Abstract
UDP-glucuronosyltransferase (UGT) 2B9, isolated from a cynomolgus monkey liver cDNA library, is 89% identical to human UGT2B7 in primary amino acid sequence, and the two expressed enzymes were previously shown to catalyze the glucuronidation of many common endogenous substrates. The purpose of the present study was to characterize the reactivity of expressed UGT2B9 with important therapeutic agents and other xenobiotics. UGT2B9, stably expressed in human embryonic kidney 293 cells, catalyzes the 3-O- and 6-O-glucuronidation of morphine and the 6-O-glucuronidation of codeine. A number of other morphinan (e.g. naloxone, naltrexone, and nalorphine) and oripavine (e.g. buprenorphine) derivatives are substrates for this enzyme. In general, morphinan derivatives are glucuronidated at higher rates, compared with oripavines; however, glucuronidation efficiency values (Vmax/KM ) for the compounds are similar. Stably expressed UGT2B9 also catalyzes the glucuronidation of profen nonsteroidal anti-inflammatory drugs, fibrate hypolipidemic agents, and straight-chain fatty acids at the carboxylic acid moiety. Monoterpenoid alcohols and propanolol are glucuronidated at aliphatic hydroxyl positions. Expressed UGT2B9 exhibits enantioselective glucuronidation for (R/S)-ibuprofen, (R/S)-propanolol, and (+)/(−)-menthol. The data suggest that monkey UGT2B9 and human UGT2B7 are functionally similar.
Glucuronidation is a major conjugation reaction that is catalyzed by numerous isoforms of UGT.1 These enzymes are localized primarily in the endoplasmic reticulum and participate in the metabolic elimination of many endogenous compounds and xenobiotics (1). Compounds with a wide variety of chemical moieties, such as amines, hydroxylated compounds, and carboxylic acids, are substrates for UGT isoforms. UGTs that are members of the UGT1 gene complex share common second through fifth exons, with at least 12 separate first exons coding for proteins with unique amino-terminal domains (2). In contrast, gene products of the UGT2 family appear to be transcribed from unique genes (3, 4).
Although much is known about the different UGTs expressed in rats, rabbits, and humans, less is known about UGTs expressed in other laboratory animals. Monkeys are commonly used in preclinical drug metabolism studies, as well as in testing of many compounds, especially opioid agonists and antagonists, with pharmacological activity in the central nervous system (5-7). Therefore, it is of interest to characterize UGTs to determine the similarities and differences in the proteins expressed in monkeys, compared with those expressed in humans. Recently, a cDNA encoding UGT2B92 has been isolated and stably expressed in HK293 cells, and the reactivity of the expressed protein toward endobiotics has been characterized (8). UGT2B9, isolated from cynomolgus monkey prostate and liver cDNA libraries, is 89% identical to human UGT2B7 in primary amino acid sequence, and the two expressed enzymes have been shown to catalyze the glucuronidation of many common endogenous substrates, such as androsterone and hyodeoxycholate (8-11). The major difference in substrate specificity between expressed human UGT2B7 and monkey UGT2B9 is the ability of the latter to catalyze the glucuronidation of 17-hydroxylated androgens such as testosterone and dihydrotestosterone (8). Coffman et al. (12) have shown that expressed human UGT2B7 protein catalyzes the 3-O- and 6-O-glucuronidation of morphine. Jinet al. (11) showed that expressed human UGT2B7 catalyzes the glucuronidation of many clinically important, carboxylic acid-containing drugs, such as profen NSAIDs, clofibrate, and valproic acid. The purpose of this study was to characterize the reactivity of expressed UGT2B9 with drugs and other xenobiotics. Specifically, the reactivity of UGT2B9 with opioids and carboxylic acid-containing drugs was examined.
Materials and Methods
Materials.
Aglycone substrates for glucuronidation assays were of the highest purity available and were purchased from Sigma Chemical Co. (St. Louis, MO) or Aldrich Chemical Co. (Milwaukee, WI). Geneticin (G418), saccharolactone, UDP-glucuronic acid, and L-α-phosphatidylcholine (type XVI-E from egg yolk) were obtained from Sigma. UDP-[U-14C]glucuronic acid (225 mCi/mmol) was purchased from ICN Radiochemicals (Costa Mesa, CA). Protein assay reagents were obtained from Bio-Rad (Richmond, CA).
