed around puberty, and aggravated by intercurrent illness, stress, fasting or after administration of certain drugs[77,78]. Physical examination and the results of routine laboratory tests are normal apart from elevated serum bilirubin and jaundice. The clinical diagnosis of GS can be established if patients have a mild, predominantly unconjugated hyperbilirubinemia and normal activity of liver enzymes. The reduced caloric intake test and phenobarbital stimulation test have low diagnostic specificity in GS subjects[79]. Histological findings in GS are mild, with a slight centrilobular accumulation of pigment with lipofuscin-like properties[80]. Ultrastructurally, hepatocytes reveal hypertrophy of smooth endoplasmic reticulum[81,82]. Since the morphological picture of GS is completely non-specific and the disorder is benign, liver biopsy is not indicated.
GS is characterised by reduced levels of UGT1A1 activity to about 25%-30% caused by homozygous, compound heterozygous, or heterozygous mutations in the UGT1A1 with autosomal recessive transmission[80].
GS is the most frequent hereditary jaundice affecting nearly 5%-10% of the Caucasian population[83]. The genetic basis of GS was first disclosed in 1995[84] as presence of the allele UGT1A1*28, characterised by insertion of TA in the TATAA box (A[TA]7TAA) in the proximal promoter of UGT1A1. UGT1A1*28 has been identified as the most frequent mutation in Caucasian GS subjects[85]. The insertion is responsible for reduction of transcription of UGT1A1 to 20% from normal and for a decrease of hepatic glucuronidation activity by 80% in a homozygous state[86]. In Caucasians and African Americans, the frequency of UGT1A1*28 allele is about 35%-40%, but it is much lower in Asians, including Koreans (13%), Chinese (16%), and Japanese (11%)[87-89]. Moreover, in the majority of Caucasian GS subjects, expression of UGT1A1 is further decreased by the presence of the second mutation T>G in gtPBREM[59,60]. In addition to the mutations in the promoter, GS may be caused by mutations in structural regions of the UGT1A1. In Asians, other variants, such as UGT1A1*6 characterised by a missense mutation involving G to A substitution at nucleotide 211 (c.211G>A) in exon 1 (also known as p.G71R), UGT1A1*7 (p.Y486D), UGT1A1*27 (p.P229Q), and UGT1A1*62 (p.F83L) have been detected[60,87-90].
In addition to biochemical defect leading to reduced glucuronidation, other factors, such as impaired hepatic (re)uptake of bilirubin (see Rotor syndrome below for the possible mechanism) or an increased load of bilirubin, seem to be necessary for clinical manifestation of GS[86,91,92].
GS is benign and GS carriers present with no liver disease. However, the mutations in the UGT1A1 identical to those recognised in GS subjects may contribute to the development of prolonged neonatal hyperbilirubinemia in breast-fed infants[93,94].
Moreover, since the process of glucuronidation is an important step in elimination of numerous endogenous and exogenous substrates, GS subjects may be more susceptible to the adverse effects of some drugs metabolised by UGT1A1, such as indinavir, atazanavir[95-99] or irinotecan[100-102].
Two types of hereditary conjugated jaundice are known as Dubin-Johnson and Rotor syndrome. Both are characterised by the presence of mixed, predominantly conjugated hyperbilirubinemia, with conjugated bilirubin more than 50% of total bilirubin.
DJS (MIM # 237500), a benign autosomal recessive disorder described in 1954 by Dubin et al[103] and Sprinz et al[104], is characterised by fluctuating mild, predominantly conjugated hyperbilirubinemia, with typical manifestation in adolescence or young adulthood. Most patients are asymptomatic except of occasional slight abdominal pain and fatigue. Urine excretion of total coproporphyrin in 24 h is normal, but 80% are represented by coproporphyrin I. Biliary excretion of anionic dyes including bromosulfophthalein (BSP), indocyanine green and cholescintigraphy radiotracers is delayed with absent or delayed filling of the gallbladder[105]. BSP clearance in DJS subjects is normal at 45 min with the second peak at 90 min[106]. Liver histology in DJS shows an accumulation of distinctive melanin-like lysosomal pigment in an otherwise normal liver that gives the organ a characteristic dark pink or even black colour. The pigment is positive in PAS and Masson-Fontana reaction with marked autofluorescence. In contrast to melanin, DJS pigment does not reduce neutral silver ammonium solution[103,107]. The amount of pigment may vary and possible transient loss may occur in coincidence with other liver diseases[108,109]. The molecular mechanism in DJS is absence or deficiency of human canalicular multispecific organic anion transporter MRP2/cMOAT caused by homozygous or compound heterozygous mutation in ABCC2 (gene ID: 1244) on chromosome 10q24[110-114]. The ABCC2 mutation alters not only MRP2-mediated transport of conjugated bilirubin but also transport of many anionic substrates as well as a wide range of drugs, such as chemotherapeutics, uricosurics, antibiotics, leukotrienes, glutathione, toxins and heavy metals. Absence of MRP2/cMOAT may result in impaired elimination and in subsequent renal toxicity of the substrates mentioned above[115-120].
