Abstract
Phenyldiazene reacted with lymphoblast-expressed CYP3A4 to give a stable phenyl-iron complex that could be induced to rearrange in situ producing approximately equal amounts of fourN-phenyl-protoporphyrin IX isomers (NB:NA:NC:ND, 01:01:02:02). In the presence of 10 mM MgCl2, the formation profile of the protoporphyrin isomers was markedly altered compared with control, favoring the NA isomer (NB:NA:NC:ND, 01:34:01:02). In addition, an investigation of MgCl2effects on CYP3A4-mediated metabolism of triazolam revealed that 10 mM MgCl2 increased the apparent Kmof triazolam 4-hydroxylation from 83 to 173 μM and reduced theVmax for the reaction from 3.4 to 2.4 min−1. Moreover, when the reaction kinetics of the oxidation of pyrene by CYP3A4 was examined in the absence of MgCl2, it was found that the substrate-velocity curve was best approximated by a sigmoidal velocity curve (Hill coefficient 1.7 ± 0.1). However, when the reaction was conducted in the presence of 10 mM MgCl2, the resulting pyrene kinetics was not sigmoidal but rather biphasic (Hill coefficient 0.80 ± 0.07). Based on the current results, it appears that CYP3A4 is conformationally sensitive to its in vitro environment and parameters, such as the presence of a divalent magnesium, can have a measurable effect on active site topography and consequently catalytic activity.
Human cytochrome P-450 3A4 (CYP3A4) is the most abundant isoform of the P-450 superfamily and is responsible for the metabolism of a significant number of drugs (Guengerich, 1995). Previously it has been demonstrated that the catalytic activity of CYP3A4 can be altered by varying incubation conditions, including buffer composition, ionic strength, and the source of reducing equivalents (Mäenpää et al., 1998). Furthermore, incubation variables such as the addition of divalent cations (e.g., Mg2+), glutathione, cytochromeb5, and lipid composition have also been shown to influence the catalytic activity of CYP3A4 (Guengerich et al., 1986; Imaoka et al., 1992; Gillam et al., 1993; Yamazaki et al., 1995). Collectively, it appears that in vitro conditions contribute to interlaboratory variability and ultimately may confound attempts to extrapolate in vitro data to the in vivo condition.
The purpose of the current study was to examine in detail the effect of one in vitro incubation parameter, Mg2+concentration, with respect to CYP3A4 active site topology and catalytic activity. This was accomplished through the use of phenyldiazene as a probe of active site conformation. Phenyldiazene is known to form a stable phenyl-iron complex and, on potassium ferricyanide treatment, the phenyl group may migrate to one of the four available pyrrole nitrogens on the porphyrin. The relative proportion of each phenylporphyrin isomer formed has been proposed to reflect the protein active site topology above heme (Raag et al., 1990). The presence of Mg2+ was found to significantly alter phenyl migration and the relative amounts of each porphyrin isomer formed, thus indicating that a protein conformational change had occurred. In addition, the effects of conformational change on catalysis were examined with two CYP3A4 substrates, triazolam and pyrene, in the presence and absence of Mg2+.
Materials and Methods
Reagents.
Phenyldiazene carboxylate azo ester was purchased from Research Organics, Inc. (Cleveland, OH). Pyrene, magnesium chloride, and 1-hydroxypyrene were purchased from Aldrich Chemical Company (Milwaukee, WI). Triazolam, 1′-hydroxytriazolam, and 4-hydroxytriazolam were obtained from Pharmacia & Upjohn (Kalamazoo, MI). Equine myoglobin was obtained from Sigma (St. Louis, MO). Human lymphoblast-expressed CYP3A4 was purchased from Gentest Corp. (Woburn, MA).
Formation of Iron-Phenyl Complex.
The formation of an iron-phenyl complex with lymphoblast-expressed CYP3A4 was achieved through a procedure similar to that of Mackman et al. (1996a,b). Briefly, 2 μl of neat phenyldiazene carboxylate azo ester was dissolved in 500 μl of 1 N NaOH and allowed to sit on ice for 15 min. A 1-μl aliquot of the phenyldiazene stock solution was introduced into 1 ml of buffer (100 mM potassium phosphate, pH = 7.4) containing 200 pmol of expressed lymphoblast CYP3A4. The mixture was allowed to sit for 15 min, after which the formation of the iron-phenyl complex was observed spectroscopically by noting the formation of a new chromophore at 478 nm with a simultaneous loss of absorbance at 416 nm (relative to a time 0 control). Due to the turbidity of the reaction mixture, the sample was made soluble before measurement with an equal volume of buffer containing 20% glycerol, 0.5% cholate, 0.4% Emulgen 911, and 0.1 mM EDTA in 50 mM phosphate (pH = 7.4; Guengerich, 1989).
