Abstract
The phase I and phase II drug-metabolizing capacity of freshly isolated and cryopreserved parenchymal cells (PC) from human, rat, and mouse liver held in suspension at 37°C for up to 120 min after thawing was compared. Although 7-ethoxycoumarin-O-deethylase activity was strongly reduced in freshly isolated as well as in cryopreserved PC from human, rat, and mouse liver after 120 min, 7-ethoxyresorufin-O-deethylase activity as well as the profile and formation rates of hydroxylated testosterone metabolites in general remained constant throughout the 2-h incubation period in cryopreserved PC from all three species and was similar to that measured in freshly isolated PC. The activity of glutathioneS-transferase (GST) and that of UDP- glucuronosyltransferase (UDP-GT) toward 4-methylumbelliferone significantly decreased, whereas the activities of UDP-GT activity toward 4-hydroxybiphenyl and sulfotransferase in cryopreserved human PC were similar to those measured in freshly isolated PC. The activities of GST, UDP-GT toward 4-methylumbelliferone, and sulfotransferase in cryopreserved rat PC showed a significant decrease when compared with the activities in freshly isolated PC. The phase II enzyme activities in cryopreserved mouse PC proved to be far more stable, being similar to the activities of freshly isolated mouse PC at all four time points measured with the exception of GST, which showed a decay from t = 60 min onward. In conclusion, phase I drug-metabolizing enzyme activities in cryopreserved human, rat, and mouse PC are very similar to those of freshly isolated PC, whereas phase II enzyme activities are affected by cryopreservation.
Many pharmacological and toxicological studies in the chemico-pharmaceutical industry as well as in basic research require the availability of freshly isolated liver parenchymal cells (PC)5 from diverse animal species. Although freshly isolated rat and mouse PC are readily available, it often happens that only a small portion of the cells obtained from these two animal species is used in a certain experiment and that the rest of the PC is discarded. Thus, a few years ago, methods for cryopreservation of rat liver PC were developed (Utesch et al., 1992; Diener et al., 1993). In this context, it was shown that the cytochrome P-450 content, the testosterone hydroxylation rate, and the activities of phenol sulfotransferase, UDP-glucuronosyl transferase (UDP-GT), cytosolic and microsomal epoxide hydrolase, as well as cytosolic glutathione S-transferase (GST), were similar in freshly isolated and cryopreserved PC determined just after thawing (Utesch et al., 1992; Diener et al., 1993; 1995).
In the case of human PC the number of liver samples available is generally low; in most cases in the past, when human PC were needed for a certain experiment, they were not immediately obtainable. In the meantime a cryopreservation protocol for human PC has been developed and the activities of phenol sulfotransferase, UDP-GT, and microsomal epoxide hydrolase were shown to be excellently maintained after thawing (Diener et al., 1994).
Taken together, the above-mentioned studies have shown that cryopreservation might help to reduce the number of laboratory animals sacrificed to obtain PC and to make the best of the erratic supply of human liver samples for the same purpose. However, in all the reports dealing with the drug-metabolizing capacity of cryopreserved human and rat hepatocytes published up to the present time, the analyses have been restricted to the situation immediately after thawing. When using isolated PC to study the in vitro metabolism of a given substance, the cytochrome P-450 status of the PC is critical: it has been known for a number of years now that the cytochrome P-450 content rapidly decreases within PC after isolation (Skett, 1994). Therefore, in the present report we have compared lactate dehydrogenase (LDH) retention, 7-ethoxycoumarin-O-deethylase (ECOD), and 7-ethoxyresorufin-O-deethylase (EROD) activities as well as the testosterone hydroxylation rate in freshly isolated and cryopreserved human, rat, and mouse PC held in suspension for up to 2 h, a time period often used to study the metabolism of compounds by isolated PC. LDH retention was chosen as an index for the viability of PC whereas ECOD, EROD, and testosterone hydroxylation were selected as parameters to monitor the status of cytochrome P-450-dependent reactions in PC. The O-deethylation of 7-ethoxycoumarin is catalyzed by a wide range of cytochrome P-450 forms including cytochromes P-450 1A1, 1A2, 2A1, 2B1, 2B2, 2B6, 2C6, 2C7, 2C11, 2C13, and 2E1 (Bayliss et al., 1994), whereas theO-deethylation of 7-ethoxyresorufin is catalyzed by cytochromes P-450 1A1 and 1A2 (Weaver et al., 1994). Testosterone hydroxylation is catalyzed by diverse cytochrome P-450 forms with a high degree of regio- and stereoselectivity (Waxman et al., 1983; Wood et al., 1983).