A rare type of hereditary mixed hyperbilirubinemia caused by the simultaneous presence of mutations characteristic for DJS and GS has been classified as dual hereditary jaundice[121]. Serum direct bilirubin concentrations in dual hereditary jaundice reach only 20%-50% of total bilirubin.
RS (MIM #237450), described in 1948 by Rotor et al[122], is characterised by mild, predominantly conjugated hyperbilirubinemia with delayed excretion of anionic dyes without re-increase of their concentration. Total urinary coproporphyrin excretion is significantly increased and the proportion of coproporphyrin I in urine is approximately 65% of the total in homozygotes and 43% in heterozygotes[123,124]. By histopathological examination, the liver tissue does not display any marked architectural or cytomorphological abnormalities and pigment is not present.
The presence of homozygous mutations in both SLCO1B1 and SLCO1B3 neighbouring genes located on chromosome 12 with complete and simultaneous deficiency of proteins OATP1B1 and OATP1B3 has recently been identified as the molecular mechanism of the syndrome[125]. The complete absence of both transporters OATP1B1 and OATP1B3 has been confirmed by immunohistochemistry in all studied Rotor subjects. Interestingly, the presence of a single functional allele of either SLCO1B1 or SLCO1B3 prevented the jaundice.
RS does not require any therapy but, with regard to the impact of OATP1B transporters on pharmacokinetics of a broad spectrum of commonly used drugs such as penicillins, statins, sartans, rifampicin, methotrexate and many others, it is assumed that RS subjects and also those with the deleterious mutations in either of the SLCO1B genes, even without full clinical expression of the syndrome, may be at increased risk for drug toxicity[125-129].
Animal models of obstructive and intrahepatic cholestasis help us to discover and understand the main principles of acquired defects in hepatobiliary transport of bile salts and other organic anions. Up and down regulation of these mechanisms can explain impaired liver uptake and excretion of the biliary constituents resulting in the cholestasis and icterus which accompanies many common acquired liver disorders[48,130,131]. A general pattern of response to cholestatic liver injury is initiated by downregulation of the basolateral membrane bound transporters NTCP and OATP1B1. The expression of several canalicular export pumps is relatively unaffected [bile salt export pump (BSEP), multidrug resistance protein 2 (MDR2)] or even upregulated (MDR1). Decreased expression of MRP2 in sepsis or in obstructive cholestasis is followed by upregulation of several MRP homologues at basolateral membrane of hepatocytes that may extrude bile salts back to the sinusoidal blood and systemic circulation. Most of these changes are believed to help prevent an accumulation of potentially toxic bile components and other substrates in the liver.
Similar patterns of expression of the bilirubin and bile salts handling proteins and mRNA are observed in cholestatic liver diseases in humans. At the stage I and II of primary biliary cirrhosis (PBC), expression and localisation of OATP1B1, OATP1B3, NTCP, MRP2, MRP3 and MDR3 are unchanged. At stage III, immunostaining intensities of the sinusoidal uptake transporters and their mRNA levels decrease. Irregular MRP2 immunostaining suggests redistribution of MRP2 into intracellular structures in the advanced stages of PBC; however, at stage III and IV, basolateral uptake transporters NTCP and OATP1B1 are downregulated. Expression of the canalicular export pumps for bile salts (BSEP) and bilirubin (MRP2) remains unchanged and the canalicular P-glycoproteins MDR1 and MDR3 and the basolateral efflux pump MRP3 are upregulated[132-135].
At the early-stages of cholestasis in extrahepatic biliary atresia, BSEP, MDR3, MRP2, NTCP/SLC10A1, SLCO1A2 and nuclear receptor farnesoid X receptor are downregulated. At the late-stages of cholestasis, farnesoid X receptor and BSEP levels returns to normal, MDR3 and MDR1 are upregulated and MRP2 is downregulated[136].
In primary sclerosing cholangitis, the level of OATP1B1 mRNA in liver tissue has been demonstrated to represent 49% of controls and the level of MRP2 mRNA dropped to 27% of controls[137].
Over the last decades, molecular basis of hyperbilirubinemia syndromes has been elucidated and mutations affecting the basolateral and apical membrane transporters responsible for accumulation of either conjugated or unconjugated bilirubin have been identified.