Stable Expression of Monkey UGT2B9 Protein.
The establishment and characterization of an HK293 cell line stably expressing UGT2B9 protein were reported previously (8). The HK293 cells expressing UGT2B9 were grown in Dulbecco’s modified Eagle’s medium containing 4.5 mM glucose, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10% fetal bovine serum, and 700 μg/ml geneticin, in a humidified incubator with an atmosphere of 5% CO2, at 37°C. Membrane preparations were made by differential ultracentrifugation using a modification of the method of Battaglia et al. (13), as described by King et al. (14). Glucuronidation activities for the substrates used in the present studies were not detected in nontransfected HK293 cell homogenates.
UGT Assays.
Membrane pellets from HK293 cells expressing UGT2B9 protein were suspended in 10 mM Tris-buffered saline (pH 7.4), containing 0.5 mM dithiothreitol, and were gently resuspended using a Potter-Elvehjem homogenizer. Opioid glucuronidation was determined using the method described by Puig and Tephly (15). The HPLC method of Svensson et al. (16) was used to separate morphine 3-O- and 6-O-glucuronides, and the corresponding fractions were collected and quantitated using scintillation counting. Glucuronidation activities toward all other aglycone substrates were analyzed using the TLC method described by Bansal and Gessner (17), as modified (18). All assay mixtures (0.1-ml final volume) contained 50 mM Tris-HCl, 10 mM MgCl2, 100 μg/ml phosphatidylcholine, 8.5 mM saccharolactone, 2.0 mM UDP-glucuronic acid (0.25 μCi/assay), and 0.5 mM aglycone substrate, unless otherwise indicated. Reactions in which the aglycone substrate was omitted served as controls, and the minimal counts obtained were subtracted from values for reactions where aglycone was present. Glucuronidation assays were conducted using pH 7.5 buffer, unless otherwise indicated. All enzymatic assays were conducted at 37°C under conditions that produced linear product formation with respect to time (0.5–2 hr) and protein concentration (up to 75 μg/0.1-ml assay).
Apparent KM values for aglycone substrates were estimated by varying the aglycone concentration from approximately one-fifth to five times the apparent KM values, using a fixed concentration (2.0 mM) of UDP-glucuronic acid. Kinetic values were determined using assay conditions yielding linear product formation with respect to protein concentration and reaction times. Kinetic parameters were calculated using the program Enzyme Kinetics (Trinity Software, Plymouth, NH).
Results
Glucuronidation of Opioids Catalyzed by UGT2B9.
Membrane preparations derived from HK293 cells stably expressing UGT2B9 protein catalyzed the glucuronidation of morphinan-based and oripavine-based opioids (table 1). Opioid glucuronidation was not found using membrane preparations from control HK293 cells. Morphine and hydromorphone were glucuronidated at high rates, whereas naloxone and naltrexone were glucuronidated at lower rates. The observation that codeine was a substrate suggested that expressed UGT2B9 catalyzes 6-O-glucuronidation of morphinan opioids. HPLC analysis confirmed that UGT2B9 catalyzed the formation of both morphine 3-O- and 6-O-glucuronide, with the rate of 3-O-glucuronide formation being about 15 times higher than that of 6-O-glucuronide formation. Removal of the N-methyl group from morphine (normorphine) and codeine (norcodeine) greatly decreased the rates of conjugation of these compounds. In general, the rates of glucuronidation for oripavine opioids are lower than those for the morphinan opioids.