Furthermore, we have analyzed the phase II drug-metabolizing enzyme capacity of cryopreserved human, rat, and mouse PC held in suspension for up to 2 h. The parameters measured were GST activity with 1-chloro-2,4-dinitrobenzene as substrate, sulfotransferase (ST) activity with 2-naphthol as substrate, and UDP-GT activity toward 4-hydroxybiphenyl (HOBI) and 4-methylumbelliferone (MUF). HOBI is metabolized by phenobarbital-inducible UDP-GT isoenzymes, whereas MUF is a substrate for the 3-methylcholanthrene inducible UDP-GT isoforms (Bock et al., 1980, 1983).
Materials and Methods
Animals.
Male Sprague-Dawley rats (200–240 g b.wt.) and male NMRI mice (30–35 g b.wt.) were obtained from Charles River Wiga (Sulzfeld, Germany).
Chemicals.
Collagenase for the isolation of human, rat, and mouse PC was purchased from Sigma (Deisenhofen, Germany), Biochrom (Berlin, Germany), or Boehringer Ingelheim Bioproducts Partnership (Heidelberg, Germany), respectively; the kit to determine LDH activity was obtained from Boehringer Mannheim (Mannheim, Germany), the HPLC-grade methanol from E. Merck (Darmstadt, Germany), and HPLC-grade tetrahydrofuran from Roth (Karlsruhe, Germany).
Isolation of Human PC.
The use of the liver samples for the experiments described in this study was approved by the Ethics Committee of Rheinland-Pfalz, Germany. Human liver samples were obtained from patients in which a liver resection was performed due to hepatic metastases from colorectal tumors. Healthy liver tissue at least 1 cm distant from the tumor was used. The resected tissue was transferred into ice-cold transport buffer (10 mM HEPES, 142 mM NaCl, 6.7 mM KCl, 1% fungizone, 1% penicillin, and 1% streptomycin). The perfusion procedure was started within 90 min of liver resection, and the ischemia phase during surgery had a duration of maximally 30 min. Whenever possible, liver samples weighing approximately 15 to 100 g were cut off in such a way that they only presented one cut surface. Three to eight cannulae were inserted into vessels of the cut surface and the tissue was first perfused with washing buffer A (10 mM HEPES, 142 mM NaCl, 6.7 mM KCl) supplemented with 0.5 mM EGTA for 20 min and then with EGTA-free washing buffer A for another 20 min. Thereafter, the liver sample was perfused with 0.05% collagenase in collagenase buffer (67 mM NaCl, 6.7 mM KCl, 10 mM HEPES, and 4.8 mM CaCl2) for 30 min in a recirculating way. At the end of the perfusion, the tissue was put into washing buffer B [Hank's balanced salt solution (HBSS) containing 10 mM HEPES and 0.2% BSA] and liver cells were gently scraped out with a spatula. The cells were filtered through gauze and PC were obtained by centrifuging the suspension at 50g. Finally, PC were resuspended in suspension buffer (modified Krebs-Henseleit buffer containing 25 mM HEPES, 0.5% glucose, 0.2% BSA, 1 mM CaCl2, 0.4 mM MgSO4, 1 μg/ml insulin, 70 μg/ml glutamine, and the amino acid mixture recommended by Seglen (1976).
Isolation of Rat PC.
The isolation of rat PC was performed according to the method of Seglen (1976) as modified by Utesch et al. (1987) and at the end of the procedure PC were taken up in suspension buffer.
Isolation of Mouse PC.
Mouse PC were isolated by a two-step collagenase perfusion technique described by Moldéus et al. (1978) with slight modifications. Briefly, the liver was first perfused with HBSS (pH 7.4) containing 0.6 mM EGTA, 13 mM HEPES, 25 mM NaHCO3, and 0.7% BSA for 10 min at a flow rate of 8 ml/min and then with 0.02% collagenase in HBSS containing 13 mM HEPES, 25 mM NaHCO3, and 4 mM CaCl2 for 6 to 8 min at the same flow rate. At the end of the perfusion, the gallbladder was first excised and the liver was put into ice-cold HBSS supplemented with 5% BSA. Liver cells were carefully scraped out with a spatula and the cells were centrifuged at 50g for 5 min at 4°C. PC were washed twice in Leibowitz L-15 medium containing 10 mM HEPES, 6 μg/ml insulin, and 5% fetal bovine serum and finally taken up in suspension buffer.
Cryopreservation.
Human (3 × 106 cells/ml), rat (3 × 106 cells/ml), and mouse PC (2 × 106 cells/ml) in suspension buffer were centrifuged at 50g for 5 min at 4°C. Supernatants were discarded and then 0.5 ml of ice-cold suspension buffer containing 10% dimethyl sulfoxide (DMSO) was added. PC were left for 5 min on ice, resuspended, and 0.5 ml of ice-cold suspension buffer containing 16% DMSO were added; the final DMSO concentration in the cell suspensions was 10%. Aliquots of 1-ml cell suspension were transferred to 2-ml cryopreservation tubes (Nunc, Wiesbaden, Germany) and frozen down by using a computer-controlled cryopreservation procedure in a BV-8 Cryoson freezing machine (Cryoson, Schöllkrippen, Germany) as described by Diener et al. (1993). The time period between the addition of DMSO and the beginning of cryopreservation was limited to 7 min. The cryopreservation tubes were held in liquid nitrogen until needed.
PC Thawing and Removal of DMSO.
Cells held in liquid nitrogen for up to 4 weeks were thawed by gentle shaking in a water bath at 37°C. Immediately after thawing, the vials were put on ice. DMSO was gradually removed: 0.5, 1, 2, 3, and 6 ml of ice-cold suspension buffer were added to 1-ml cell suspension at 5-min intervals. Thereafter, the cells were centrifuged at 50g for 5 min at 4°C, the supernatant was discarded, and PC were resuspended in ice-cold suspension buffer.
Biochemical Assays.
Freshly isolated and thawed PC were left for 0, 30, 60, or 120 min in suspension buffer at 37°C and then homogenized by sonification. The protein content of the cell homogenates was determined as described byBradford (1969). LDH retention was quantified by determining LDH activity in the supernatant and in the cell homogenate after a 1-h incubation of 1 × 106 cells in 1-ml suspension buffer; enzyme activity was measured with the LDH kit from Boehringer Mannheim. ECOD activity was determined by a fluorometric assays according to Lake (1987). EROD activity was measured as described previously by Burke and Mayer (1974) with the modifications introduced by Lubet et al. (1985). The testosterone hydroxylation assay was performed according to Oesch et al. (1992). GST activity was determined with 1-chloro-2,4-dinitrobenzene as substrate by a spectrophotometric assay according to Habig et al. (1974). UDP-GT activity with HOBI and MUF as substrates was measured as described previously by Bock et al. (1980, 1983). ST activity with 2-naphthol as substrate was performed according to Arand et al. (1987).
Results
Human Liver PC.
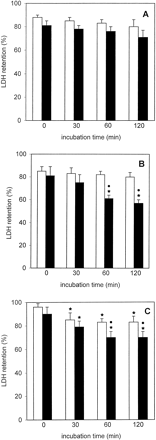
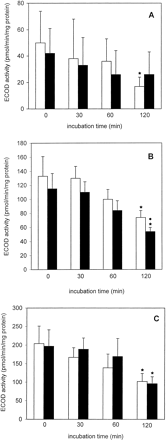
LDH retention was slightly lower in cryopreserved than in freshly isolated PC throughout the 2-h incubation period; this difference was not statistically significant (Fig. 1A). Furthermore, a slight decline in LDH retention was observed in freshly isolated as well as in cryopreserved PC, when the t = 0 and the t = 120 min values were compared (Fig. 1A). The ECOD activity of freshly isolated PC decreased to a statistically significant extent (to about 34%) at t = 120 min (Fig.2A). Furthermore, at each measured time point, ECOD activities were similar in freshly isolated and cryopreserved PC (Fig. 2A). EROD activities remained fairly constant during the 120-min incubation period in freshly isolated and cryopreserved PC and were similar at each time point in the two groups (Fig. 3A). After incubating freshly isolated and cryopreserved PC with testosterone the HPLC analysis revealed the formation of 6β- and 2β-hydroxytestosterone (OHT) (Table 1). In the case of freshly isolated PC the formation rates of 6β-OHT and 2β-OHT att = 120 min decreased to about 54 and 51%, respectively, of the corresponding t = 0 values, whereas in the case of cryopreserved PC no such decline was observed.
LDH retention in freshly isolated ■ and cryopreserved ▪ human (A), rat (B), and mouse (C) liver PC held in suspension at 37°C for up to 2 h.
LDH retention is defined as the quotient between LDH activity in the cells and total activity (i.e., activity in the cells + activity in the supernatant) × 100. Data are expressed as mean ± S.D. of four independent human and rat PC isolations and their corresponding cryopreserved counterparts. In the case of mouse PC, cells from three to four mice were pooled and the results are expressed as mean ± S.D. of four such cell pools. ∗, significantly different from the corresponding t = 0 value (p < .05, Dunnett's test). ●, significantly different from the corresponding value of freshly isolated cells at the given time point (p < .05, Dunnett's test).
Ethoxycoumarin-O-deethylase activity in freshly isolated ■ and cryopreserved ▪ human (A), rat (B), and mouse (C) liver PC held in suspension at 37°C for up to 2 h.
Data are expressed as mean ± S.D. of four independent human and rat PC isolations and their corresponding cryopreserved counterparts. In the case of mouse PC, cells from three to four mice were pooled and the results are expressed as mean ± S.D. of four such cell pools. ∗, significantly different from the correspondingt = 0 value (p < .05, Dunnett's test). ●, significantly different from the corresponding value of freshly isolated cells at the given time point (p < .05, Dunnett's test).
EROD activity in freshly isolated ■ and cryopreserved ▪ human (A), rat (B), and mouse (C) liver PC held in suspension at 37°C for up to 2 h.
Data are expressed as mean ± S.D. of four independent human and rat PC isolations and their corresponding cryopreserved counterparts. In the case of mouse PC, cells from three to four mice were pooled and the results are expressed as mean ± S.D. of four such cell pools.
Formation of hydroxylated testosterone metabolites in freshly isolated and cryopreserved human liver PC held in suspension at 37°C for up to 2 h
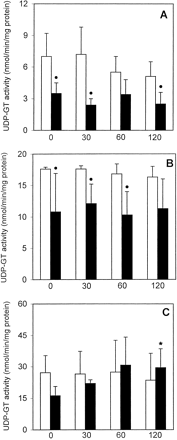
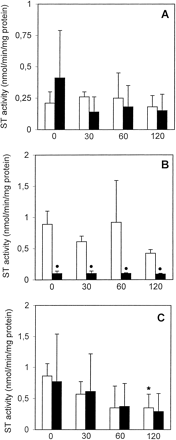
GST activity in freshly isolated PC remained stable during the 120-min incubation period, whereas cryopreserved PC showed a statistically significant decrease in GST activity of about 70 to 80% when compared with freshly isolated PC at each given time point (Fig.4A). UDP-GT activity toward MUF in freshly isolated PC was not significantly altered for up to 2 h, whereas it decreased to a statistically significant degree in cryopreserved PC (39–66% decrease when compared with freshly isolated PC; Fig. 5A). In contrast, UDP-GT activity with HOBI as substrate decreased by about 46% after 2 h in freshly isolated PC (when compared with the t = 0 value); interestingly, this activity remained constant in cryopreserved PC and was similar to that in freshly isolated PC throughout the 2-h incubation period (Fig. 6A). ST activity was similar in freshly isolated as well as cryopreserved PC and did not decrease with time (Fig. 7A).
GST activity in freshly isolated ■ and cryopreserved ▪ human (A), rat (B), and mouse (C) liver PC held in suspension at 37°C for up to 2 h.
Data are expressed as mean ± S.D. of four independent human and rat PC isolations and their corresponding cryopreserved counterparts. In the case of mouse PC, cells from three to four mice were pooled and the results are expressed as mean ± S.D. of four such cell pools. ●, significantly different from the corresponding value of freshly isolated cells at the given time point (p < .05, Dunnett's test).
UDP-GT activity toward MUF in freshly isolated ■ and cryopreserved ▪ human (A), rat (B), and mouse (C) liver PC held in suspension at 37°C for up to 2 h.
Data are expressed as mean ± S.D. of four independent human and rat PC isolations and their corresponding cryopreserved counterparts. In the case of mouse PC, cells from three to four mice were pooled and the results are expressed as mean ± S.D. of four such cell pools. ∗, significantly different from the correspondingt = 0 value (p < .05, Dunnett's test). ●, significantly different from the corresponding value of freshly isolated cells at the given time point (p < .05, Dunnett's test).
UDP-GT activity toward HOBI in freshly isolated ■ and cryopreserved ▪ human (A), rat (B), and mouse (C) liver PC held in suspension at 37°C for up to 2 h.
Data are expressed as mean ± S.D. of four independent human and rat PC isolations and their corresponding cryopreserved counterparts. In the case of mouse PC, cells from three to four mice were pooled and the results are expressed as mean ± S.D. of four such cell pools. ∗, significantly different from the correspondingt = 0 value (p < .05, Dunnett's test).
ST activity in freshly isolated ■ and cryopreserved ▪ human (A), rat (B), and mouse (C) liver PC held in suspension at 37°C for up to 2 h.
Data are expressed as mean ± S.D. of four independent human and rat PC isolations and their corresponding cryopreserved counterparts. In the case of mouse PC,cells from three to four mice were pooled and the results are expressed as mean ± S.D. of four such cell pools. ∗, significantly different from the correspondingt = 0 value (p < .05, Dunnett's test). ●, significantly different from the corresponding value of freshly isolated cells at the given time point (p < .05, Dunnett's test).
Rat Liver PC.
LDH retention in freshly isolated PC incubated up to 2 h at 37°C remained unchanged, whereas this parameter was significantly reduced in cryopreserved PC at t = 60 or 120 min when compared with cryopreserved PC at t = 0 and to freshly isolated PC at t = 60 and 120 min (Fig. 1B). ECOD activities in freshly isolated and cryopreserved PC declined time dependently to about 55 and 47%, respectively, of the t = 0 values and the activity was significantly lower in cryopreserved than in freshly isolated PC (Fig. 2B). In contrast, EROD activities were similar in the two groups and did not decrease during the 2-h incubation period (Fig. 3B). The main hydroxylated testosterone metabolites formed in freshly isolated and cryopreserved PC were 16α-, 2α-, and 6β-OHT, whereas 2β-, 7α-, and 15β-OHT were present in minor amounts (Table 2). In freshly isolated PC, 6β- and 2β-OHT formation rates significantly decreased with time whereas those of the other four metabolites mentioned above remained fairly constant during the 2-h incubation period (Table 2). In cryopreserved PC 6β-, 2β-, 16α-, and 2α-OHT formation rates decreased with time, although, as in the case of freshly isolated PC, only 6β- and 2β-OHT formation rates were reduced to statistically significant levels (Table 2).
Formation of hydroxylated testosterone metabolites in freshly isolated and cryopreserved rat liver PC held in suspension at 37°C for up to 2 h
GST activity in freshly isolated PC remained stable for up to 2 h, whereas that of cryopreserved PC decreased by about 30 to 54% when compared with freshly isolated PC (Fig. 4B). UDP-GT activity toward MUF was not altered in freshly isolated PC for up to 120 min, whereas in cryopreserved PC it decreased to about 61 to 69% of the values measured in freshly isolated PC (Fig. 5B). UDP-GT activity toward HOBI was similar and remained stable for up to 2 h in freshly isolated as well as cryopreserved PC (Fig. 6B). In freshly isolated PC, ST activity after 120 min decreased to about 53% of the value att = 0, whereas in cryopreserved PC it was already very low immediately after thawing, and remained that low throughout the 2-h incubation period (Fig. 7B).
Mouse Liver PC.
After 120 min LDH retention decreased to about 77 and 86% of thet = 0 values in freshly isolated and cryopreserved PC, respectively (Fig. 1C). Interestingly, the LDH retention rates were consistently higher in the cryopreserved than in the freshly isolated PC throughout the 2-h incubation period. ECOD activities were similar in freshly isolated and cryopreserved PC at the four time-points measured and in both cases ECOD activities at t = 120 min were reduced to about 50% of the corresponding t = 0 values (Fig. 2C). In contrast, EROD activities were similar and did not significantly decrease in freshly isolated and cryopreserved PC (Fig. 3C). Incubation of freshly isolated and cryopreserved PC with testosterone led to the formation of 6β-, 15α-, 6α-, 16α-, 7α-, 2α-, and 2β-OHT, 6β-OHT being the major metabolite in both experimental groups (Table 3). The formation rates of the seven testosterone metabolites mentioned above at each time point were similar in freshly isolated and cryopreserved PC and did not decrease to a statistically significant level during the 2-h incubation period (Table 3).
Formation of hydroxylated testosterone metabolites in freshly isolated and cryopreserved mouse liver PC held in suspension at 37°C for up to 2 h
GST activity remained stable in freshly isolated PC for up to 2 h, whereas in cryopreserved PC it was reduced, albeit not to a statistically significant extent, at all four time points (Fig. 4C). UDP-GT activity toward MUF did not significantly decrease with time in freshly isolated PC, whereas in cryopreserved PC this activity was reduced immediately after thawing, and recovered within the 2-h incubation period (Fig. 5C). In contrast, UDP-GT activity toward HOBI remained fairly stable in freshly isolated as well as cryopreserved PC for up to 2 h (Fig. 6C). A time-dependent decrease in ST activity was observed in both experimental groups (Fig. 7C).
Discussion
The aim of the present study was to analyze the drug-metabolizing capacity of cryopreserved human, rat, and mouse liver PC held in suspension at 37°C for up to 2 h. A prerequisite to accomplish this task was the establishment of isolation procedures that made routinely available fresh PC fractions with a high viability index (i.e., LDH retention rate >80%) from all three species. In this context it was consistently observed that those human liver samples weighing less than 100 g, showing only one cut surface, and being perfused simultaneously with three to eight cannulae yielded the highest number of PC with the expected viability. By using liver samples with the above-mentioned characteristics it could be demonstrated that the viability of freshly isolated human PC fluctuated between 80 and 90% throughout the 2-h incubation period. In the case of cryopreserved human PC the viability ranged between 70 and 80% and was slightly lower than that of freshly isolated human PC at each time point analyzed, thus showing that the cryopreservation procedure only led to a minimal additional damage of PC. Furthermore, the viability of the cryopreserved human PC was consistently higher than that obtained in previous studies by Loretz et al. (1989) and by our own group (Diener et al., 1994). This is most probably due to the optimized PC isolation procedure, because the cryopreservation protocol used herein was identical with that of Diener et al. (1994).
ECOD and EROD activities were similar in freshly isolated and cryopreserved human PC at all four time points studied and within the same range of values reported previously by Loretz et al. (1989) for human PC immediately after thawing and by Kern et al. (1997) for human PC cultured between collagen gel layers. Furthermore, as in the case ofLoretz et al. (1989) and Kern et al. (1997), ECOD activity was shown to decrease with time whereas EROD activity remained constant for up to 2 h. In humans, 7-ethoxycoumarin is primarily deethylated by cytochromes P-450 1A2, 2B6, and 2E1, whereas 7-ethoxyresorufin is mainly deethylated by cytochrome P-450 1A2. The results obtained in this study suggest that the individual cytochromes P-450 are not equally stable when held in suspension and that at least cytochrome P-450 1A2 is very well conserved. The testosterone hydroxylation analysis revealed that 6β-OHT and 2β-OHT were formed at similar rates in freshly isolated and cryopreserved PC during the 2-h incubation period, and the formation rates were within the range of those reported previously by Kern et al. (1997) for human PC sandwich cultures and by Mäenpää et al. (1991) and Kimonen et al. (1995) for human liver microsomes. Because formation of 6β-OHT and 2β-OHT is catalyzed by cytochrome P-450 3A4, these results show that, as in the case of cytochrome P-450 1A2, cryopreservation does not affect cytochrome P-450 3A4 expression.
As in the case of human PC, EROD activity in freshly isolated and cryopreserved rat and mouse PC remained fairly stable during the 2-h incubation period, whereas ECOD activity in freshly isolated and cryopreserved rat and mouse PC decreased to about one-half of the activity measured at t = 0. In earlier studies byNovicki et al. (1982) and Jackson et al. (1985) it was consistently reported that a faster decline in cytochrome P-450-dependent activities was observed in cryopreserved than in freshly isolated rat PC. The fact that in the present study no such faster decline was observed demonstrates that a clear improvement in the cryopreservation and thawing steps has been achieved with the experimental system presented in this report. The three main hydroxylated metabolites formed in freshly isolated and cryopreserved rat PC were 16α-, 2α-, and 6β-OHT, whereas 2β-, 7α-, and 15β-OHT represented quantitatively minor metabolites. Because 16α- and 2α-hydroxylation of testosterone are catalyzed by cytochrome P-450 2C11, the data presented herein show that cytochrome P-450 2C11 is a main cytochrome P-450 in male rat liver, and that it is not affected by cryopreservation. In mouse PC 6β-, 15α-, 6α-, 16α-, 7α-, 2α-, and 2β-OHT were detected and their formation rates remained constant throughout the 2-h incubation period. No 16β-OHT could be detected in freshly isolated and cryopreserved rat and mouse liver PC, which is in accordance with the fact that cytochrome P-450 2B1 is absent or only present in extremely low amounts in uninduced rat liver PC (Steinberg et al., 1987).
The activity of GST and that of UDP-GT toward MUF were significantly reduced after cryopreservation of human PC, whereas the activity of UDP-GT toward HOBI and that of ST were similar to those measured in freshly isolated PC. At first glance one could argue that the decreases in GST and UDP-GT activities observed in cryopreserved PC are an expression of liver cell damage induced by the cryopreservation procedure. However, in the present report we have shown that: 1) LDH retention rates in freshly isolated human PC during the 120-min incubation period were within the range of 80 to 90%; and 2) in cryopreserved human PC, LDH retention rates remained stable and were similar to those measured in freshly isolated PC for up to 2 h. Hence, the decrease in GST and UDP-GT activity cannot be ascribed to an extensive damage of PC, leading to cell death, elicited by the cryopreservation step.
One possible explanation for the decrease in GST and UDP-GT activities could be that, although the cells have not died off, the plasma membrane might have been rendered leaky to one or the other cofactor needed by the above-mentioned enzymes. In fact, in a recent study,Swales and Utesch (1998) have shown that glucuronidation and sulfation in cryopreserved dog PC are strongly reduced when compared with freshly isolated dog PC and that these activities recover when the cell homogenates are supplemented with UDP-glucuronic acid or with 3′-phosphoadenosine 5′-phosphosulphate. Because in our case we are interested in using whole human PC and because cofactors such as glutathione or UDP-glucuronic acid are not able to get into intact PC, one can foresee that supplementation of the medium with glutathione or UDP-glucuronic acid will be of no help. The recovery, viability, and metabolic capacity of PC after cryopreservation depends to a great extent on the quality of the cells to be frozen. Therefore, at the present time we are currently testing whether centrifugation of PC in a Percoll gradient before cryopreservation might help to isolate a PC fraction particularly resistant to the freezing procedure. Preliminary results obtained with dog PC by Swales and Utesch (1998) suggest that this is a promising approach.
Although leakage of enzyme cofactors out of cryopreserved human PC might in part explain the decrease in the activities of GST and UDP-GT (toward MUF), the fact that UDP-GT activity toward HOBI remained unchanged when compared with the corresponding activity in freshly isolated PC shows that protein denaturation within PC may also contribute to the loss of enzyme activity. When measuring GST and UDP-GT activities in the homogenized cells exogenous cofactors are added in saturating (i.e., nonlimiting) concentrations. Thus, under these experimental conditions a decrease in the enzyme activity is not related to a cofactor decrease, but indicates that particular enzymes are being inactivated.
The activities of GST, UDP-GT (toward MUF), and ST in cryopreserved rat PC immediately after thawing were about 30, 40, and 90% lower, respectively, than those in freshly isolated PC at t = 0. However, the three above-mentioned activities in the cryopreserved PC did not decrease to a significant extent between t = 0 and t = 120 min. Thus, the phase II enzymes of rat PC were affected in a similar way to those of human PC. In contrast, the phase II enzyme activities in cryopreserved mouse PC proved to be far more stable, being at all four time points measured similar to the activities of freshly isolated mouse PC with the exception of GST, which showed a decay from t = 60 min onwards.
In conclusion, the results presented in this study show that cryopreserved PC from human, rat, and mouse liver held in suspension for up to 2 h maintain their phase I drug-metabolizing enzyme capacity at a relatively constant level, and that in the particular case of ECOD activity, which declines with time in all three cases, this decline occurs to the same extent in freshly isolated and in cryopreserved PC. Regarding phase I drug-metabolizing enzyme capacity, suspensions of cryopreserved PC might prove to be a valid experimental alternative in those cases in which livers are not readily available (humans) or in which the number of animals sacrificed should be drastically reduced (rats, mice). However, GST, UDP-GT (with MUF as substrate), and ST in human and rat PC, in contrast to mouse PC, are affected by cryopreservation. Thus the cryopreservation procedure will have to be further optimized before validation.
Footnotes
-
Send reprint requests to: Dr. Pablo Steinberg, Institut für Ernährungswissenschaft, Universität Potsdam, Arthur Scheunert-Allee 114–116, 14558 Bergholz-Rehbruecke. Germany. E-mail: steinber{at}rz.uni-potsdam.de
-
↵1 Current address: Institut für Toxikologie, Universität Mainz, Obere Zahlbacher Str. 67, 55131 Mainz.
-
↵2 Current address: Lehrstuhl für Ernährungstoxikologie, Institut für Ernährungswissenschaft, Universität Potsdam, Arthur Scheunert-Alle 114–116, 14558 Bergholz-Rehbrücke.
-
↵3 Current address: Klinik und Poliklinik für Allgemein- und Abdominalchirurgie, Universität Mainz, Langenbeckstr. 1, 55131 Mainz.
-
↵4 Current address: Chirurgische Abteilung, Stadtkrankenhaus Rüsselsheim, August Bebel Str. 59, 65428 Rüsselsheim, Germany.
-
This study was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Germany (Grant 0311258).
- Abbreviations used are::
- PC
- parenchymal cells
- ECOD
- 7-ethoxycoumarin-O-deethylase
- EROD
- 7-ethoxyresorufin-O-deethylase
- GST
- glutathioneS-transferase
- UDP-GT
- UDP-glucuronosyltransferase
- MUF
- 4-methylumbelliferone
- HOBI
- 4-hydroxybiphenyl
- ST
- sulfotransferase
- LDH
- lactate dehydrogenase
- HBSS
- Hank's balanced salt solution
- DMSO
- dimethyl sulfoxide
- OHT
- hydroxytestosterone
- Received May 10, 1999.
- Accepted September 1, 1999.
- The American Society for Pharmacology and Experimental Therapeutics