Article Text
Abstract
Background: Many drugs are unlicensed in children and consequently their doses have been scaled down from those used in adults.
Objective: To compare the performance of three scaling models in predicting maintenance doses for children from those used in adults.
Methods: Three scaling models based on body weight (BW), body surface area (BSA) and BW0.75 were used to predict maintenance doses across the paediatric age band from the equivalent adult doses for 30 different drugs. The predicted doses were compared with those in the British National Formulary for children 2006 (BNFc). Percentage error and mean squared prediction error were used as a measure of precision, and mean prediction error was used as a measure of bias.
Results: In the 1-month and 12-month age groups, the different approaches ranked on their bias (least bias first) were BW<BW0.75<BSA and on their precision (most precise first) were BW>BW0.75>BSA. The BSA and BW0.75 methods predicted doses up to 2.86-fold higher than the BNFc in the 1-month and 1-year age group. In the 7-year and 12-year age groups, BW0.75 and BSA performed better than BW for precision and bias, and no predictions were more than 1.8-fold higher than the BNFc. The BW method tended to also under-predict dose across the paediatric age range.
Conclusions: Dose scaling should only be used as a last resort for determining a suitable dose in children. No single method was suitable across the entire paediatric age range.
Statistics from Altmetric.com
The use of unlicensed medicines in children is a widespread problem in many countries1 2 and has been associated with an increase in the incidence of adverse drug reactions.3 4 For many of these drugs, proper dose optimisation studies have not been performed in the paediatric age range. Consequently, initial doses for children have often been derived by scaling from the adult dosage and then titrating according to clinical response. As clinical experience with use of medicines in children has been gained, more formalised paediatric dosage books have been published, culminating in the recent publication of the first British National Formulary for Children (BNFc).5
When doses are not listed in the BNFc, there is a UK network of paediatric medicines information centres that may be able to help. In addition, there are a number of useful online sources of information on drug prescribing in children: the Neonatal and Paediatric Pharmacist Group website6 message board allows information to be sought from paediatric pharmacists nationally; DIAL, a paediatric drug information service run by Alder Hey Children’s hospital.7 The National Electronic Library for Medicines8 and emedicine9 are also useful online sources of paediatric dosage information. Although information is often available on the use of a specific drug in children, there still occasionally arises the need to make dosage decisions about drug treatments previously untried in paediatrics. A number of formulae have been proposed for scaling adult doses to children, but Clark’s body weight (BW) and body surface area (BSA) rules (equations (1) and (2), respectively) are most often applied in the clinical situation. The BSA dose scaling method is perceived to be more accurate, as BSA correlates with the development of physiological systems.


where DoseP = dose in paediatrics, DoseA = dose in adults, BWP and BSAP are paediatric values, and BWA and BSAA are adult values for these variables, respectively.
Another type of model often used to scale pharmacokinetic parameters between adults and children are the allometric power models. The background to the development of these models and their application to paediatric anaesthesia dosing have been reviewed by Anderson and Meakin.10 The general equation for these models is:

where Y is the biological characteristic to be predicted, and a and b are an empirically derived constant and exponent, respectively.
Many anatomical variables such as blood volume have a weight exponent of b = 1, metabolic variables such as basal metabolic rate have a value of b = 0.75, and time-related variables such as heart rate, a value exponent of b = 0.25.11 In terms of scaling drugs pharmacokinetic parameters from adults to children, the weight exponents will be 0.75 for a metabolic parameter such as drug clearance (CL) as shown in equation (4):

where CLP and CLA are clearance values in paediatric and adult subjects, respectively.
Because the steady-state maintenance dose of any drug is determined by the product of plasma concentration and CL (CP × CL) and is not dependent on volume of distribution, dose can be substituted for clearance, and paediatric doses can be scaled from the adult value as shown in equation (5). This assumes that the therapeutic maintenance plasma concentration in adults is the same as in neonates, infants and children.