Glucuronidation of opioids catalyzed by expressed UGT2B9
Kinetic analysis of opioid glucuronidation catalyzed by expressed UGT2B9 is shown in table 2. Although morphinan derivatives are glucuronidated at higher rates, compared with oripavines, the glucuronidation efficiency value (Vmax/KM ) of morphine is similar to that of buprenorphine. The results show that the efficiency of glucuronidation for nalorphine was highest and that the efficiencies for the other opioids were comparable. The results also show that the N-alkyl side chain length appears to be a major influence on the apparent KM . Compounds with an N-methyl substituent (e.g., hydromorphone, dihydromorphine, and morphine) have higher apparentKM values than do compounds with longer N-alkyl substitutions, such as naloxone, nalorphine, and buprenorphine.
Kinetics of glucuronide formation for selected opioids in membrane preparations from HK293 cells stably expressing UGT2B9 protein
Glucuronidation of Carboxylic Acid-Containing Drugs.
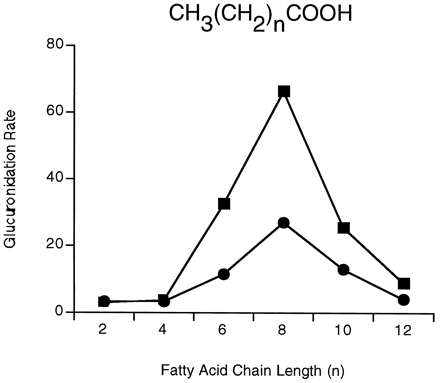
A number of clinically important drugs were tested to determine whether they were substrates for expressed UGT2B9. Several carboxylic acid-containing NSAIDs were substrates for expressed UGT2B9 protein (table 3). Of the profen NSAIDs tested,S-ibuprofen exhibited the highest glucuronidation rate. Of the two fibrate hypolipidemic agents tested, the glucuronidation rate for ciprofibrate was higher than that for clofibrate. Other carboxylic acid-containing drugs, such as valproic acid, furosemide, and diflunisal, were also substrates for expressed UGT2B9. Interestingly, expressed UGT2B9 also catalyzed the glucuronidation of straight-chain saturated fatty acids (fig. 1). Of the fatty acids tested, n-decanoic acid was the best substrate. Fatty acids with longer or shorter alkyl chains were glucuronidated at lower rates than decanoic acid. Short-chain carboxylic acids with aromatic substitutions one or two carbons removed from the carboxylic acid moiety (2-naphthylacetic acid, triphenylacetic acid, and 3,3,3-triphenylpropionic acid) were glucuronidated at low rates (12, 6, and 10 pmol/min/mg protein, respectively).
Glucuronidation of drugs catalyzed by expressed UGT2B9
Glucuronidation of n-alkyl fatty acids by expressed UGT2B9.
Glucuronidation of straight-chain fatty acids catalyzed by expressed UGT2B9 was conducted using pH 6.4 bis-Tris (▪) or pH 7.5 Tris (•) buffer. Glucuronidation rates are given in picomoles of glucuronide formed per minute per milligram of protein. The results represent the mean values obtained using three different membrane preparations, and the SDs are contained within the data points.
Stably expressed UGT2B9 also catalyzed the glucuronidation of drugs and xenobiotics with aliphatic hydroxyl groups. Propanolol, oxazepam (table3), and monoterpenoid alcohols (table 4) are weak substrates for the expressed protein. Of the monoterpenoid alcohols tested, borneol had the highest glucuronidation rate for expressed UGT2B9. Indeed, of all the compounds tested in this study, borneol exhibited the highest glucuronidation rate.
Glucuronidation of monoterpenoid alcohols catalyzed by expressed UGT2B9
Of the other drugs tested, measurable glucuronidation activity was not detected for acetaminophen, probenecid, phenolphthalein, hexafluoro-2-propanol, and the 4-hydroxy metabolite of tamoxifen. The reactivity of expressed UGT2B9 toward coumarins and flavonoids was also examined. 4-Methylumbelliferone was glucuronidated at a moderate rate (48 pmol/min/mg protein), but glucuronidation of umbelliferone, scopoletin, esculetin, and 4-hydroxycoumarin was not detected. Likewise, glucuronidation of 7-hydroxyflavone, apigenin, naringenin, chrysin, and galangin by UGT2B9 was not detected. The only flavonoids found to be substrates for expressed UGT2B9 were fisetin (111 pmol/min/mg), quercetin (142 pmol/min/mg), and genistein (18 pmol/min/mg). Sapogenins, such as tigogenin and hecogenin, were not substrates for UGT2B9.