Except for GS, the majority of inherited hyperbilirubinemia syndromes are rare autosomal recessive disorders with a low prevalence in the general population and, apart from CN syndrome type I and some cases of CN type II in neonatal period, mostly not requiring further therapy. Nonetheless, the enzyme and transport systems involved in bilirubin metabolism may play an important role in the elimination and disposition processes of many other endogenous and exogenous substrates including hormones, drugs, toxins and heavy metals[102,138]. Dysfunction or absence of these systems, including selected ABC transporters and OATPs, may alter pharmacokinetics and pharmacodynamics of many biologically active agents, affect penetration of the substrates into various tissues and lead to their intracellular accumulation with a subsequent increase of organ toxicity[126,127,128]. In addition, the absence of the functional transport proteins involved in hepatobiliary and enterohepatic circulation may involve drug disposition, drug-drug or drug-food interactions and result in decreased effectiveness or even resistance to a diverse spectrum of chemotherapeutic agents and xenobiotics[139-141]. Individuals with mutations in the responsible gene or genes with the fully expressed phenotype of the corresponding hyperbilirubinemia syndrome, as well as subjects carrying mutations without clinical manifestation of hyperbilirubinemia under normal conditions, may be more susceptible to the adverse effects of some drugs and metabolites[142,143].
Clarifying the molecular genetic basis of hereditary hyperbilirubinemia syndromes together with the discoveries of the major systems essential for the metabolism and transport of bilirubin and other endogenous and exogenous substrates represent a substantial contribution to the current knowledge of the heme degradation pathway. Further investigation of how bilirubin transport proteins and their variations affect pharmacokinetics of drugs may be of significant clinical importance.
P- Reviewers Marin JJG, Ruiz-Gaspa S, Teng RJ S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Stadeler G. Uber die Farbstoffe der Galle. Anaalen d Chemie u Pharmacie. 1864;132:323-354. [Cited in This Article: ] |
| 2. | London IM, West R, Shemin D, Rittenberg D. On the origin of bile pigment in normal man. J Biol Chem. 1950;184:351-358. [PubMed] [Cited in This Article: ] |
| 3. | Berk PD, Howe RB, Bloomer JR, Berlin NI. Studies of bilirubin kinetics in normal adults. J Clin Invest. 1969;48:2176-2190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 173] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748-755. [PubMed] [Cited in This Article: ] |
| 5. | Schmid R. The identification of direct-reacting bilirubin as bilirubin glucuronide. J Biol Chem. 1957;229:881-888. [PubMed] [Cited in This Article: ] |
| 6. | Schmid R. Direct-reacting bilirubin, bilirubin glucuronide, in serum, bile and urine. Science. 1956;124:76-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 162] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Gorski JP, Kasper CB. Purification and properties of microsomal UDP-glucuronosyltransferase from rat liver. J Biol Chem. 1977;252:1336-1343. [PubMed] [Cited in This Article: ] |
| 8. | Burchell B. Purification of UDP-glucuronyltransferase from untreated rat liver. FEBS Lett. 1977;78:101-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Fisher MB, Paine MF, Strelevitz TJ, Wrighton SA. The role of hepatic and extrahepatic UDP-glucuronosyltransferases in human drug metabolism. Drug Metab Rev. 2001;33:273-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Kokudo N, Takahashi S, Sugitani K, Okazaki T, Nozawa M. Supplement of liver enzyme by intestinal and kidney transplants in congenitally enzyme-deficient rat. Microsurgery. 1999;19:103-107. [PubMed] [Cited in This Article: ] |
| 11. | Owens IS, Basu NK, Banerjee R. UDP-glucuronosyltransferases: gene structures of UGT1 and UGT2 families. Methods Enzymol. 2005;400:1-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Burchell B. Identification and purification of mutiple forms of UDP-glucuronosyltransferase. Rev Biochem Toxicol. 1981;3:1. [Cited in This Article: ] |
| 13. | Roy Chowdhury J, Roy Chowdhury N, Falany CN, Tephly TR, Arias IM. Isolation and characterization of multiple forms of rat liver UDP-glucuronate glucuronosyltransferase. Biochem J. 1986;233:827-837. [PubMed] [Cited in This Article: ] |
| 14. | Burchell B, Brierley CH, Monaghan G, Clarke DJ. The structure and function of the UDP-glucuronosyltransferase gene family. Adv Pharmacol. 1998;42:335-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1179] [Cited by in F6Publishing: 1085] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 16. | Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD. Organ distribution of multidrug resistance proteins 1, 2, and 3 (Mrp1, 2, and 3) mRNA and hepatic induction of Mrp3 by constitutive androstane receptor activators in rats. J Pharmacol Exp Ther. 2002;300:97-104. [PubMed] [Cited in This Article: ] |
