Abstract
Transporters on the plasma membrane of tumor cells are promising molecular “Trojan horses” to deliver drugs and imaging agents into cancer cells. Radioiodine-labeled meta-iodobenzylguanidine (mIBG) is used as a diagnostic agent (123I-mIBG) and a targeted radiotherapy (131I-mIBG) for neuroendocrine cancers. mIBG enters cancer cells through the norepinephrine transporter (NET) where the radioactive decay of 131I causes DNA damage, cell death, and tumor necrosis. mIBG is predominantly eliminated unchanged by the kidney. Despite its selective uptake by neuroendocrine tumors, mIBG accumulates in several normal tissues and leads to tissue-specific radiation toxicities. Emerging evidences suggest that the polyspecific organic cation transporters play important roles in systemic disposition and tissue-specific uptake of mIBG. In particular, human organic cation transporter 2 (hOCT2) and multidrug and toxin extrusion proteins 1 and 2-K (hMATE1/2-K) likely mediate renal secretion of mIBG, whereas hOCT1 and hOCT3 may contribute to mIBG uptake into normal tissues such as the liver, salivary glands, and heart. This mini-review focuses on the clinical applications of mIBG in neuroendocrine cancers and the differential roles of NET, OCT, and MATE transporters in mIBG disposition, response and toxicity. Understanding the molecular mechanisms governing mIBG transport in cancer and normal cells is a critical step for developing strategies to optimize the efficacy of 131I-mIBG while minimizing toxicity in normal tissues.
SIGNIFICANCE STATEMENT Radiolabeled meta-iodobenzylguanidine (mIBG) has been used as a diagnostic tool and as radiotherapy for neuroendocrine cancers and other diseases. Norepinephrine transporter, organic cation transporters, and multidrug and toxin extrusion proteins play differential roles in tumor targeting, systemic elimination, and accumulation in normal tissues. The clinical use of mIBG as a radiopharmaceutical in cancer diagnosis and treatment can be further improved by taking a holistic approach considering mIBG transporters in both cancer and normal tissues.
Introduction
Cancer continues to be a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020 (Ferlay et al., 2020). Precise detection and diagnosis of specific tumor types and disease stages is essential for designing the appropriate and effective regimens for treatment. Further, cancer treatment often involves radiotherapy and chemotherapy, which not only kills cancer cells but also affects healthy cells. Treatment-related toxicity is frequently a dose-limiting factor and represents a major challenge in clinical management of cancer. Hence, a major goal in cancer drug development and therapy is to increase tumor-specific drug uptake while reducing uptake into normal tissues to minimize toxicities.
Transporters on the plasma membrane of tumor cells represent promising molecular “Trojan horses” to deliver drugs and imaging agents into cancer cells. Transporter-mediated uptake has been exploited successfully in nuclear medicine to deliver several radiopharmaceuticals for cancer imaging and treatment (Zhang and Wang, 2020). For instance, the use of 131I radiotherapy to treat thyroid cancer is highly dependent on the selective expression of the sodium/iodine symporter on these cells (Kogai and Brent, 2012). The use of 18F fluorodeoxyglucose (18F-FDG) as a cancer imaging agent is due to the Warburg effect and glucose transporter 1-mediated uptake in rapidly growing tumor cells (Ancey et al., 2018; Zhang and Wang, 2020). Radiolabeled meta-iodobenzylguanidine (mIBG) is designed to target the norepinephrine transporter (NET), which is highly expressed in neuroendocrine tumors (DuBois et al., 2012; Streby et al., 2015; Zhang and Wang, 2020). Despite its selective uptake by cancer cells, mIBG also accumulates in several normal tissues such as liver, heart, salivary glands, and intestines and leads to tissue-specific radiation toxicities (Matthay et al., 2006; Bleeker et al., 2013; Parisi et al., 2016; Pryma et al., 2019). Emerging evidences suggest that the polyspecific organic cation transporters are the major transporters driving the systemic elimination and tissue-specific disposition of mIBG in normal organs. In this review, we focus on the clinical pharmacology of mIBG and the impact of transporters on mIBG disposition in both tumor and normal cells. It is hoped that through analyzing the case of mIBG, much can be learned about the challenges and opportunities of targeting membrane transporters in cancer diagnosis and treatment.
History and Development of mIBG
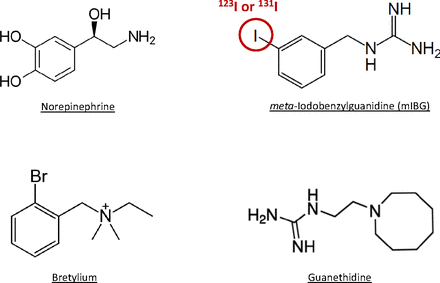
Historically, mIBG is a structural analog of norepinephrine (Fig. 1) and was first developed as a noninvasive imaging agent to detect and characterize irregularities in the adrenal glands. The adrenal gland is comprised of two embryologically distinct tissues, the cortex and medulla (Willenberg and Bornstein, 2017; Dutt et al., 2021). The cortex arises from the mesoderm and is involved in steroidogenesis. The medulla is derived from the neuroectoderm and is responsible for producing catecholamines (epinephrine, norepinephrine) involved in the “fight-or-flight” response. Early imaging efforts exploited the physiologic roles of these tissues through the use of radioiodinated cholesterols and radiolabeled catecholamines that can accumulate in the adrenal system (Fowler et al., 1973; Beierwaltes et al., 1978). Since the adrenal medulla functions like a specialized sympathetic ganglion, compounds with an affinity for adrenergic nerves would be expected to accumulate in the adrenal system. In the early 1960s, two antihypertensive drugs, guanethidine and bretylium (Fig. 1), entered the US market and were potent and selective adrenergic blocking agents. This led to the development of benzylguanidines as potential antiadrenergic agents by combining the halogenated benzyl group of bretylium and the guanidine group in guanethidine (Short and Darby, 1967).