Kinetic Analysis of the Glucuronidation of Xenobiotics by Expressed UGT2B9.
Apparent enantioselectivity was observed for the glucuronidation of (R/S)-ibuprofen, (R/S)-propanolol, and (+)/(−)-menthol, but not for (R/S)-β-citronellol (tables 3 and 4). Kinetic analysis was conducted for some of these compounds to determine whether the differences in glucuronidation rates for the enantiomers were due to different affinities of the compounds for the expressed enzyme. The results of these studies are shown in table5. No significant differences were found in the apparent KM values for (R/S)-ibuprofen, (R/S)-propanolol, and (+)/(−)-menthol, suggesting that the stereoisomers interact with the expressed protein in similar manners. The higher glucuronidation rates observed for (S)-ibuprofen, (R)-propanolol, and (−)-menthol, compared with their enantiomers, can be explained entirely by their higher Vmax values.
Kinetics of glucuronide formation for selected xenobiotics in membrane preparations from HK293 cells stably expressing UGT2B9 protein
pH Effects on the Glucuronidation of Opioids and Carboxylic Acids Catalyzed by Expressed UGT2B9.
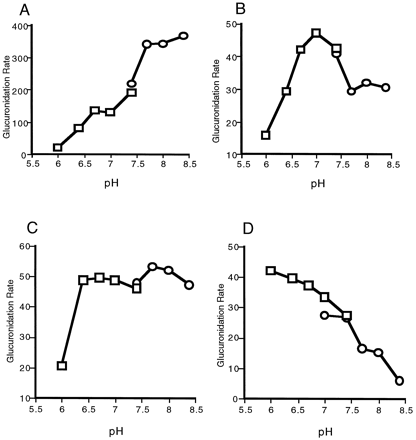
Opioids and carboxylic acids represent very diverse classes of substrates when their pKa values are considered. Glucuronidation of the basic opioids by expressed UGT2B9 membrane preparations exhibited three types of pH dependence. The pH curves for morphine (fig. 2A), hydromorphone, and codeine were similar, in that glucuronidation rates increased with increasing pH up to about pH 7.7 and then plateaued. In contrast, the glucuronidation rates for buprenorphine (fig.2B) and norbuprenorphine peaked at pH 7.0 and then declined. The glucuronidation of naltrexone (fig. 2C) and naloxone exhibited very little pH dependence in the range of pH 6.7–8.4. These results suggest that, for morphinan opioids, the ionization potential of the N-methyl substituent contributes to the binding of the substrate to the active site of the enzyme. LongerN-alkyl substitutions are more likely to be unionized over the range of pH values used in the present study.
pH optima for the glucuronidation of selected substrates.
The glucuronidation pH optima for morphine (A), buprenorphine (B), naltrexone (C), and naproxen (D) were determined using bis-Tris (□) or Tris (○) buffers, as described in Materials and Methods.
The glucuronidation of carboxylic acids catalyzed by expressed UGT2B9 was also found to be pH dependent. Whereas pH 7.4 was found to be optimum for the glucuronidation of the ibuprofen enantiomers, naproxen (fig. 2D) and the intermediate-chain fatty acids were glucuronidated at higher rates at low pH (about pH 6.4). Kinetic analysis of naproxen glucuronidation showed that at pH 6.4 the apparentKM was lower and theVmax was higher than the values obtained at pH 7.5 (table 5). This resulted in an approximately 10-fold increase in the glucuronidation efficiency at pH 6.4. These results suggest that the unionized forms of carboxylic acids are the preferred forms of the substrates.