| 17. | Gartung C, Matern S. Molecular regulation of sinusoidal liver bile acid transporters during cholestasis. Yale J Biol Med. 1997;70:355-363. [PubMed] [Cited in This Article: ] |
| 18. | van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120:2942-2952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Iusuf D, van de Steeg E, Schinkel AH. Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol Sci. 2012;33:100-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | König J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000;275:23161-23168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 372] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | König J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278:G156-G164. [PubMed] [Cited in This Article: ] |
| 22. | Iusuf D, van de Steeg E, Schinkel AH. Hepatocyte hopping of OATP1B substrates contributes to efficient hepatic detoxification. Clin Pharmacol Ther. 2012;92:559-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Zhang W, He YJ, Gan Z, Fan L, Li Q, Wang A, Liu ZQ, Deng S, Huang YF, Xu LY. OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin Exp Pharmacol Physiol. 2007;34:1240-1244. [PubMed] [Cited in This Article: ] |
| 24. | Sanna S, Busonero F, Maschio A, McArdle PF, Usala G, Dei M, Lai S, Mulas A, Piras MG, Perseu L. Common variants in the SLCO1B3 locus are associated with bilirubin levels and unconjugated hyperbilirubinemia. Hum Mol Genet. 2009;18:2711-2718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371:897-905. [PubMed] [Cited in This Article: ] |
| 26. | Macias RI, Marin JJ, Serrano MA. Excretion of biliary compounds during intrauterine life. World J Gastroenterol. 2009;15:817-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2595] [Cited by in F6Publishing: 2556] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 28. | Dennery PA, McDonagh AF, Spitz DR, Rodgers PA, Stevenson DK. Hyperbilirubinemia results in reduced oxidative injury in neonatal Gunn rats exposed to hyperoxia. Free Radic Biol Med. 1995;19:395-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Mayer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 2000;46:1723-1727. [PubMed] [Cited in This Article: ] |
| 30. | Ollinger R, Kogler P, Troppmair J, Hermann M, Wurm M, Drasche A, Königsrainer I, Amberger A, Weiss H, Ofner D. Bilirubin inhibits tumor cell growth via activation of ERK. Cell Cycle. 2007;6:3078-3085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Lacko M, Roelofs HM, Te Morsche RH, Voogd AC, Ophuis MB, Peters WH, Manni JJ. Genetic polymorphism in the conjugating enzyme UGT1A1 and the risk of head and neck cancer. Int J Cancer. 2010;127:2815-2821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Horsfall LJ, Rait G, Walters K, Swallow DM, Pereira SP, Nazareth I, Petersen I. Serum bilirubin and risk of respiratory disease and death. JAMA. 2011;305:691-697. [PubMed] [Cited in This Article: ] |
| 33. | Keshavan P, Schwemberger SJ, Smith DL, Babcock GF, Zucker SD. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int J Cancer. 2004;112:433-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Hervieux J. De l’ictere des nouveau-nes. Paris: These Med 1847; . [Cited in This Article: ] |
| 35. | Schmorl G. Zur Kenntnis des ikterus neonatatorum, inbesondere der dabei auftreten den gehirnveranderungen. Verh Dtsch Ges Pathol. 1903;6:109. [Cited in This Article: ] |
| 36. | Schiff D, Chan G, Poznansky MJ. Bilirubin toxicity in neural cell lines N115 and NBR10A. Pediatr Res. 1985;19:908-911. [PubMed] [Cited in This Article: ] |
| 37. | Mustafa MG, Cowger ML, King TE. Effects of bilirubin on mitochondrial reactions. J Biol Chem. 1969;244:6403-6414. [PubMed] [Cited in This Article: ] |
| 38. | Diamond I, Schmid R. Oxidative phosphorylation in experimental bilirubin encephalopathy. Science. 1967;155:1288-1289. [PubMed] [Cited in This Article: ] |
| 39. | Strumia E. [Effect of bilirubin on some hydrolases]. Boll Soc Ital Biol Sper. 1959;35:2160-2162. [PubMed] [Cited in This Article: ] |
| 40. | Flitman R, Worth MH. Inhibition of hepatic alcohol dehydrogenase by bilirubin. J Biol Chem. 1966;241:669-672. [PubMed] [Cited in This Article: ] |
| 41. | Katoh R, Kashiwamata S, Niwa F. Studies on cellular toxicity of bilirubin: Effect on the carbohydrate metabolism in the young rat brain. Brain Res. 1975;83:81-92. [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Greenfield S, Majumdar AP. Bilirubin encephalopathy: effect on protein synthesis in the brain of the Gunn rat. J Neurol Sci. 1974;22:83-89. [PubMed] [Cited in This Article: ] |
| 43. | Majumdar AP. Bilirubin encephalopathy: effect on RNA polymerase activity and chromatin template activity in the brain of the Gunn rat. Neurobiology. 1974;4:425-431. [PubMed] [Cited in This Article: ] |