Chemical structure of mIBG and compounds that inspired the development of benzylguanidines. The red circle indicates the position of 123I or 131I labeling on mIBG.
In the late 1970s, Dr. Beierwaltes’ group at the University of Michigan set out to develop a radiopharmaceutical that could image the adrenal medulla and its neoplasms (Wieland et al., 1980). They radiolabeled the benzylguanidines with 131I and investigated their accumulation in the adrenal glands and other tissues. It was found that para-iodobenzylguanidine (pIBG) and meta-iodobenzylguanidine (mIBG) were highly concentrated in the adrenal glands of dogs for up to 192 hours. The compounds also accumulated in other tissues (e.g., liver, kidney, heart, and lung) but their concentrations decreased over time with minimal to no radiation after 48 hours. Although pIBG had greater accumulation in the adrenal glands, mIBG was shown to have less accumulation in the thyroid gland, have less in vivo deiodination, and be more metabolically stable (Wieland et al., 1980, 1984). Therefore, mIBG was selected for further development as a diagnostic imaging agent for the adrenal medulla and related cancers.
After its success in multiple imaging studies and shown to be safe following intravenous administration, 131I-mIBG (iobenguane) was approved by the US Food and Drug Administration (FDA) in 1994 to diagnose neuroendocrine cancers. However, there are limitations to the use of 131I-mIBG as an imaging agent. The conventional gamma camera inefficiently captures the principal photon (364 keV) emitted by the radioactive decay of the 131I isotope, leading to scatter and image noise (Liu et al., 2013). In addition, 90% of radioactive decay for the 131I isotope is via β-emission, and its decay half-life (8 days) does not contribute to favorable image formation. In contrast, the principal photon of the 123I isotope (159 keV) is more suited for imaging with conventional gamma cameras and has a relatively shorter decay half-life (13 hours) compared with 131I. The improved image resolution, better dosimetric profile, and single photon emission computed tomography (SPECT) capabilities led to FDA approval of 123I-mIBG (AdreView) in 2008.
Due to its adrenergic imaging capabilities, 123I-mIBG has also been successfully applied for cardiac imaging and was approved by the FDA in 2013 to image the sympathetic innervation of the myocardium. In addition, due to its selective accumulation in neuroendocrine tumor cells, mIBG was tested as a targeted radiotherapy to treat several neuroendocrine cancers (Parisi et al., 2016; Pryma et al., 2019). In this case, the β emission by 131I is more favorable than 123I, as the stronger radiation emission can penetrate surrounding cancer cells and result in DNA damage and cell death. High dose 131I-mIBG (Azedra; Progenics Pharmaceuticals, Inc., New York, NY) was approved by the FDA in 2018 and proved efficacious in the treatment of advanced pheochromocytoma and paraganglioma. Lastly, 131I-mIBG is under investigation in multiple clinical trials for the treatment of neuroblastoma (Parisi et al., 2016; DuBois et al., 2021).
Clinical Uses of mIBG
Neuroblastoma
Neuroblastoma is the most common extracranial solid tumor in children and accounts for over 15% of all pediatric cancer mortalities in the US (Maris et al., 2007). Most patients are diagnosed at infancy, with a median age at diagnosis of 19 months (Ward et al., 2014). Neuroblastoma is a malignancy of the sympathoadrenal lineage of the neural crest cells and can arise anywhere in the sympathetic nervous system. Approximately 70% of primary tumors occur within the abdominal region, with at least half of the primary tumors arising in the adrenal gland. Unfortunately, approximately half of the patients are metastatic at diagnosis, most commonly to the bone and bone marrow, lymph nodes, and liver (DuBois et al., 1999).
Neuroblastoma is heterogenous in its clinical presentation and can spontaneously regress or progress as an aggressive metastatic cancer. Patients with neuroblastoma are stratified as low, intermediate, or high-risk based on age, tumor stage, and biologic factors, and the classification affects prognosis and treatment strategies (Park et al., 2008). Low- and intermediate-risk patients undergo surgery and/or chemotherapy with survival rates of greater than 90% (Irwin and Park, 2015). In contrast, high-risk neuroblastoma patients undergo a combination of surgery, autologous transplantation, chemotherapy, and whole-body radiation with survival rates of less than 40%–50% (Matthay et al., 1999; Parisi et al., 2016).