Discussion
UGT2B9 is a simian enzyme whose cDNA has been recently isolated from liver and prostate and expressed in HK293 cells, to investigate the substrate specificity of the encoded protein. Bélangeret al. (8) showed that UGT2B9 mRNA is expressed in liver and in steroid target tissues such as ovary, epididymis, testis, and prostate and that the expressed enzyme demonstrated a wide substrate specificity for C18, C19, and C21 steroids and bile acids. The major difference in substrate specificity between expressed human UGT2B7 and monkey UGT2B9 is the ability of the latter to catalyze the glucuronidation of 17-hydroxylated androgens, such as testosterone and dihydrotestosterone. Among the C18 steroids glucuronidated by expressed UGT2B9 are the catechol estrogens 4-hydroxyestrone and 4-hydroxyestradiol. Recent studies in our laboratory have suggested that human UGTs that catalyze the glucuronidation of 4-hydroxylated catechol estrogens also catalyze the glucuronidation of morphine (19). In the present study, we found that expressed UGT2B9 catalyzes the glucuronidation of opioids at the aromatic 3- and aliphatic 6-hydroxyl positions, that of profen NSAIDs, fibrate hypolipidemic agents, and straight-chain fatty acids at the carboxylic acid moiety, and that of monoterpenoid alcohols at aliphatic hydroxyl groups.
In general, the rates of glucuronidation for oripavine opioids are lower than those for morphinan opioids, suggesting that these compounds may be poorer substrates for the enzyme. However, kinetic analysis showed that the glucuronidation efficiency values (Vmax/KM ) of the compounds are similar. The rate of morphine 6-O-glucuronide formation catalyzed by expressed UGT2B9 (6%), compared with the rate of morphine 3-O-glucuronide formation, is similar to the comparable value found by Rane et al. (20) for rhesus monkey liver microsomes (4%). Using expressed human UGT2B7, the rate of morphine 6-O-glucuronide formation was found to be about 13% of the rate of morphine 3-O-glucuronide formation (12), and the ratio obtained was similar to that observed in human liver microsomes. Because the morphine 6-O-glucuronidation rate was low for expressed monkey UGT2B9, it was not possible to accurately determine the apparentKM for morphine for the 6-O-glucuronidation reaction. However, for human morphine glucuronidation catalyzed by UGT2B7, the apparentKM for morphine for 6-O-glucuronide formation is similar to that for 3-O-glucuronidation (12).
Morphinan-based opioid glucuronidation catalyzed by expressed UGT2B9 demonstrates opioid structure-activity relationships similar to those found for expressed rat UGT2B1, except that rat UGT2B1 does not catalyze morphine 6-O-glucuronide formation (14). Sanchezet al. (21) showed that the presence and length of theN-alkyl side chain of opioids affect the ability of the compounds to interact with the UGT opioid binding site. They found that N-demethylated opioids were poor inhibitors of morphine glucuronidation in rat and rabbit liver microsomes. In contrast, they found that opioids with longer N-alkyl side chains had low Ki values for the inhibition of morphine glucuronidation. In the present study, we have shown that normorphine and norcodeine are glucuronidated at very low rates (1/100 and 1/25, respectively), compared with theirN-methylated analogues. The very low glucuronidation rates for these compounds made it impossible to accurately determine the kinetic values for these compounds, but the data suggest that the demethylated compounds are poor substrates. Saturation of the 7,8-double bond of the morphinan ring structure resulted in compounds (i.e. dihydromorphine and hydromorphone) that exhibited much higher apparent KM values and similar or somewhat higher Vmax values, compared with morphine. Oxymorphone, naloxone, and naltrexone differ only in theirN-alkyl substituents (N-methyl,N-allyl, and N-cyclopropylmethyl, respectively). As predicted by the results of Sanchez et al. (21), naloxone and naltrexone have apparent KM values 1 order of magnitude lower than that for the N-methylated oxymorphone. Similar results have recently been observed for expressed human UGT2B7 (12, 22).