| 44. | Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003;38:345-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773:283-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803-1823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 323] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 47. | Thompson R, Jansen PL. Genetic defects in hepatocanalicular transport. Semin Liver Dis. 2000;20:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Lee J, Boyer JL. Molecular alterations in hepatocyte transport mechanisms in acquired cholestatic liver disorders. Semin Liver Dis. 2000;20:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Gunn CH. Hereditary acholuric jaundice in a new mutant strain of rats. J Hered. 1938;29:137-139. [Cited in This Article: ] |
| 50. | Roy Chowdhury J, Van ES HHG, Roy Chowdhury N. Gunn rat: An animal model of deficiency of bilirubin conjugation. Hepatic Transport and Bile Secretion: Physiology and Pathophysiology. New York: Raven Press 1992; 713. [Cited in This Article: ] |
| 51. | Nguyen N, Bonzo JA, Chen S, Chouinard S, Kelner MJ, Hardiman G, Bélanger A, Tukey RH. Disruption of the ugt1 locus in mice resembles human Crigler-Najjar type I disease. J Biol Chem. 2008;283:7901-7911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Portman OW, Roy Chowdhury J, Roy Chowdhury N, Alexander M, Cornelius CE, Arias IM. A nonhuman primate model of Gilbert’s syndrome. Hepatology. 1984;4:175-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Portman OW, Alexander M, Cornelius CE, Chowdhury JR, Chowdhury NR, Arias IM. The effects of nutrition on unconjugated plasma bilirubin concentrations in squirrel monkeys. Hepatology. 1984;4:454-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Jansen PL, van Klinken JW, van Gelder M, Ottenhoff R, Elferink RP. Preserved organic anion transport in mutant TR- rats with a hepatobiliary secretion defect. Am J Physiol. 1993;265:G445-G452. [PubMed] [Cited in This Article: ] |
| 55. | Kawaguchi A, Nozaki Y, Hosokawa S, Tagaya O, Mikami T, Wakabayashi T. [Establishment of hyperbilirubinuria rat mutant--a new animal model for jaundice]. Jikken Dobutsu. 1994;43:37-44. [PubMed] [Cited in This Article: ] |
| 56. | Yamazaki K, Mikami T, Hosokawa S, Tagaya O, Nozaki Y, Kawaguchi A, Funami H, Katoh H, Yamamoto N, Wakabayashi T. A new mutant rat with hyperbilirubinuria (hyb). J Hered. 1995;86:314-317. [PubMed] [Cited in This Article: ] |
| 57. | Cornelius CE, Arias IM, Osburn BI. Hepatic pigmentation with photosensitivity: A Syndrome in corriedale sheep resembling dubin-johnson syndrome in man. J Am Vet Med Assoc. 1965;146:709-713. [PubMed] [Cited in This Article: ] |
| 58. | Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2). J Pharmacol Exp Ther. 2006;317:579-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Sugatani J, Yamakawa K, Yoshinari K, Machida T, Takagi H, Mori M, Kakizaki S, Sueyoshi T, Negishi M, Miwa M. Identification of a defect in the UGT1A1 gene promoter and its association with hyperbilirubinemia. Biochem Biophys Res Commun. 2002;292:492-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, Owens IS, Negishi M, Sueyoshi T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CA-R. Hepatology. 2001;33:1232-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 303] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 61. | Crigler JF, Najjar VA. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics. 1952;10:169-180. [PubMed] [Cited in This Article: ] |
| 62. | Ritter JK, Yeatman MT, Ferreira P, Owens IS. Identification of a genetic alteration in the code for bilirubin UDP-glucuronosyltransferase in the UGT1 gene complex of a Crigler-Najjar type I patient. J Clin Invest. 1992;90:150-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 114] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Jansen PL. Diagnosis and management of Crigler-Najjar syndrome. Eur J Pediatr. 1999;158 Suppl 2:S89-S94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Karon M, Imach D, Schwartz A. Effective phototherapy in congenital nonobstructive, nonhemolytic jaundice. N Engl J Med. 1970;282:377-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Shevell MI, Bernard B, Adelson JW, Doody DP, Laberge JM, Guttman FM. Crigler-Najjar syndrome type I: treatment by home phototherapy followed by orthotopic hepatic transplantation. J Pediatr. 1987;110:429-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 826] [Cited by in F6Publishing: 834] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 67. | Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, Najarian JS. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976;192:892-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 236] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Ilan Y, Attavar P, Takahashi M, Davidson A, Horwitz MS, Guida J, Chowdhury NR, Chowdhury JR. Induction of central tolerance by intrathymic inoculation of adenoviral antigens into the host thymus permits long-term gene therapy in Gunn rats. J Clin Invest. 1996;98:2640-2647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Takahashi M, Ilan Y, Chowdhury NR, Guida J, Horwitz M, Chowdhury JR. Long term correction of bilirubin-UDP-glucuronosyltransferase deficiency in Gunn rats by administration of a recombinant adenovirus during the neonatal period. J Biol Chem. 1996;271:26536-26542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Arias IM. Chronic unconjugated hyperbilirubinemia without overt signs of hemolysis in adolescents and adults. J Clin Invest. 1962;41:2233-2245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Gollan JL, Huang SN, Billing B, Sherlock S. Prolonged survival in three brothers with severe type 2 Crigler-Najjar syndrome. Ultrastructural and metabolic studies. Gastroenterology. 1975;68:1543-1555. [PubMed] [Cited in This Article: ] |