Induction of Phenyl Migration.
A control sample was generated by addition of three 2-μl aliquots of potassium ferricyanide (100 mM in water) to the reaction mixture, which was then allowed to sit for 20 min between each addition. Experimental samples followed the same protocol except that MgCl2 (final concentration 10 mM) was added (subsequent to iron-phenyl complex formation) and allowed to sit for 15 min, before the addition of potassium ferricyanide aliquots. After the potassium ferricyanide-induced shift, 200 μl of 38 M urea was added to each sample for a final concentration of 8 M urea, followed by vortexing. Next, 50 μl of concentrated acetic acid was added followed by additional vortexing. The porphyrin was extracted into ether by three separate extractions (1 ml). The organic layer was collected and dried down to a white residue under nitrogen. Each sample was then redissolved in 50 μl of acetonitrile and 50 μl of water. The samples were then placed at 4°C for 30 min, and the resulting white particulate was pelleted by centrifugation in a 1.5-ml Eppendorf tube. The supernatant was removed and transferred to 0.5-ml autoinjection vials for HPLC-electrospray ion-trap mass spectrometry analysis.
HPLC-Electrospray Ion-Trap Mass Spectrometry Analysis.
Separation was achieved on a YMC MCB-01-5 column (2.1 × 150 mm; YMC, Inc., Wilmington, NC), and solvent was delivered at a flow rate of 0.3 ml/min by a Perkin-Elmer series 200 LC pump (Norwalk, CT). The N-phenylporphyrins were eluted using a gradient of 60% water/40% acetonitrile containing 0.1% trifluoroacetic acid to 100% acetonitrile containing 0.1% trifluoroacetic acid over 15 min. Porphyrin elution was monitored by electrospray ion-trap mass spectrometry (Finnigan LCQ, San Jose, CA) in single ion monitoring mode (639 Da). The identity of each porphyrin regioisomer was confirmed by a comparison of retention times of known standards. The standards were generated from equine myoglobin as previously described (Swanson and Ortiz de Montellano, 1991).
Enzymatic Assay.
Kinetic experiments were done in duplicate under conditions where less than 10% substrate depletion occurred. Velocity determinations with expressed enzyme contained 15 pmol (triazolam) or 2.5 pmol (pyrene) of CYP3A4, 100 mM potassium phosphate buffer (pH = 7.4), 1 mM EDTA, 1 mM NADPH, and varying concentrations of substrate in a 0.5-ml incubation volume. Substrate was introduced to the incubation in less than 5 μl (1% total volume) of acetonitrile. Reactions were preincubated at 37°C for 5 min, and catalysis was initiated by the introduction of NADPH. The incubations were terminated by the addition of 100 μl of acetonitrile with a total incubation time of 10 min for triazolam and 5 min for pyrene. The contents of each incubation tube were directly transferred to a 1.5-ml microfuge tube, capped, and centrifuged at 14,000g for 10 min. The resulting supernatant was removed and transferred to 1.5-ml autoinjection vials for HPLC analysis.
HPLC Analysis.
HPLC chromatography was achieved with a PE410 pump (Perkin-Elmer), PE ISS-200 autosampler (Perkin-Elmer), a Waters 474 scanning fluorescence detector (Milford, MA), and a Spectrafocus UV detector (Spectra-Physics, Mountain View, CA).
Triazolam.
Triazolam metabolites were eluted isocratically on a 5-μm C18 column, 4.6 × 250 mm (Zorbax, Chides Ford, PA), at 2.0 ml/min with a total run time of 15 min. The mobile phase consisted of 50 mM phosphate buffer (pH = 8.0), methanol, and acetonitrile in a 50:45:5 mixture, respectively. Metabolites were monitored by absorbance at 220 nm and quantified with standard curves generated from authentic metabolite standards.