123I-mIBG is routinely used in the clinic to diagnose neuroblastoma by detecting primary tumors and to identify sites of metastasis (Matthay et al., 2010). In a cohort of 66 confirmed neuroblastoma cases, 123I-mIBG scintigraphy had a sensitivity of 88% and specificity of 83% for diagnosing neuroblastoma (Vik et al., 2009). When combined with planar and SPECT imaging, the sensitivity rose to 91%. With the inclusion of histopathology information, the sensitivity and specificity rose to 93% and 92%, respectively (Vik et al., 2009). Of note, false positive interpretations in this cohort were due to atypical adrenal or normal tissue uptake. Another study had similar results, with false negative rates of approximately 8% in 196 patients with pathologically proven neuroblastoma (Biasotti et al., 2000). Patients with bone metastasis at diagnosis had increased false negative rates of up to 21% without additional diagnostic tests (Gordon et al., 1990). Nevertheless, 123I-mIBG scintigraphy has proven to be an essential imaging agent for diagnosing and monitoring disease progression of neuroblastoma.
The use of 131I-mIBG as a therapeutic agent began in the early 1980s and was predominantly focused on the treatment of relapsed or refractory neuroblastoma (Treuner et al., 1988). Subsequently, multiple strategies have been undertaken to improve the clinical outcomes of relapsed or refractory neuroblastoma. These strategies include: the use of 131I-mIBG as a monotherapy, as a combination therapy with radiosensitizers, in conjunction with bone marrow transplantation, and as part of the induction therapy with concomitant chemotherapeutic regimens (Matthay et al., 1999; Johnson et al., 2011; DuBois et al., 2015; Yanik et al., 2015). These strategies have shown complete response rates similar to or better than the standard of care for high-risk neuroblastoma patients. Additionally, 131I-mIBG has been investigated as a front-line treatment of patients with neuroblastoma and has shown a response rate of up to 73%, higher than the typical response for high-risk patients (de Kraker et al., 2008).
Toxicity is a major concern for 131I-mIBG therapy. Toxicities are associated with the radioactive 131I whereas nonradiolabeled mIBG is nontoxic. Hematologic toxicities are the most common and severe toxicities, but patients are rescued by autologous stem cell infusion (Bleeker et al., 2013). Thyroid uptake of 123/131I-mIBG is usually blocked by coadministration with potassium iodine or Lugol’s solution to saturate the sodium-iodine symporter (Matthay et al., 2007; Parisi et al., 2016). However, radiation to the thyroid is still observed in patients and can lead to hypothyroidism months after treatment (Van Santen et al., 2002). Transient cardiac toxicities can occur during 131I-mIBG infusion and require continuous and automated blood pressure monitoring (Parisi et al., 2016). Tachycardia and hypertension are the most common cardiac toxicities in patients with neuroblastoma and occur in approximately 10% of neuroblastoma patients receiving 131I-mIBG therapy (Shusterman et al., 2011). Other significant toxicities include gastrointestinal disturbances (nausea, vomiting), sialadenitis (swelling of the salivary glands), and hepatic-related toxicities (Bleeker et al., 2013; Parisi et al., 2016). When 131I-mIBG is used in combination with other anticancer drugs, improvements to the response rates are variable and the 131I-mIBG associated toxicities can be exacerbated (Matthay et al., 2006).
Pheochromocytoma and Paraganglioma
In addition to neuroblastoma, 123I-mIBG has been successfully used in the localization and evaluation of other neuroendocrine cancers, in particular pheochromocytoma and paraganglioma. Unlike neuroblastoma, sporadic forms of pheochromocytoma and paraganglioma are usually diagnosed in individuals aged 40–50 years and are rare in children (Neumann et al., 1993). Pheochromocytoma and paraganglioma arise from the chromaffin cells that produce catecholamines in the adrenal medulla or accessory adrenal tissue (Lenders et al., 2005). The two tumor types cannot be differentiated based on histology alone; hence, anatomic location of the tumors is used to distinguish between them (Lam, 2017). Based on the 2017 World Health Organization (WHO) classification, pheochromocytoma is an adrenal tumor, whereas paraganglioma is an extra-adrenal tumor. Approximately 80% of patients with pheochromocytoma present with primary tumors in the adrenal medulla and frequently produce excessive levels of catecholamines (Eisenhofer et al., 2003). The rest of primary tumors are typically localized in the paravertebral sympathetic ganglia of the abdominal region and typically do not present with hypersecretion of catecholamines. Pheochromocytoma and paraganglioma are both rare cancers with a prevalence of 0.3% in patients with hypertension (Anderson et al., 1994). However, fatal complications preceded diagnosis in a significant proportion of patients with pheochromocytoma, as evidenced in over 8000 autopsies, indicating that the cancer is often undetected and left untreated (Lo et al., 2000).
123I-mIBG scintigraphic imaging has been proved to successfully help diagnose pheochromocytoma. 123I-mIBG SPECT imaging has a reported sensitivity of 83%–100% and a specificity of 95%–100% for detecting pheochromocytomas (Nielsen et al., 1996). However, 123I-mIBG has a lower sensitivity (56%–75%) for detecting the extra-adrenal paragangliomas and in patients with genetic mutations of succinate dehydrogenase, an enzyme involved in the Krebs cycle and associated with metastasis (Wiseman et al., 2009). Nonetheless, patients who exhibit positive 123I-mIBG scintigraphy may benefit from 131I-mIBG radiotherapy (Rao et al., 2019).