| 72. | Moghrabi N, Clarke DJ, Boxer M, Burchell B. Identification of an A-to-G missense mutation in exon 2 of the UGT1 gene complex that causes Crigler-Najjar syndrome type 2. Genomics. 1993;18:171-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Hunter JO, Thompson RP, Dunn PM, Williams R. Inheritance of type 2 Crigler-Najjar hyperbilirubinaemia. Gut. 1973;14:46-49. [PubMed] [Cited in This Article: ] |
| 74. | Labrune P, Myara A, Hennion C, Gout JP, Trivin F, Odievre M. Crigler-Najjar type II disease inheritance: a family study. J Inherit Metab Dis. 1989;12:302-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Seppen J, Bosma PJ, Goldhoorn BG, Bakker CT, Chowdhury JR, Chowdhury NR, Jansen PL, Oude Elferink RP. Discrimination between Crigler-Najjar type I and II by expression of mutant bilirubin uridine diphosphate-glucuronosyltransferase. J Clin Invest. 1994;94:2385-2391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 123] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Gilbert A, Lereboullet P. La cholemie simple familiale. Semaine Medicale. 1901;21:241-243. [Cited in This Article: ] |
| 77. | Nixon JC, Monahan GJ. Gilbert’s disease and the bilirubin tolerance test. Can Med Assoc J. 1967;96:370-373. [PubMed] [Cited in This Article: ] |
| 78. | Schmid R. Gilbert’s syndrome--a legitimate genetic anomaly? N Engl J Med. 1995;333:1217-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Thomsen HF, Hardt F, Juhl E. Diagnosis of Gilbert’s syndrome. Reliability of the caloric restriction and phenobarbital stimulation tests. Scand J Gastroenterol. 1981;16:699-703. [PubMed] [Cited in This Article: ] |
| 80. | Barth RF, Grimley PM, Berk PD, Bloomer JR, Howe RB. Excess lipofuscin accumulation in constitutional hepatic dysfunction (Gilbert’s syndrome). Light and electron microscopic observations. Arch Pathol. 1971;91:41-47. [PubMed] [Cited in This Article: ] |
| 81. | Dawson J, Seymour CA, Peters TJ. Gilbert’s syndrome: analytical subcellular fractionation of liver biopsy specimens. Enzyme activities, organelle pathology and evidence for subpopulations of the syndrome. Clin Sci (Lond). 1979;57:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Black M, Billing BH. Hepatic bilirubin udp-glucuronyl transferase activity in liver disease and gilbert’s syndrome. N Engl J Med. 1969;280:1266-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 199] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Owens D, Evans J. Population studies on Gilbert’s syndrome. J Med Genet. 1975;12:152-156. [PubMed] [Cited in This Article: ] |
| 84. | Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1077] [Cited by in F6Publishing: 1094] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 85. | Hsieh TY, Shiu TY, Huang SM, Lin HH, Lee TC, Chen PJ, Chu HC, Chang WK, Jeng KS, Lai MM. Molecular pathogenesis of Gilbert’s syndrome: decreased TATA-binding protein binding affinity of UGT1A1 gene promoter. Pharmacogenet Genomics. 2007;17:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Burchell B, Hume R. Molecular genetic basis of Gilbert’s syndrome. J Gastroenterol Hepatol. 1999;14:960-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA. 1998;95:8170-8174. [PubMed] [Cited in This Article: ] |
| 88. | Ki CS, Lee KA, Lee SY, Kim HJ, Cho SS, Park JH, Cho S, Sohn KM, Kim JW. Haplotype structure of the UDP-glucuronosyltransferase 1A1 (UGT1A1) gene and its relationship to serum total bilirubin concentration in a male Korean population. Clin Chem. 2003;49:2078-2081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Ando Y, Chida M, Nakayama K, Saka H, Kamataki T. The UGT1A1*28 allele is relatively rare in a Japanese population. Pharmacogenetics. 1998;8:357-360. [PubMed] [Cited in This Article: ] |
| 90. | Takeuchi K, Kobayashi Y, Tamaki S, Ishihara T, Maruo Y, Araki J, Mifuji R, Itani T, Kuroda M, Sato H. Genetic polymorphisms of bilirubin uridine diphosphate-glucuronosyltransferase gene in Japanese patients with Crigler-Najjar syndrome or Gilbert’s syndrome as well as in healthy Japanese subjects. J Gastroenterol Hepatol. 2004;19:1023-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Udomuksorn W, Elliot DJ, Lewis BC, Mackenzie PI, Yoovathaworn K, Miners JO. Influence of mutations associated with Gilbert and Crigler-Najjar type II syndromes on the glucuronidation kinetics of bilirubin and other UDP-glucuronosyltransferase 1A substrates. Pharmacogenet Genomics. 2007;17:1017-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Kadakol A, Ghosh SS, Sappal BS, Sharma G, Chowdhury JR, Chowdhury NR. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat. 2000;16:297-306. [PubMed] [Cited in This Article: ] |