The aim of this study was to compare the performance of the three scaling models (BW, BSA and BW0.75) in predicting maintenance drug doses across the paediatric age range from standard adult doses. The emphasis is on predicting safe and effective doses in children as compared with those in the BNFc.
METHODS
Prediction of maintenance doses in children
Thirty representative drugs used for the long-term maintenance treatment of a variety of clinical conditions in children were selected. Table 1 shows the standard adult doses and licensed indications in adults/children for the specific drugs studied.
The standard adult doses were selected according to those listed in the BNF 5112 and, as far as practically possible, were chosen for the equivalent indication as likely to be used in children—for example, furosemide for the treatment of oedema.
Adult doses were scaled to paediatric doses using equations (1), (2) and (5) for the BW, BSA and BW0.75 models, respectively. The ages selected for scaling were 1 month, 1 year, 7 years and 12 years to reflect the paediatric age band classifications of the International Conference on Harmonisation.13 Table 2 shows the 50th centile population values for BW and BSA for these age bands.
Comparison of the allometric models
The predicted doses from allometric scaling were compared at a defined age with doses derived from the BNFc. This was chosen as the comparator reference as it is the recommended UK national source for paediatric doses. Percentage error was calculated using equation (6).
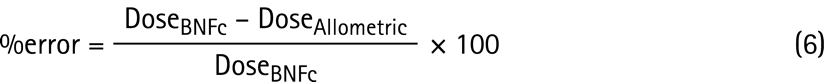
The %error data are presented as the percentage of drugs where the predicted dose is within ±25% and ±50% of the BNFc dose. In addition, the percentage of predicted doses within twofold (half the dose to twice the dose) of the BNFc was also calculated.
Mean squared prediction error (mse) and mean predicted error (me) were used as measures of precision and bias, respectively.14 Precision is a measure of how close the predicted dose is to the recommended BNFc dose, and bias is a measure of the distribution of the data (does the model under-predict or over-predict?). Because of the range of doses of drugs studied, both precision and bias were calculated on the basis of the deviation of the predicted dose/BNFc dose ratio from 1 (where a ratio of 1 is a perfect prediction). Thus pe, the prediction error for dose, is defined in equation (7).

From this equation, precision and bias are calculated from equations (8) and (9), respectively, where N is the number of observations.