The goals for 131I-mIBG as treatment of pheochromocytoma are focused on alleviating symptoms, lowering blood pressure, reducing catecholamine secretion, and ultimately shrinking tumors with minimal systemic toxicity (Beierwaltes, 1987). The early noncontrolled clinical trials with 131I-mIBG for treating pheochromocytoma failed to achieve complete remission in most patients but produced an improvement in sustained remission or stable disease for up to 2 years (Loh et al., 1997). More recent studies have used 131I-mIBG therapy in patients with predominantly nonresectable and malignant pheochromocytoma and paraganglioma (Kotecka-Blicharz et al., 2018; Pryma et al., 2019; Wakabayashi et al., 2019). These studies showed high rates of stable disease in patients treated with 131I-mIBG therapy and a significant reduction in the use of hypertension medication. In addition, the use of 131I-mIBG therapy to reduce tumor size prior to surgery in a case study of two patients highlighted the potential of 131I-mIBG as an adjunctive therapy with surgery for pheochromocytoma (Dhingra and Halkar, 2020). The toxicities in pheochromocytoma patients given 131I-mIBG were similar to those in neuroblastoma patients, with hematologic toxicities being the most common and reported hypothyroidism, hypertension, dry mouth, and gastrointestinal disturbances.
Pharmacokinetics of mIBG
mIBG is administered as an intravenous infusion over 1–2 minutes for 123I-mIBG diagnostic imaging and 60–120 minutes for 131I-mIBG radiotherapy (Parisi et al., 2016). The blood concentrations of mIBG rapidly decrease in a biphasic manner with a half-life of the initial phase of approximately 0.2–0.3 hours and a long terminal half-life greater than 38 hours (Coleman et al., 2009; Chin et al., 2014). The amount of mIBG in the blood after 5 minutes corresponds to about 10% of the dose. mIBG has a large volume of distribution of 3–5 l/kg, indicating a substantial distribution into tissues. After 1 hour, only 2%–4% of the dose remains within the vascular compartment.
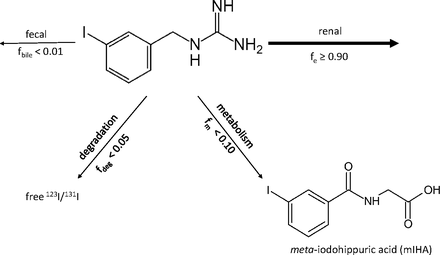
The systemic elimination pathways of mIBG are summarized in Fig. 2. The majority (>90%) of the administered dose is excreted into the urine unchanged, with 50% of the dose excreted into the urine within 24 hours (Lashford et al., 1988; Blake et al., 1989; Parisi et al., 2016). Both glomerular filtration and tubular secretion are involved in mIBG elimination by the kidney (Ehninger et al., 1987; López Quiñones et al., 2020). Fecal excretion accounts for less than 1% of the total mIBG dose, whereas dissociation of the 123I/131I from mIBG accounts for less than 5% of the total dose. Metabolism of mIBG accounts for less than 10% of the dose, with the major metabolite being meta-iodohippuric acid (Mangner et al., 1986; Coleman et al., 2009; Chin et al., 2014).
mIBG exhibits extensive physiologic distribution into normal tissues. Based on whole-body scintigraphy scans, mIBG distributes and accumulates in the salivary glands, liver, intestines, thyroid, lungs, spleen, muscle, urinary bladder, and adrenal glands (Nakajo et al., 1983; Coleman et al., 2009; Chin et al., 2014). The salivary glands, a highly sympathetically innervated tissue, exhibit the highest radiation exposure of mIBG and can consistently be visualized in scintigraphic images. The tissue distribution and accumulation are similar between 123I-mIBG and 131I-mIBG (Nakajo et al., 1983; Parisi et al., 1992).
Elimination pathways of mIBG in humans. The majority of mIBG (>90% of administered dose) is excreted unchanged in urine. Other pathways such as fecal elimination, chemical degradation, and metabolism are minor routes in systemic elimination of mIBG.
mIBG Disposition in Cancer Cells and the Role of NET
The broad clinical applications of radioiodinated mIBG are due to its structural similarities to norepinephrine (Fig. 1) and its active uptake by the Na+-dependent norepinephrine transporter (NET; SLC6A2) expressed in neurons and neuroendocrine cancer cells (Streby et al., 2015). With a pKa of ∼13, mIBG exists predominantly as a cation at physiologic pH. Although nonsaturable, passive transport has been suggested for mIBG; active transport is at least 50-fold more efficient than passive diffusion at lower mIBG concentrations (Mairs et al., 1995; Streby et al., 2015). Once inside the cells, the gamma radiation emitted by the radioactive isotope of iodine allows for imaging of the tumor and—in the case of 131I—emission of beta particles can lead to DNA damage and cell death. Unlike norepinephrine, metabolism does not contribute to the elimination of mIBG in the neuroendocrine cells, as mIBG is not a substrate of monoamine oxidase (MAO) or catechol-O-methyltransferase (COMT) (Graefe et al., 1999). However, mIBG can be transported out of the cancer cells by efflux mechanisms. As the tumor killing efficiency is directly related to how much and how long the radioactive 131I stays in cancer cells, tumor-selective uptake, retention, and efflux are the main determinants of the clinical efficacy of 131I-mIBG.