| 93. | Monaghan G, McLellan A, McGeehan A, Li Volti S, Mollica F, Salemi I, Din Z, Cassidy A, Hume R, Burchell B. Gilbert’s syndrome is a contributory factor in prolonged unconjugated hyperbilirubinemia of the newborn. J Pediatr. 1999;134:441-446. [PubMed] [Cited in This Article: ] |
| 94. | Maruo Y, Nishizawa K, Sato H, Sawa H, Shimada M. Prolonged unconjugated hyperbilirubinemia associated with breast milk and mutations of the bilirubin uridine diphosphate- glucuronosyltransferase gene. Pediatrics. 2000;106:E59. [PubMed] [Cited in This Article: ] |
| 95. | Burchell B, Soars M, Monaghan G, Cassidy A, Smith D, Ethell B. Drug-mediated toxicity caused by genetic deficiency of UDP-glucuronosyltransferases. Toxicol Lett. 2000;112-113:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Maruo Y, Iwai M, Mori A, Sato H, Takeuchi Y. Polymorphism of UDP-glucuronosyltransferase and drug metabolism. Curr Drug Metab. 2005;6:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Rotger M, Taffe P, Bleiber G, Gunthard HF, Furrer H, Vernazza P, Drechsler H, Bernasconi E, Rickenbach M, Telenti A. Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. J Infect Dis. 2005;192:1381-1386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 98. | Zucker SD, Qin X, Rouster SD, Yu F, Green RM, Keshavan P, Feinberg J, Sherman KE. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci USA. 2001;98:12671-12676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 99. | Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 100. | Ando Y, Saka H, Asai G, Sugiura S, Shimokata K, Kamataki T. UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol. 1998;9:845-847. [PubMed] [Cited in This Article: ] |
| 101. | Iyer L, Das S, Janisch L, Wen M, Ramírez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 566] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 102. | Strassburg CP. Pharmacogenetics of Gilbert’s syndrome. Pharmacogenomics. 2008;9:703-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 103. | Dubin IN, Johnson FB. Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Medicine (Baltimore). 1954;33:155-197. [PubMed] [Cited in This Article: ] |
| 104. | Sprinz H, Nelson RS. Persistent non-hemolytic hyperbilirubinemia associated with lipochrome-like pigment in liver cells: report of four cases. Ann Intern Med. 1954;41:952-962. [PubMed] [Cited in This Article: ] |
| 105. | Shani M, Seligsohn U, Gilon E, Sheba C, Adam A. Dubin-Johnson syndrome in Israel. I. Clinical, laboratory, and genetic aspects of 101 cases. Q J Med. 1970;39:549-567. [PubMed] [Cited in This Article: ] |
| 106. | Erlinger S, Dhumeaux D, Desjeux JF, Benhamou JP. Hepatic handling of unconjugated dyes in the Dubin-Johnson syndrome. Gastroenterology. 1973;64:106-110. [PubMed] [Cited in This Article: ] |
| 107. | Swartz HM, Chen K, Roth JA. Further evidence that the pigment in the Dubin-Johnson syndrome is not melanin. Pigment Cell Res. 1987;1:69-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 108. | Hunter FM, Sparks RD, Flinner RL. Hepatitis with resulting mobilization of hepatic pigment in a patient with dubin-johnson syndrome. Gastroenterology. 1964;47:631-635. [PubMed] [Cited in This Article: ] |
| 109. | Watanabe S, Nishioka M, Kodama T, Ando K, Numa Y, Fukumoto Y, Okita K, Takemoto T, Mizuta M. Clinicopathological studies of the Dubin-Johnson syndrome complicated with chronic hepatitis. Gastroenterol Jpn. 1982;17:576-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 110. | Kartenbeck J, Leuschner U, Mayer R, Keppler D. Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology. 1996;23:1061-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 111. | Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 597] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 112. | Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539-1542. [PubMed] [Cited in This Article: ] |
| 113. | Wada M, Toh S, Taniguchi K, Nakamura T, Uchiumi T, Kohno K, Yoshida I, Kimura A, Sakisaka S, Adachi Y. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum Mol Genet. 1998;7:203-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 114. | Toh S, Wada M, Uchiumi T, Inokuchi A, Makino Y, Horie Y, Adachi Y, Sakisaka S, Kuwano M. Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/cMOAT) and mutations in the ATP-binding-cassette region in Dubin-Johnson syndrome. Am J Hum Genet. 1999;64:739-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 180] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 115. | Uchiumi T, Hinoshita E, Haga S, Nakamura T, Tanaka T, Toh S, Furukawa M, Kawabe T, Wada M, Kagotani K. Isolation of a novel human canalicular multispecific organic anion transporter, cMOAT2/MRP3, and its expression in cisplatin-resistant cancer cells with decreased ATP-dependent drug transport. Biochem Biophys Res Commun. 1998;252:103-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Hulot JS, Villard E, Maguy A, Morel V, Mir L, Tostivint I, William-Faltaos D, Fernandez C, Hatem S, Deray G. A mutation in the drug transporter gene ABCC2 associated with impaired methotrexate elimination. Pharmacogenet Genomics. 2005;15:277-285. [PubMed] [Cited in This Article: ] |