Pyrene.
1-Hydroxypyrene separation was achieved from a gradient with a mobile phase consisting of water (solvent A) and 75% methanol/25% acetonitrile (solvent B). The column used was a 5-μm C18 column, 4.6 × 150 mm (Zorbax), that was first equilibrated with 30% A and 70% B at 2.0 ml/min for 5 min. Subsequent to equilibration, the mobile phase was linearly increased to 95% B over a period of 5 min. The formation of 1-hydroxypyrene was quantified by fluorescence spectrophotometry (excitation = 340 nm, emission = 390 nm), and resulting peak areas were compared with a standard curve.
Mathematical Analysis.
Data were plotted and fit by nonlinear regression using the Prism program by GraphPad (San Diego, CA). Triazolam substrate-velocity data were fit using the standard Michaelis-Menten model:
Results
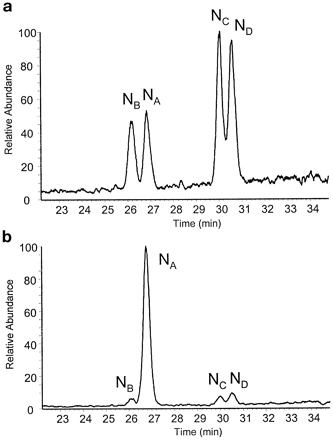
In accordance with previous human P-450 work, reaction of phenyldiazene with CYP3A4 yielded an UV spectrum that was characteristic of a stable phenyl-iron complex (absorption at 476–480 nm, data not shown). Subsequent addition of potassium ferricyanide induced migration of the phenyl moiety from the heme-bound iron to one of the four available pyrrole nitrogens on the surrounding porphyrin ring (Mackman et al., 1996a,b; Dierks et al., 1998). The relative ratios for phenyl migration to each pyrrole ring in the CYP3A4 heme are depicted in Fig. 1. In the absence of Mg2+, Fig. 1a indicates that similar amounts of all the isomers were formed (NB:NA:NC:ND, 01:01:02:02). In contrast, when the reaction was conducted in the presence of 10 mM MgCl2, migration was altered such that adduction to CYP3A4 heme pyrrole ring A was dramatically favored (NB:NA:NC:ND, 01:34:01:02) as seen in Fig. 1b.
Liquid chromatography-mass spectrometry profile depicting the relative amounts of each N-phenyl-protoporphyrin IX isomer formed in the absence of MgCl2 (a) and the presence of 10 mM MgCl2(b).
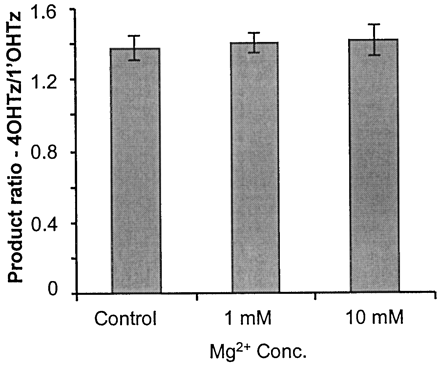
In light of the topographical changes induced by Mg2+ on the CYP3A4 active site, studies were conducted to examine the effect of Mg2+ on CYP3A4 catalytic function using the metabolism of triazolam and pyrene as reporter substrates for CYP3A4 catalytic activity. Triazolam is metabolized in both the C-1′ (1′OHTz) and C-4 (4OHTz) positions by CYP3A4. However, without the coexpression of cytochromeb5, the CYP3A4 lymphoblast system did not produce measurable amounts of 1′OHTz metabolite at lower substrate concentrations (compared with the baculovirus-expressed system supplemented with cytochrome b5, data not shown). Therefore, kinetic parameters were only determined for 4OHTz formation. In the absence of magnesium, it was found that the apparentKm for 4OHTz formation was 83 μM with aVmax of 3.4 ± 0.39 min−1. However, when 10 mM MgCl2 was present in the incubations, theKm was increased to 173 μM and theVmax decreased to 2.4 ± 0.30 min−1. This represented a 3-fold reduction in the enzymatic intrinsic clearance (Vmax/Km). Interestingly, at higher substrate concentrations, it was possible to monitor 1′OHTz formation, and in Fig. 2both 1 and 10 mM MgCl2 had no effect on the product ratio of 4OHTz/1′OHTz at 120 μM triazolam.