Currently, it is believed that NET is the major transporter driving mIBG uptake into neuroendocrine cancer cells. NET is highly expressed in most neuroendocrine tumors derived from neural crest and chromaffin cells of the adrenal medulla (DuBois et al., 2012; Streby et al., 2015; Zhang and Wang, 2020). NET is expressed in ∼90% neuroblastoma tumor samples, and its expression has been correlated with mIBG avidity in 123I-mIBG imaging (Carlin et al., 2003; DuBois et al., 2012; Streby et al., 2015). Due to the major role NET has in mIBG uptake, drugs known to interfere with NET function such as tricyclic antidepressants and norepinephrine reuptake inhibitors (e.g., amitriptyline, bupropion, duloxetine, venlafaxine) should be discontinued during treatment with mIBG according to the Azedra (2018) (131I-mIBG) drug label. Coadministration of such medications could lead to reduction in mIBG uptake in tumor tissue and therefore reduced efficacy and/or false negative imaging results. On the other hand, several studies have explored approaches aimed at enhancing NET expression in tumor cells with the goal of increasing 131I-mIBG efficacy (More et al., 2011; DuBois et al., 2015; Streby et al., 2015). For instance, More et al. (2011) showed that vorinostat, a histone deacetylase (HDAC) inhibitor, increased tumor NET expression and mIBG uptake in preclinical models of neuroblastoma. The specific mechanism by which vorinostat upregulates NET expression has not been well defined but is thought to relate to its inhibitory effect on HDACs (More et al., 2011). HDACs can act as transcription repressors for some genes, and inhibition of HDACs could selectively increase gene transcription by opening the chromatin and making it more accessible to transcriptional factors (More et al., 2011). The use of vorinostat as a radiation sensitizer for 131I-mIBG therapy has been recently explored in clinical studies with promising results (DuBois et al., 2015, 2021).
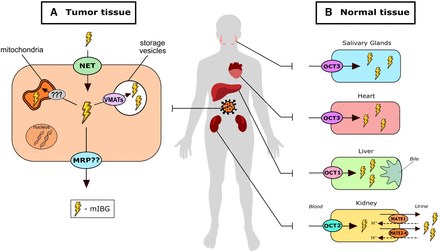
After gaining entry into tumor cells through NET, mIBG can be retained in the cytosol or further transported into intracellular organelles (Fig. 3A). For example, mIBG can be further taken up into secretory vesicles via the vesicular monoamine transporters 1 and 2 (VMAT1/2; SLC27A1/2). It was reported that in patients with high-risk metastatic neuroblastoma, VMAT1 and 2 were expressed in 62% and 75% of tumors, respectively (Temple et al., 2016). In addition, the expression level of VMAT2 but not that of VMAT1 correlated with avidity for mIBG in neuroblastoma (Temple et al., 2016). However, other studies suggested that only ∼7% of mIBG is contained in secretory vesicles in neuroblastoma cells (Lashford et al., 1991) and that the stimulation of vesicular release by calcium-dependent process and use of reserpine—a classic VMAT inhibitor—were demonstrated to have slight to no effect in mIBG retention in neuroblastoma cells (Smets et al., 1990; Servidei et al., 1995; Streby et al., 2015). Interestingly, in neuroblastoma the majority of mIBG appears to remain within the cytoplasm and is seemingly concentrated in the mitochondria through an unknown mechanism (Gaze et al., 1991; Lashford et al., 1991). In more differentiated neuroendocrine tumors such as pheochromocytoma and paragangliomas, intracellular mIBG is actively transported by VMAT1/2 after entering the cancer cells, leading to accumulation of mIBG in storage vesicles (Kölby et al., 2003). Such difference in intracellular disposition is possibly due to neuroblastoma cells having fewer storage vesicles than more differentiated tumors (Smets et al., 1990; Montaldo et al., 1991; Streby et al., 2015).
Proposed roles of transporters in tumor disposition, normal tissue disposition, and systemic elimination of mIBG. (A) In neuroendocrine tumors, mIBG uptake is mediated by NET. In pheochromocytoma and paraganglioma, uptake into storage vesicles by VMATs is believed to play a significant role in intracellular retention of mIBG. In neuroblastoma, however, most of mIBG is found in the cytoplasm and concentrates within the mitochondria through a currently unknown mechanism. Efflux by MRPs was suggested as a possible tumor efflux mechanism; however, clinical evidence of such is limited. (B) The polyspecific organic cation transporters OCT1–3 and MATE1/2-K are believed to play a role in normal tissue distribution and in systemic elimination of mIBG. Tissue expression pattern of such transporters indicates that OCT3 may be involved in mIBG accumulation in salivary glands and heart, whereas OCT1 may be involved in mIBG liver uptake. The OCT2-MATEs pathway is believed to play a major role in active secretion of mIBG in the renal proximal tubule cells.
The efflux of mIBG out of the cancer cells is thought to be through carrier-mediated efflux or by passive diffusion. In cultured neuroblastoma cell lines, efflux of mIBG was shown to be saturable, temperature-sensitive, and inducible by high extracellular potassium concentrations or the addition of norepinephrine or unlabeled mIBG (Servidei et al., 1995). This has led to the speculation that mIBG release from tumor cells efflux is mediated by a carrier, most likely NET working in a reverse mode. However, Lashford et al. (1991) suggested that after local release from the tumor cells, mIBG can undergo reuptake by NET and be transported back into cancer cells. Therefore, mIBG retention in cancer cells may depend on a dynamic equilibrium between active reuptake and efflux processes (Streby et al., 2015). Recently, Kobayashi et al. (2020) reported that mIBG is a substrate of multidrug resistance protein 1 and 4 (MRP1/4; ABCC1/4), which may contribute to the efflux of mIBG in neuroendocrine cancer cells. The clinical significance of the MRP transporters in 131I-mIBG efficacy and treatment resistance has yet to be investigated.