RESULTS
Tables 3 and 4 give a summary of results for dose prediction. In the 1-month and 12-month age groups, the different approaches ranked on their bias (least bias first) were BW<BW0.75<BSA, and ranked on their precision (most precise first) they were BW>BW0.75>BSA. The BSA and BW0.75 methods predicted doses up to 2.86-fold and 2.66-fold higher, respectively, compared with the BNFc in the 1-month age group, and up to 2.17-fold and 2-fold higher, respectively, in the 1-year age group. Overall, the BW method tended to under-predict dose in the 1-month and 1-year age groups for 90% (27/30) of drugs, with predicted doses being less than 50% of the BNFc dose for 10% and 17% of drugs, respectively. In contrast, the BSA and BW0.75 methods generally over-predicted for 93% of drugs in both of these groups. In the 7-year age group, rankings for bias were BW0.75<BSA<BW and rankings for precision were BW0.75 >BSA>BW. In the 12-year age group, rankings for bias were BSA>BW0.75<BW and rankings for precision were BW0.75 = BSA>BW. In both of these age groups, no predictions from any method were more than 1.8-fold higher than the BNFc dose and only one predicted dose was less than 50% of the BNFc dose. Again the BW method tended to under-predict the dose for 90% of drugs in the 12-year age group compared with the BSA (53% of drugs) and BW0.75 (63% of drugs) scaling methods.
DISCUSSION
The simple weight-based model (equation (1)) tends to give the best estimates of maintenance dose in subjects 12 months and younger, as indicated by precision and bias. This is in agreement with a recent study by Mahmood,15 who evaluated the ability of five different allometric models to predict CL across the paediatric age range and showed the BW model to give the best results in infants and neonates. However, also in agreement with other studies, the BW model tends to under-predict doses across the paediatric age range. Anderson and Ellis16 reported that the BW model under-predicted CL in subjects weighing <47 kg by 10% or more compared with the BW0.75 model, with the error increasing to 50% as body weight decreased to the neonatal range. For drugs with a narrow therapeutic index, such as gentamicin and phenytoin, doses were under-predicted by ∼30% in this study, which may mean that therapeutic plasma concentrations would not be reached.
The BSA model over-predicts dose in the neonatal and infant groups, again in agreement with other studies10 17 that showed it to over-predict drug clearance by at least 10% in children weighing <20 kg. Although for most drugs the therapeutic dose ratio between unwanted and wanted effects is >2, for 40% of the drugs in this study, the over-prediction was more than twofold, which could have clinical implications. For drugs with a narrow therapeutic index, the twofold tolerance limit is too wide. In this study, the BSA model predicted doses of gentamicin and phenytoin in neonates that were1.8-fold and 1.7-fold higher, respectively, than recommended in the neonatal group. In the case of a drug such as gentamicin, scaling by the BSA method could lead to nephrotoxicity and ototoxicity. Because phenytoin undergoes zero-order pharmacokinetics, the scaled dose could result in very high plasma concentrations and the associated side effects of nystagmus, ataxia and dysarthria.
The BW0.75 model over-predicts dose in children aged 1 year or less but performed better than the BSA model. Both the BSA and BW0.75 models performed reasonably well in the 7-year and 12-year age groups, with all but two predicted doses being within 50% of the BNFc dose. For all ages, the problems of dose scaling were no more apparent with renally than hepatically cleared drugs.
What is already known on this topic
No single scaling method is suitable for scaling drug clearance in children.
Scaling methods based on body weight tend to under-predict clearance prediction in children, and those based on body surface area can over-predict drug clearance in young children.
What this study adds
The scaling of paediatric drug doses from adults is investigated for the first time, and the relative risks of using different scaling methods in different ages are demonstrated.
Recommendations are made for the use of scaling methods at different ages.
Up until the 50th edition (2005), the BNF suggests that “children’s doses may be calculated from adult doses by using age, body weight or body surface area or by a combination of these factors”, and it is only in the March 2006 BNF 51 that this advice is quite correctly changed to “consult BNFc or seek advice from medicines information centre”. Clearly, the results of this study indicate that the use of dose scaling methods, especially in the neonatal and infant age ranges, is problematic. However, situations may still arise that require the use of a medicine that is unlicensed for children and for which there is no dosage information available through the paediatric medicines information networks. The risk–benefits for the drug treatment, the therapeutic index of the drug, its toxicity profile in adults, the route by which the drug is cleared, and, not least, the age of the child should all be carefully considered. Clinical judgement is needed, and, in case of doubt, advice should be sought from a paediatric clinical pharmacologist/pharmacist. Mahmood15 has suggested that an error of 50% or less between scaled and actual values may be a safe level but that further evaluation is needed. Thus, scaling using body weight alone may be safer in the neonatal and infant age range in terms of avoiding toxicity: the possibility exists that an under-dose will be administered, but this dose can then be titrated up according to clinical response. Scaling using the BSA or BW0.75 method would seem reasonable in children above 2 years of age, but even so should still be used with caution.
So why do the dose scaling methods described in this study perform so poorly in neonates and infants? From birth onwards, changes in pharmacokinetics and pharmacodynamics occur as a consequence of organ maturation, changes in body composition, and the ontogeny of drug elimination pathways. Because of the non-monotonic nature of some of the age-related changes, simple allometric scaling methods as described in this paper often fail to predict pharmacokinetics parameters, especially in children below 2 years of age.10 15
The most obvious way forward in the long term is to lessen the need for dose scaling by performing properly conducted clinical studies, including pharmacokinetic/pharmacodynamic studies, on all medicines likely to be used in children. The Medicines for Children Research Network, part of a European network, has been established so that adequately powered paediatric studies can be performed on both new and existing drugs. Further to this, European legislation18 along the lines of the Best Medicines for Children Act in the USA19 will require pharmaceutical companies to test new drugs in children with safeguards in place to prevent unnecessary studies being performed.
In the short to medium term, more robust methods of predicting drug dosage across the paediatric age range are required. This may be achieved through the development and validation of paediatric physiologically based pharmacokinetic (PBPK) models. Paediatric PBPK models take into account the existing demographic, physiological and biochemical information, including the ontogeny of drug elimination pathways, to assess the exposure of neonates, infants and children to drugs and xenobiotics. Thus, they offer a more mechanistic approach to drug dose determination than simple allometric scaling.
PBPK models have previously been applied to predict the exposure of children to inhaled volatile organic chemicals20 21 and the exposure of the fetus in utero to environmental chemicals.22 PBPK models have also been used to predict the pharmacokinetics of specific drugs in children.23–25 The usefulness of these models relies on further research into paediatric pharmacology—for instance, very little is known about how drug transporters develop with age—and also on their independent validation by clinical groups. Such models may also be of use to the pharmaceutical industry in the design of clinical studies in children.26
In conclusion, the results of this study suggest that dose scaling should only be used as a last resort for determining dose in children, and no single extrapolation method is suitable across the entire paediatric age range. Careful consideration of all prescribing factors is needed in all cases, but simple BW scaling is perhaps the safest option for neonates and infants, and either BSA or BW0.75 scaling in older children. In all cases, doses should then be titrated according to response.
REFERENCES
Footnotes
Competing interests: None.