Expressed monkey UGT2B9 catalyzes the stereoselective glucuronidation of menthol, ibuprofen, and propanolol. Similarly to the results reported using expressed human UGT2B7 (11), expressed monkey UGT2B9 preferentially glucuronidated the S-enantiomer of ibuprofen. The S/R ratio of 3.7/1 obtained using standard assay conditions was similar to the VmaxS/R ratio of 4/1 obtained using optimal kinetic assay conditions. Likewise, the (−)/(+)-menthol glucuronidation ratio of 2.6/1 under standard assay conditions was the same as theVmax (−)/(+)-menthol glucuronidation ratio of 2.8/1. In contrast, the VmaxS/R glucuronidation ratio for propanolol of 1/2.6 was somewhat different from the ratio of 1/1.7 obtained under standard assay conditions. These results are probably explained by the fact that for the propanolol standard glucuronidation assays the substrate concentration (0.5 mM) is only slightly higher that the apparentKM values for (S)- and (R)-propanolol (about 0.4 mM), whereas for ibuprofen and menthol the standard assay substrate concentration is 4–5 times the apparent KM values. Although large differences are observed in the Vmax values of the enantiomers studied, the apparentKM values are not different. These results suggest that the stereoisomers interact similarly with the enzyme but that the energetics of the reactions are different.
In addition to the carboxylic acid-containing profen NSAIDs and fibrates, expressed simian UGT2B9 catalyzes the glucuronidation of fatty acids. Fatty acids have also been shown to be substrates for rat UGT2B1, an enzyme that also catalyzes opioid glucuronidation (23). Similarly to the results of Pritchard et al. (23) using rat UGT2B1, UGT2B9 glucuronidates n-decanoic acid at higher rates, compared with compounds with shorter and longer side chains. The glucuronidation of fatty acids by other UGTs has not been investigated. Caprylic, decanoic, and dodecanoic acids were glucuronidated at higher rates at pH 6.4, compared with pH 7.5, suggesting that the unionized carboxylic group is the preferred substrate. The disappearance of pH dependence for the glucuronidation of fatty acids with side chains longer than decanoic acid is probably due to the increasing lipophilicity of these compounds. With highly lipophilic fatty acids, glucuronidation may be less pH dependent. Short-chain fatty acids are quite water soluble, and the lower pH may be necessary for a significant amount of the compound to be in the unionized, and therefore more lipophilic, form.
Glucuronidation of naproxen at pH 6.5 results in a lower apparentKM for the substrate and a higherVmax, compared with assays conducted at pH 7.5. This resulted in a 10-fold increase in the glucuronidation efficiency for naproxen at pH 6.5. It is unclear which values are more physiologically relevant, because the microenvironment at the active site of UGTs is not known. Therefore, pH dependence for UGT substrates must be determined to identify the appropriate kinetic values.
Simian UGT2B9 and human UGT2B7 are 89% identical in primary amino acid sequence, and expressed UGT2B9 was previously shown to catalyze the glucuronidation of many C18, C19, and C20 steroids and bile acids (8). The substrate specificity of monkey UGT2B9 for endogenous compounds and xenobiotics is clearly different from those observed for human UGT2B4 (10), UGT2B15 (18), and UGT2B17 (24). Of the human UGTs that have been characterized to date, UGT2B9 appears to be most similar to UGT2B7, based on substrate specificity of the expressed enzymes for endogenous compounds and xenobiotics.
Acknowledgments
We thank Martin Beaulieu and Eric Lévesque for isolation of the UGT2B9 cDNA.
Footnotes
-
Send reprint requests to: Dr. Thomas R. Tephly, Department of Pharmacology, 2–452 Bowen Science Building, The University of Iowa, Iowa City, IA 52242.
-
This work was supported by National Institutes of Health Grant GM26221 (T.R.T.), the Medical Research Council of Canada, Fonds de la Recherche en Santé du Québec (950031–103) (D.W.H.), and Endoresearch.
-
↵2 In a previously published abstract (22), this transferase was referred to as UGT2B18; however, the UGT Nomenclature Committee has since revised some of the UGT nomenclature and has designated this monkey transferase as UGT2B9.
- Abbreviations used are::
- UGT
- UDP-glucuronosyltransferase
- HK293 cells
- human embryonic kidney 293 cells
- NSAIDs
- nonsteroidal anti-inflammatory drugs
- Received June 19, 1997.
- Accepted August 5, 1997.
- The American Society for Pharmacology and Experimental Therapeutics