mIBG Disposition in Normal Tissues and the Emerging Roles of Organic Cation Transporters
The distribution and accumulation of radioactive mIBG in normal tissues can complicate the findings from 123I-mIBG imaging and the efficacy and safety of 131I-mIBG radiotherapy for neuroendocrine cancers. Accumulation of 123I-mIBG into normal tissues may interfere with tumor imaging, leading to an increased rate of false negatives for diagnosing neuroendocrine cancers (Eisenhofer et al., 2003; Vik et al., 2009; Parisi et al., 2016). For therapeutic use, uptake of 131I-mIBG into normal tissues can compete with tumor uptake, leading to reduced antitumor efficacy and increased tissue-specific toxicities. Importantly, the high uptake and accumulation in normal tissues are frequently associated with common and sometimes severe radiation-induced toxicities (Bleeker et al., 2013).
Compared with neuroendocrine cancer cells, much less is known on mIBG disposition in normal tissues. NET is predominantly expressed in neurons and neuroendocrine cells but is also expressed in the lung and thus may contribute to the pulmonary accumulation of mIBG (Torres et al., 2003). Although the closely related dopamine transporter (DAT; SLC6A3) and serotonin transporter (SERT; SLC6A4) (Table 1) were thought to not transport mIBG (Glowniak et al., 1993), recent evidence suggests that mIBG is a substrate of human SERT and that the transporter may contribute to the platelet accumulation of mIBG, which may lead to the common hematologic toxicities in 131I-mIBG therapy (Blom et al., 2020).
Characteristics of the monoamine and polyspecific organic cation transporters
Increasing evidence suggest that the polyspecific organic cation transporters play a major role in disposition of mIBG in normal tissues and organs. mIBG contains a guanidine moiety, which is a strong organic base (pKa of ∼13) (Chen et al., 2019). Hence at physiologic pH, mIBG exists as an organic cation with limited passive permeability. The polyspecific organic cation transporters are a group of solute carriers that mediate the cellular uptake and efflux of structurally diverse organic cations. This group consists of the organic cation transporter 1–3 (OCT1–3; SLC22A1–3), the plasma membrane monoamine transporter (PMAT; SLC29A4), and the multidrug and toxin extrusion protein 1 and 2-K (MATE1/2-K; SLC47A1/2). The transport mediated by the OCTs and PMAT is electrogenic and sodium-independent (Table 1) (Koepsell, 2004; Wagner et al., 2016; Wang, 2016). On the other hand, transport by the MATEs is through an electroneutral exchange between an H+ and a cationic substrate (Otsuka et al., 2005). The tissue distribution and the general roles of OCTs and MATEs in drug disposition are well established (Table 1). In this section, we focus on discussing the roles that these transporters may play in systemic elimination and tissue-specific disposition of mIBG.
hOCT2/hMATEs in Renal Handling of mIBG
The renal handling of drugs is mainly governed by glomerular filtration, tubular secretion, and reabsorption. The tubular secretion of drugs is mediated by various drug transporters located in the basolateral and apical membranes of renal proximal tubular cells. The major organic cation transporters expressed in the human kidney are hOCT2 and hMATE1/2-K (Li et al., 2006; Morrissey et al., 2013; Yin and Wang, 2016) (Table 1). Organic cations in circulation are transported into renal tubular cells by the electrogenic hOCT2. The organic cations are then secreted into the renal lumen by hMATE 1 and 2-K, which are driven by the inwardly directed proton gradient (Li et al., 2006; Morrissey et al., 2013; Yin and Wang, 2016). Multiple cationic drugs (e.g., metformin, atenolol) undergo active secretion across the renal proximal tubule cells through the hOCT2 and hMATE1/2-K pathway (Giacomini et al., 2010; Yin and Wang, 2016).
mIBG is predominantly eliminated by the kidney, with more than 90% of the administered dose excreted unchanged in the urine (Lashford et al., 1988) (Fig. 2). Although the FDA labels for AdreView (123I-mIBG) and Azedra (2018) (131I-mIBG) only mention glomerulus filtration as a major process for renal elimination of mIBG, we have previously estimated a high contribution of tubular secretion using data from available pharmacokinetic studies (Ehninger et al., 1987; Blake et al., 1989; López Quiñones et al., 2020). In a cohort of patients with neuroblastoma, the renal clearance of mIBG was reported to be 226 ml/min with a mean glomerular filtration rate of 94 ml/min (Blake et al., 1989). Considering the plasma protein binding of mIBG (61%) as stated in the FDA label for Azedra (2018) (131I-mIBG), the estimated glomerular filtration clearance is about 37 ml/min, which contributes to only 16% of the total renal clearance of mIBG. Thus, approximately 84% of the administered dose of mIBG is estimated to be eliminated through tubular secretion in the kidney.