| 117. | Ahmed S, Vo NT, Thalhammer T, Thalhammer F, Gattringer KB, Jäger W. Involvement of Mrp2 (Abcc2) in biliary excretion of moxifloxacin and its metabolites in the isolated perfused rat liver. J Pharm Pharmacol. 2008;60:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Pedersen JM, Matsson P, Bergström CA, Norinder U, Hoogstraate J, Artursson P. Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2). J Med Chem. 2008;51:3275-3287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 119. | Jedlitschky G, Hoffmann U, Kroemer HK. Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin Drug Metab Toxicol. 2006;2:351-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 120. | Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981-2039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 121. | Cebecauerova D, Jirasek T, Budisova L, Mandys V, Volf V, Novotna Z, Subhanova I, Hrebicek M, Elleder M, Jirsa M. Dual hereditary jaundice: simultaneous occurrence of mutations causing Gilbert’s and Dubin-Johnson syndrome. Gastroenterology. 2005;129:315-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Rotor B, Manahan L, Florentin A. Familial non-hemolytic jaundice with direct van den Bergh reaction. Acta medica Philippina. 1948;5:37-49. [Cited in This Article: ] |
| 123. | Wolkoff AW, Wolpert E, Pascasio FN, Arias IM. Rotor’s syndrome. A distinct inheritable pathophysiologic entity. Am J Med. 1976;60:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 77] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Wolpert E, Pascasio FM, Wolkoff AW, Arias IM. Abnormal sulfobromophthalein metabolism in Rotor’s syndrome and obligate heterozygotes. N Engl J Med. 1977;296:1099-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | van de Steeg E, Stránecký V, Hartmannová H, Nosková L, Hřebíček M, Wagenaar E, van Esch A, de Waart DR, Oude Elferink RP, Kenworthy KE. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest. 2012;122:519-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 126. | Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 664] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 127. | Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. The effect of SLCO1B1 polymorphism on repaglinide pharmacokinetics persists over a wide dose range. Br J Clin Pharmacol. 2008;66:818-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 128. | Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 458] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 129. | Iusuf D, Sparidans RW, van Esch A, Hobbs M, Kenworthy KE, van de Steeg E, Wagenaar E, Beijnen JH, Schinkel AH. Organic anion-transporting polypeptides 1a/1b control the hepatic uptake of pravastatin in mice. Mol Pharm. 2012;9:2497-2504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 130. | Trauner M, Fickert P, Stauber RE. [New molecular aspects of cholestatic liver diseases]. Z Gastroenterol. 1999;37:639-647. [PubMed] [Cited in This Article: ] |
| 131. | Wagner M, Trauner M. Transcriptional regulation of hepatobiliary transport systems in health and disease: implications for a rationale approach to the treatment of intrahepatic cholestasis. Ann Hepatol. 2005;4:77-99. [PubMed] [Cited in This Article: ] |
| 132. | Kojima H, Nies AT, König J, Hagmann W, Spring H, Uemura M, Fukui H, Keppler D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatol. 2003;39:693-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 133. | Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 134. | Pauli-Magnus C, Kerb R, Fattinger K, Lang T, Anwald B, Kullak-Ublick GA, Beuers U, Meier PJ. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 135. | Takeyama Y, Sakisaka S. Hepatobiliary membrane transporters in primary biliary cirrhosis. Hepatol Res. 2012;42:120-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 136. | Chen HL, Liu YJ, Chen HL, Wu SH, Ni YH, Ho MC, Lai HS, Hsu WM, Hsu HY, Tseng HC. Expression of hepatocyte transporters and nuclear receptors in children with early and late-stage biliary atresia. Pediatr Res. 2008;63:667-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 137. | Oswald M, Kullak-Ublick GA, Paumgartner G, Beuers U. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver. 2001;21:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 138. | Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106:97-132. [PubMed] [Cited in This Article: ] |
| 139. | Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 532] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 140. | Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, Haglund U, Artursson P. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem. 2012;55:4740-4763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 141. | Shitara Y. Clinical importance of OATP1B1 and OATP1B3 in drug-drug interactions. Drug Metab Pharmacokinet. 2011;26:220-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 142. | Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44:152-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 143. | Sadée W, Dai Z. Pharmacogenetics/genomics and personalized medicine. Hum Mol Genet. 2005;14 Spec No. 2:R207-R214. [PubMed] [Cited in This Article: ] |