Relative product ratio of 4OHTz/1′OHTz (120 μM triazolam) with increasing MgCl2 concentration on the x-axis.
Values were determined from assays done in triplicate.
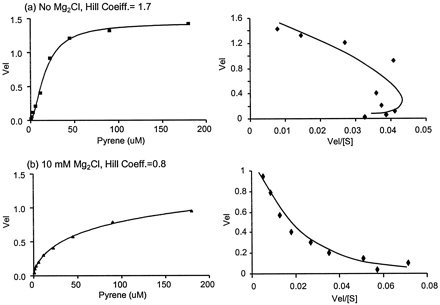
Pyrene incubations conducted in the presence of CYP3A4 resulted in the formation of a single metabolite (data not shown). Based on identical HPLC retention compared with an authentic standard and characterization by mass spectrometry and 1H NMR, the identity of this metabolite was confirmed as 1-hydroxypyrene. The substrate-velocity curve for pyrene oxidation was found to be sigmoidal in appearance and was fit by the Hill equation (coefficient = 1.7 ± 0.1) rather than the standard Michaelis-Menten equation, which describes hyperbolic kinetics (Fig.3a). It is important to note that in the current context the Hill equation was used as a descriptor of the shape of the substrate-velocity curve and no mechanistic meaning was implied by its use. A replot of the data in Eadie-Hofstee format is also presented in Fig. 3, and from the plot there is apparent nonlinearity at low pyrene concentrations. However, when 10 mM MgCl2 was present in the incubations, the substrate-velocity curve was markedly altered at low pyrene concentrations such that the replot is curved in the opposite direction compared with control (Fig. 3b). The altered substrate-velocity curve was biphasic and resulted in a lower coefficient when fit to the Hill equation (coefficient = 0.80 ± 0.07).
Substrate-velocity curves and corresponding Eadie-Hofstee plots of pyrene metabolism.
The incubations were under identical conditions except for: a, no MgCl2; and b, 10 mM MgCl2. Velocity is in units of minutes−1 and concentration of [S] is expressed in micromolar.
Discussion
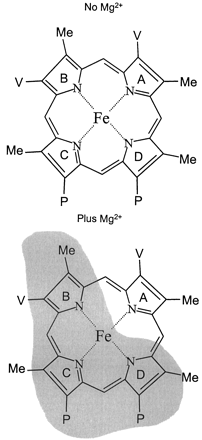
Phenyldiazene active site topological analysis of human P-450 enzyme has been previously reported (Mackman et al., 1996a,b;Dierks et al., 1998). Due to the potential effects of membrane composition on CYP3A4 activity (Imaoka et al., 1992), a human lymphoblast expression system was chosen over an insect expression system to more closely simulate mammalian membrane composition. Within this system, it was found that the formation of phenyl-phorphyrin isomers was dramatically altered in the presence of MgCl2 (Fig. 1a). The data presented in Fig. 1, panel a, suggest that in the absence of MgCl2 the CYP3A4 active site immediately surrounding the heme was relatively open, allowing near equal phenyl migration to any of the four pyrrole rings. However, when MgCl2 was present (panel b), there was an apparent restriction in the space above the heme that blocked free migration to pyrrole rings B, C, and D in Fig.4. The altered phenyl migration suggests that magnesium was either bound in the CYP3A4 active site or had conferred a conformational change to the enzyme.
Heme pyrrole rings are labeled, and the shaded region indicates the proposed space above the heme that is masked in the presence of 10 mM MgCl2.
Based on evidence in the literature, there is no precedent to suggest that magnesium may bind within the CYP3A4 active site. Moreover, Tamura et al. (1990) reported that divalent cations affected protein-protein interactions and/or the reduction rate of soluble cytochromeb5. Furthermore, Yamazaki et al. (1995), in a detailed study, concluded that MgCl2 stimulated electron transfer from NADPH-P-450 reductase to cytochromeb5 and CYP3A4 in both microsomes and reconstituted systems. Consistent with the notion that magnesium modulates P-450 catalysis through protein-protein interactions and/or enhanced rates of electron transfer, the results of the current study suggest that MgCl2-induced alteration of the phenyl migration pattern was a result of a CYP3A4 conformational change in the space directly above the heme as depicted in Fig. 4.
Further characterization of MgCl2 effects on CYP3A4 catalytic activity was studied through the metabolism of triazolam. Triazolam incubations conducted in the presence of magnesium ion resulted in an increase in the apparentKm for triazolam 4-hydroxylation (83 → 173 μM), along with a decrease in theVmax (3.4 → 2.4 min−1). Although there was a 3-fold change in the intrinsic clearance (Vmax/Km), the ratio of 4OHTz to 1′OHTz formation at elevated triazolam concentration (120 μM) was not effected (Fig. 2). Previously, Michaelis-Menten arguments have been proposed that indicate that metabolite ratios are enzyme-specific (Porter et al., 1976). One early study concluded that metabolite ratios for cytochrome P-450s are regioselectively controlled by a combination of protein-substrate contacts and substrate chemical reactivity (White et al., 1984). Therefore, because Mg2+ appears to only perturb enzymatic function and not triazolam metabolite ratios, a gross morphological change of the active site was not implied. These data combined with the altered phenyl migration data suggest that the observed protein conformational change induced by Mg2+ must involve structural elements immediately surrounding the heme and that these elements are not critical determinants in triazolam oxidation. It was unfortunate that due to the insensitivity of the triazolam assay (in the absence of cytochrome b5) product ratio effects at lower substrate concentrations could not be experimentally determined.
An alternative CYP3A4 substrate, pyrene, offered a sensitive assay that allowed confident investigation of the lower portion (low substrate concentration) of the substrate velocity curve, where atypical kinetics is most evident. In the absence of MgCl2, pyrene kinetics was characterized by a sigmoidal substrate-velocity curve (Fig. 3a). However, when 10 mM MgCl2 was present, the resulting Eadie-Hofstee plot was curved in a manner indicative of a biphasic substrate-velocity curve (Fig. 3b), which may arise by a variety of mechanisms. One possibility is that magnesium influences CYP3A4 conformation such that two or more distinct forms of the enzyme are present. A kinetic model that assumes two separate protein conformations can yield biphasic or sigmoidal substrate-velocity curves and thus provides a reasonable model for the observed changes in Fig. 3(Segal, 1975). Alternatively, a mechanism based on multiple substrate binding has been hypothesized to account for the atypical substrate-velocity curves generated in P-450 metabolism (Korzekwa et al., 1998). In agreement with the conformational model, this mechanism may also produce curves that are biphasic or sigmoidal depending on the relative magnitudes of the kinetic constants within the model.
In conclusion, the catalytic activity of CYP3A4 can be modulated by a number of factors that may activate and/or inhibit catalysis under certain conditions. This report investigates one factor, MgCl2, and finds evidence that its modulation of CYP3A4 catalytic activity involves a conformational change of the enzyme. Such a result is concordant with previous studies (Koley et al., 1995), which suggest that CYP3A4 tertiary structure is flexible, existing in multiple conformations that can also be affected by the binding of substrate. In light of the current findings, it is important to consider the levels of Mg2+ used in in vitro experiments. Examples from the drug metabolism literature indicate that in vitro P-450 incubation assays may be devoid of magnesium or may utilize concentrations up to 10 mM MgCl2, or greater. In addition, in vivo estimates of hepatic cytosolic levels of free Mg2+ are reported to be about 0.5 mM, and the total cell content is approximately 25 mM (Corkey et al., 1986). Therefore, depending on the protein content used in a particular assay, Mg2+within the range of 0 to 10 mM may or may not reflect in vivo concentrations (e.g., Mg2+ is highly protein bound). Thus, Mg2+ levels should be carefully considered when extrapolating CYP3A4 in vitro data to the in vivo condition and when comparing kinetic results obtained in separate laboratories.
Footnotes
-
Send reprint requests to: Larry C. Wienkers, Pharmacia Corporation, 301 Henrietta St., Kalamazoo, MI 49007. E-mail:Larry.C.Wienkers{at}am.pnu.com
- Abbreviations used are::
- CYP or P-450
- cytochrome P-450
- 4OHTz
- 4-hydroxytriazolam
- 1′OHTz
- 1′-hydroxytriazolam
- Received April 24, 2000.
- Accepted June 19, 2000.
- The American Society for Pharmacology and Experimental Therapeutics