We recently demonstrated that mIBG is efficiently transported by hOCT2, hMATE1, and hMATE2-K with apparent affinity (Km) values of 17.2 ± 2.8, 17.7 ± 10.9, and 12.6 ± 5.6 μM, respectively, which are comparable to the Km of hNET (8.7 ± 1.4 μM) under the same experimental condition (López Quiñones et al., 2020) (Table 1). Importantly, in hOCT2/hMATE1 double-transfected Madin-Darby canine kidney (MDCK) cells, mIBG was transported across the monolayer, with a higher permeability in the basal-to-apical direction than in the apical-to-basal direction and also when compared with control cells. These data suggest that hOCT2/hMATE1 in proximal tubule cells greatly facilitates the renal secretion of mIBG from the circulation into the renal lumen (Fig. 3B). Despite a substantial increase in basal-to-apical permeability, intracellular mIBG accumulation in hOCT2/hMATE1 cells was only marginally increased, suggesting that hOCT2-mediated uptake, but not hMATE-mediated efflux, is likely the rate-limiting step in the secretion process. In line with our observations, 123I-mIBG whole-body imaging shows rapid and substantial bladder accumulation of 123I-mIBG but only minimal to moderate kidney accumulation in patients with neuroblastoma and in healthy adults (Lashford et al., 1988; Chin et al., 2014).
The identification of the hOCT2-hMATE1/2-K pathway as the major route for systemic elimination of mIBG has important implications for the clinical use of mIBG because changes to the transport of mIBG by hOCT2 and/or hMATE could alter the pharmacokinetics and toxicity of radiolabeled mIBG. For instance, inhibition of the basolateral hOCT2 may reduce mIBG renal clearance, resulting in an increased systemic exposure that would result in greater radioactive exposure to the patients. Inhibition of hMATE-mediated efflux may increase intracellular accumulation of radioactive mIBG in proximal tubular cells, leading to increased risk of nephrotoxicity. The impact of hOCT2 and hMATE1/2-K on the pharmacokinetics and pharmacodynamics of 131I-mIBG warrants further investigation. In particular, the drug interaction potentials with renal mIBG transporters should be considered during 131I-mIBG therapy.
hOCT1 and hOCT3 in Tissue Uptake of mIBG
The liver, heart, salivary glands, and intestines are the major physiologic sites of high mIBG accumulation (Coleman et al., 2009; Chin et al., 2014). Consistently, cardiac, hepatic, and salivary gland toxicities have been observed with high-dose 131I-mIBG therapy (Modak et al., 2008; Bleeker et al., 2013; Parisi et al., 2016). In humans, hOCT1 is predominantly expressed in the liver and localized to the sinusoidal membrane of hepatocytes (Giacomini et al., 2010). In addition, hOCT1 has been detected at low expression levels in other tissues, such as the adrenal gland and small intestine (Gorboulev et al., 1997; Koepsell, 2004; Wagner et al., 2016). mIBG is a substrate of OCT1 with a Km value of 19.5 ± 6.9 μM as determined in HEK293 cells transfected with human OCT1 (López Quiñones et al., 2020). Although the liver is not a site for mIBG elimination, hepatic accumulation accounts for approximately 30% of the administered dose (Chin et al., 2014; Parisi et al., 2016). Using OCT1−/− mice, a 75% decrease was observed in the liver concentrations of mIBG 30 minutes after an intravenous injection, suggesting that OCT1 indeed contributes to the hepatic accumulation of mIBG in vivo (Jonker et al., 2001) (Fig. 3B). By selectively targeting OCT1 with a potent inhibitor, distribution of mIBG into the liver could be reduced, which may lead to improvements in the image resolution and toxicity profile of radioactive mIBG.
OCT3 is broadly expressed in various tissues, including salivary glands, heart, skeletal muscle, and placenta (Table 1). OCT3 is the major organic cation transporter expressed in both mouse and human salivary glands (Lee et al., 2014). OCT3 is localized on both the apical and basolateral membrane of the acinar cells lining the salivary glands, suggesting that OCT3 is involved in both uptake into the salivary glands and secretion into saliva of organic cations. OCT3 expression in the heart is localized to vascular endothelial cells and myocardiocytes (Grube et al., 2011; Solbach et al., 2011). Using Oct3−/− mice, we previously demonstrated an important role of OCT3 in the uptake and transport of organic cationic drugs such as metformin and methamphetamine in salivary glands, skeletal muscle, and heart in vivo (Lee et al., 2014, 2018; Wagner et al., 2018).
mIBG is efficiently transported by hOCT3 with a Km value of 14.5 ± 7.1 μM that is comparable to hOCT1, hOCT2, and hMATE1/2-K (López Quiñones et al., 2020). These in vitro transport data alongside the tissue expression of OCT3 suggest that hOCT3 is an important determinant for mIBG uptake and accumulation in salivary glands and heart as illustrated in Fig. 3B. Bayer et al. (2016) previously proposed to improve tumor selective uptake of mIBG by reducing OCT3-mediated tissue uptake. In non–tumor-bearing mice, corticosteroids were shown to decrease mIBG uptake in a few tissues, including small intestine, kidney, and heart; whereas in a neuroblastoma patient dosed with hydrocortisone prior to 123I-mIBG scan, liver and heart uptake of mIBG was reduced in comparison to other patients (Bayer et al., 2016). However, the nonselective inhibitory nature of corticosteroids makes it difficult to specifically interpret the impact of OCT3 on mIBG disposition in vivo. Our laboratory recently showed that crizotinib, a tyrosine kinase inhibitor used to treat neuroblastoma, preferentially inhibits hOCT3 (López Quiñones et al., 2020). Hence, crizotinib could be further explored to specifically block peripheral tissue uptake of 131I-mIBG without affecting its systemic elimination or tumor uptake.
In addition to OCTs and MATEs, another polyspecific organic cation transporter, PMAT, was cloned and characterized by our laboratory (Engel et al., 2004). The transport of small organic cations by PMAT is electrogenic and pH sensitive (Duan and Wang, 2010; Zhou et al., 2010). PMAT is highly expressed in the brain and also has varied expression in the kidney, small intestine, and heart (Dahlin et al., 2007; Xia et al., 2007; Zhou et al., 2007; Duan and Wang, 2013; Vieira and Wang, 2021). Furthermore, PMAT was identified in an exploratory study as one of the top 25 membrane protein genes overexpressed in neuroblastoma tumor samples (Orentas et al., 2012). The role of PMAT in mIBG uptake in tumor and in normal tissue is currently under investigation in our laboratory.
Perspectives and Future Directions
Transporters on the plasma membrane of tumor cells are promising molecular “Trojan horses” to deliver drugs and imaging agents into cancer cells. Nevertheless, even with molecules designed to target tumor transporters, accumulation can still occur in normal tissues, raising concerns for tissue toxicity or decreased sensitivity in tumor detection. Although the same carrier transporting these agents into tumor cells may also contribute to their uptake in normal tissues, this is not always the case. As illustrated for mIBG in this review, tumor-targeting of this radiopharmaceutical is primarily mediated by NET expressed in neuroendocrine cancer cells, whereas its systemic elimination and accumulation in nontarget tissues are likely mediated by various polyspecific organic cation transporters (Fig. 3). Although the in vivo impact of various organic cation transporters in mIBG pharmacology remains to be validated through clinical studies, the prospect that tumor and normal cells employ a different set of transporters to transport mIBG is exciting and suggests a range of opportunities for future exploration to improve the clinical use of mIBG.
One obvious application is to selectively inhibit hOCT3 and/or hOCT1 to reduce 131I-mIBG accumulation and toxicity in peripheral organs. As normal tissues also compete with tumor cells in mIBG uptake, one added advantage for this approach is that it may also increase mIBG uptake in tumor cells. A critical challenge in this approach is to identify a highly specific inhibitor that does not interfere with either NET-mediated tumor uptake or hOCT2/hMATEs-mediated renal elimination. Similarly, enhancing NET expression in tumor cells (e.g., through the use of histone deacetylase inhibitor) is being explored clinically to increase 131I-mIBG efficacy. For these endeavors, the effect of the NET enhancers on mIBG transporters in normal tissues should also be investigated, as changes in the expression and activity of these transporters could alter the disposition and toxicity of 131I-mIBG.
The impact of hOCT2 and hMATE1/2-K on the pharmacokinetics and pharmacodynamics of mIBG warrants further investigation. In particular, the drug interaction potentials with renal mIBG transporters should be considered during 131I-mIBG therapy. Patients with cancer often take multiple drugs to treat comorbidities, control pains, or relieve severe side effects associated with chemotherapy or radiation therapy. Thus, potential drug interactions at the transporter sites should be carefully considered. Currently, the use of clinical NET inhibitors such as tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors are avoided before and during mIBG imaging and therapy. Similarly, the potential impact of clinical inhibitors of hOCT2 and/or hMATE1/2-K should be considered. As tubular secretion of mIBG is the dominating pathway for systemic elimination of mIBG, blockage of this pathway may result in a change in 131I-mIBG pharmacokinetics and pharmacodynamics. In particular, inhibition of hMATE-mediated efflux may increase intracellular accumulation of 131I-mIBG in renal proximal tubule cells, leading to nephrotoxicity.
The discovery of mIBG as a substrate of polyspecific organic cation transporters also raises the possibility of using 123I-mIBG as a radiotracer to image OCTs and MATEs activities in humans. When used as a diagnostic agent, 123I-mIBG is given at a low dose, has a shorter radiation decay half-life, and emits low energy radiation. There are little radiation-associated toxicities for low dose 123I-mIBG, suggesting that it may be used as a noninvasive imaging agent to detect changes in tissue expression or activities of hOCTs or hMATEs. Additionally, mIBG exhibits minimal metabolism and does not interact with adrenergic receptors. These characteristics are worth exploring in clinical drug-drug interaction (DDI) studies using 123I-mIBG as a probe.
Conclusions
In this mini review, we discussed the clinical applications of mIBG and the roles of transporters as drivers of mIBG disposition, efficacy, and toxicity. Elucidating the transport mechanisms of mIBG in tumor and normal tissues has practical and translational values in the development of new clinical strategies to improve the diagnostic and therapeutic utility of radiolabeled mIBG. The clinical use of mIBG as a radiopharmaceutical in cancer diagnosis and treatment can be further improved by taking a holistic approach considering mIBG transporters in both cancer and normal tissues.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: López Quiñones, Vieira, Wang.
Footnotes
- Received October 1, 2021.
- Accepted February 17, 2022.
This work was supported by National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM066233] (to J.W.).
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- FDA
- US Food and Drug Administration
- HDAC
- histone deacetylase
- Km
- apparent affinity
- MATE
- multidrug and toxin extrusion protein
- mIBG
- meta-iodobenzylguanidine
- MRP
- multidrug resistance protein
- NET
- norepinephrine transporter
- OCT
- organic cation transporter
- PMAT
- plasma membrane monoamine transporter
- SERT
- serotonin transporter
- SPECT
- single photon emission computed tomography
- VMAT
- vesicular monoamine transporter
- Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics