Abstract
Most human hepatocyte cell lines lack a substantial set of liver-specific functions, especially major cytochrome P450 (P450)-related enzyme activities, making them unrepresentative of in vivo hepatocytes. We have used the HepaRG cells, derived from a human hepatocellular carcinoma, which exhibit a high differentiation pattern after 2 weeks at confluency to determine whether they could mimic human hepatocytes for drug metabolism and toxicity studies. We show that when passaged at low density, these cells reversed to an undifferentiated morphology, actively divided, and, after having reached confluency, formed typical hepatocyte-like colonies surrounded by biliary epithelial-like cells. By contrast, when seeded at high density, hepatocyte-like clusters retained their typical differentiated morphology. Transcripts of various nuclear receptors (aryl hydrocarbon receptor, pregnane X receptor, constitutive androstane receptor, peroxisome proliferator-activated receptor α), P450s (CYP1A2, 2C9, 2D6, 2E1, 3A4), phase 2 enzymes (UGT1A1, GSTA1, GSTA4, GSTM1), and other liver-specific functions were estimated by reverse transcriptase-quantitative polymerase chain reaction and were found to be expressed, for most of them, at comparable levels in both confluent differentiated and high-density differentiated HepaRG cells and in cultured primary human hepatocytes. For several transcripts, the levels were strongly increased in the presence of 2% dimethyl sulfoxide. Measurement of basal activities of several P450s and their response to prototypical inducers as well as analysis of metabolic profiles and cytotoxicity of several compounds confirmed the functional resemblance of HepaRG cells to primary cultured human hepatocytes. In conclusion, HepaRG cells constitute the first human hepatoma cell line expressing high levels of the major P450s involved in xenobiotic metabolism and represent a reliable surrogate to human hepatocytes for drug metabolism and toxicity studies.
Human primary hepatocytes and immortalized hepatocytes are widely used for xenobiotic metabolism, toxicity studies, and the design of bioartificial liver devices; however, both systems have limitations (Guillouzo et al., 1993; Maurel, 1996; Guillouzo, 1998; LeCluyse, 2001). Primary hepatocytes have scarce and unpredictable availability, limited growth activity and lifespan, and undergo early phenotypic alterations. Huge variations in functional activities, especially P450 levels, as well as in magnitude of P450 induction after treatment with prototypical inducers, have been reported from one human hepatocyte population to another (Guillouzo and Chesne, 1996; Madan et al., 2003). Moreover, all P450s are not similarly maintained with time in culture. Although various culture conditions, such as the use of sophisticated media, extracellular matrices as supports or sandwich systems, addition of DMSO, or coculturing with other cell types, may improve to some extent the maintenance of P450s and other liver-specific functions, these are usually already markedly and differently decreased early after hepatocyte seeding. Nevertheless, primary hepatocytes have proved to be the most suitable model for investigating induction of P450s by chemical inducers and metabolic profiles of new drugs (Guillouzo et al., 1993; Maurel, 1996; Guillouzo, 1998). Hepatocyte cell lines, mainly originated from tumors, have indefinite proliferative capacity, but they lack a variable and substantial set of liver-specific functions, making them unsuitable as representative of in vivo liver parenchymal cells. In particular, P450 expression is usually very low or undetectable in human hepatoma cells. Even the human hepatoma HepG2 cells, which have retained several liver-specific functions, contain little P450 with the exception of the isoforms expressed in fetal cells such as CYP1A1 and CYP3A7 (Sassa et al., 1987; Ogino et al., 2002). Stably transfected liver cell lines have been established by using eukaryotic P450 expression vectors under the control of inducible/constitutive promoters (Pfeifer et al., 1993), and reexpression of P450s has been obtained in human hepatoma cells by transfection of liver-specific transcription factors such as c/EBP-α (Jover et al., 1998). However, these cell lines do not mimic regulation of gene expression observed in normal hepatocytes (e.g., P450 induction by phenobarbital) (Zelko and Negishi, 2000).
Recently, we have obtained a new human hepatoma cell line derived from a hepatocellular carcinoma, named HepaRG, which exhibits extensive differentiation after 2 weeks at confluency and has the unique property to be susceptible to hepatitis B virus infection (Gripon et al., 2002). In the present study, we have analyzed expression of the main drug-metabolizing enzymes in HepaRG cells under different culture conditions. Our results demonstrate that, in conditions in which cells attain a differentiated hepatocyte-like morphology, they retain a unique set of drug-metabolizing enzymes at levels comparable to those measured in normal human hepatocytes in primary culture.
Materials and Methods
Chemicals. Aflatoxin B1 (AFB1), aflatoxin M1 (AFM1), aflatoxin P1 (AFP1), chlorpromazine, amiodarone hydrochloride, acetaminophen, testosterone, 6β-hydroxytestosterone, chlorzoxazone, 3-methylcholanthrene, chlorpropamide, resorufin, 7-ethoxyresorufin, methylthiazoletetrazolium (MTT), DMSO, rifampicin, salicylamide, isoniazid, dexamethasone, and insulin were purchased from Sigma (St. Quentin Fallavier, France). Metabolites of acetaminophen were kindly provided by Biopredic (Rennes, France). Aflatoxin-glutathione conjugate (AFB1-GSH) and aflatoxin B1-dialcohol were kindly supplied by Pr. F. P. Guengerich (Vanderbilt University, Nashville, TN). 6-Hydroxychlorzoxazone was obtained from Ultrafine Chemicals (Manchester, UK). Williams' E medium was purchased from Laboratories Eurobio (Les Ulis, France). Fetal calf serum (FCS) was obtained from Perbio (Brebières, France). Penicillin, streptomycin, minimum essential medium α, and nonessential amino acids were obtained from Invitrogen (Carlsbad, CA). Hydrocortisone hemisuccinate was obtained from Upjohn Pharmacia (Guyancourt, France). All other chemicals were of the highest quality available.
Cell Cultures. HepaRG cells were obtained from a liver tumor of a female patient suffering from hepatocarcinoma (Gripon et al., 2002). Briefly, small tumor pieces were digested with 0.025% collagenase D diluted in Hepes buffer supplemented with 0.075% CaCl2. The cell population was suspended in Williams' E medium added with 10% FCS, 5 μg/ml insulin, and 5 × 10–7 M hydrocortisone hemisuccinate and distributed in several dishes. The most hepatocyte-like populations were selected and passaged by gentle trypsinization. After three passages, cell aliquots were cryopreserved. Induction of differentiation was obtained by treating confluent cells with the culture medium containing 2% DMSO and 5 × 10–5 M hydrocortisone hemisuccinate.
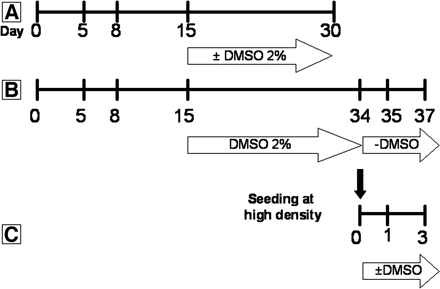
For the present studies, HepaRG cells [for availability of HepaRG cells, contact christiane.guillouzo{at}rennes.inserm.fr (academic laboratories) or christophe.chesne{at}biopredic.com (industrial laboratories)] were detached by gentle trypsinization and seeded at a density of either 2.6 × 104 cells/cm2 (low density) or 0.45 × 106 differentiated cells/cm2 (high density). Low density cells were first seeded in the growth medium composed of Williams' E medium supplemented with 10% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 5 μg/ml insulin, 2 mM glutamine and 5 × 10–5 M hydrocortisone hemisuccinate. After 2 weeks they were shifted to the same culture medium supplemented with 2% DMSO (differentiation medium) for 2 more weeks (confluent DMSO-treated cells) (Gripon et al., 2002). The medium was renewed every 2 to 3 days. High density differentiated HepaRG cells were directly seeded in the differentiation medium.
Human hepatocytes from adult donors undergoing resection for primary and secondary tumors were obtained by perfusion of histologically normal liver fragments (Guguen-Guillouzo et al., 1982). Briefly, hepatocytes were seeded at a density of 110,000 cells/cm2 in 24-well plates in Williams' E medium supplemented with 10% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 1 μg/ml insulin, 2 mM glutamine, and 1 μg/ml bovine serum albumin. The medium was discarded 12 h after seeding and cells were thereafter maintained in serum-free medium supplemented with 10–7 M dexamethasone. The medium was renewed every day. Human hepatocytes were used either freshly isolated or 3 to 5 days after seeding. HepG2 cells were seeded at a density of 100,000 cells/cm2 in 24-well plates and were used at the time they reached confluency. The growth medium was composed of minimum essential medium α supplemented with 10% FCS, nonessential amino acids, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Isolation of RNA and RT-qPCR Analysis. Total RNA was extracted from 106 HepaRG cells, 106 HepG2 cells, or 106 human hepatocytes with the SV total RNA isolation system (Promega, Madison, WI), which directly included a DNase treatment step. RNAs were reverse-transcribed into cDNA using a High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). Real-time quantitative PCR for all genes except for PPARα was performed by the fluorescent dye SYBR Green methodology using the SYBR Green PCR Master Mix (Applied Biosystems) and the ABI Prism 7000 (Applied Biosystems). Table 1 shows primer pairs for each transcript chosen with Primers 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), except for PPARα (Assay-on-demand, Applied Biosystems) and CYP2D6 (gift from Biopredic). For PPARα, we detected 5-carboxyfluorescein fluorescence of the TaqMan probe using the TaqMan Universal PCR Master Mix kit (Applied Biosystems). The amplification curves were read with the ABI Prism 7000 SDS software using the comparative cycle threshold method. The relative quantification of the steady-state mRNA levels was calculated after normalization of the total amount of cDNA tested by an active reference, 18S RNA. Furthermore, a dissociation curve was performed after the PCR to verify the specificity of the amplification. Results were expressed as a percentage of mRNA levels measured in freshly isolated hepatocytes arbitrarily set at 100%.
Primer sequences used for RT-qPCR
Determination of Drug-Metabolizing Enzyme Activities and Metabolic Profiles. For the determination of P450-related activities, HepaRG cells and primary human hepatocytes were incubated with specific substrates in phenol red-free medium deprived of either FCS and 2% DMSO or only FCS. The substrate concentrations were 200 μM testosterone, 300 μM chlorzoxazone, 0.2 mM tolbutamide, and 5 μM ethoxyresofurin. For induction studies, Hep-aRG cells were first exposed to either 25 or 50 μM rifampicin or 50 μM isoniazid for 72 h, or to 5 μM 3-methylcholanthrene for 24 h before incubation with the specific substrate. These concentrations of substrates and inducers are the ones usually used with primary human hepatocyte cultures (Langouet et al., 1995; Guillouzo and Chesne, 1996; Madan et al., 2003). 6β-Hydroxylation of testosterone, tolbutamide 4-hydroxylation, and chlorzoxazone 6-hydoxylation were estimated by HPLC analysis (Guillouzo and Chesne, 1996). Resorufin formation by 7-ethoxyresorufin O-deethylation was quantified by fluorimetry with a fluorescence plate reader (SpectraMAX Gemini; Molecular Devices, Sunnyvale, CA) after a 30-min incubation with ethoxyresorufin in the presence of 3 μM salicylamide to inhibit phase II enzyme activities (Burke and Mayer, 1983). Fluorescence was determined at λex 544 nm and λem 584 nm.
Acetaminophen and AFB1 metabolites were analyzed by HPLC. Metabolites of acetaminophen were determined as previously described (Guillouzo and Chesne, 1996). Acetaminophen was incubated at 2 mM for 10 h before medium harvesting. AFB1 metabolism was analyzed as follows. After an 8-h incubation with the mycotoxin at 5 μM (Langouet et al., 1995), aliquots of media were collected and centrifuged 2 min at 10,000 rpm; then, 90 μl of sample were injected into the HPLC system. An Interchrom C18 column (250 × 4.6 mm, 5 μm) was used for the chromatographic separation. The mobile phase consisted of two solvents, A (0.01 M ammonium acetate, pH 6.5) and B (acetonitrile), with the following gradient: 0 min, 15% B; 23 min, 23% B; 43 min, 53% B; 45 min, 100% B; 47 min, 100% B; 47.5 min, 15% B; 52 min, 15% B. All the gradients were linear and the flow rate was set at 1 ml/min. Fluorescence detection was monitored at excitation and emission wavelengths of 430 nm and 370 nm, respectively.
The HPLC apparatus consisted of an Agilent 1100 Series high performance liquid chromatograph equipped with an autosampler and Agilent 1100 Series fluorescence and UV detectors. A computer running Agilent 1100 software (ChemStation) was used to integrate and calculate the separated peak areas and to plot metabolite patterns. Metabolites tested were identified by comparison of retention times, and quantifications were estimated from calibration curves.
Evaluation of Drug Cytotoxicity. The effects of four reference hepatotoxic compounds, namely acetaminophen, chlorpromazine, amiodarone, and AFB1, were evaluated on HepaRG and HepG2 cells after either 24 or 72 h of exposure. Incubations were performed with medium deprived of 2% DMSO and FCS. At the end of the incubation time, cultures were observed by phase-contrast microscopy using an Olympus 1 × 70. Then, the medium was discarded and replaced with FCS-free medium containing 1 mg MTT/ml. After 2 h, formozan crystals were dissolved in DMSO and the intensity of color was determined at 540 nm using a microplate reader (iEMS Reader MF; Labsystems, Helsinki, Finland).
Statistical Analysis. Data are presented as means ± S.D. Each value corresponded to a different cell culture. Student's t test was applied to samples for statistical analysis. Data were considered significantly different when p < 0.05.
Results
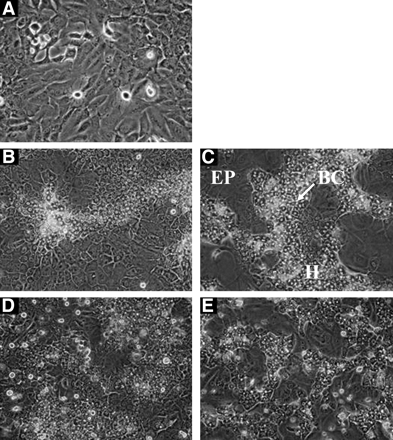
Behavior of HepaRG Cells in Culture. When seeded at low density onto plastic, HepaRG cells took a morphology of elongated undifferentiated cells characterized by a clear cytoplasm and actively divided until they reached confluency, i.e., around day 10 (Fig. 1). Then, they progressively formed typical colonies of granular epithelial cells surrounded by more flattened and clearer epithelial cells. Addition of DMSO was followed by the appearance of a denser cytoplasm and a morphology resembling that of typical normal hepatocytes in primary culture, with the formation of bile canaliculus-like structures. At this stage, hepatocyte-like cells represent 30 to 40% of the population. When seeded at high density after differentiation, HepaRG cells retained their hepatocyte-like morphology. This particular condition allowed us to concentrate the hepatocyte-like population.
Expression of Specific Genes Analyzed by RT-qPCR. Transcripts of six P450s (CYP1A2, 2B6, 2C9, 2D6, 2E1, and 3A4), four nuclear receptors (AhR, PXR, CAR, and PPARα), four phase 2 enzymes (UGT1A1, GSTA1, GSTA4, and GSTM1), three liver-specific proteins (albumin, haptoglobin, and aldolase B), as well as AFP, glutathione-related enzymes (γ-glutamylcysteine synthase regulatory subunit, γ-glutamylcysteine synthase catalytic subunit, glutathione synthase, and glutathione reductase), and thioredoxin were analyzed by RT-qPCR in HepaRG cells at different times after seeding and in the different culture conditions described in Fig. 2. For comparison, the same transcripts were analyzed in HepG2 cells, in freshly isolated human hepatocytes, and, in addition, for several major genes in primary human hepatocytes at various times of culture. As shown in Tables 2 and 3, all the mRNAs analyzed, including those from P450s, were expressed in HepaRG cells. However, transcripts of P450s and the CAR nuclear receptor were detectable in low density seeded cells only after 8 days. mRNA levels were usually slightly higher in HepaRG cells seeded at high density than in those seeded at low density and attaining differentiation after 2 weeks at confluency, probably due to a greater proportion of differentiated hepatocyte-like cells in the former. Furthermore, the highest values were frequently observed in differentiated cells exposed to 2% DMSO. However, some differences were evidenced: mRNA levels of CYP2B6, CYP2E1, CYP3A4, and to a lesser extent, CAR, UGT1A1, GSTA4, GSTM1, and GSTA1/A2, were markedly increased in the presence of DMSO, whereas those of CYP1A2, CYP2C9, CYP2D6, PXR, PPARα, AhR, albumin, aldolase B, and haptoglobin were only slightly modulated. Compared to those measured in primary human hepatocytes (Table 4), with the exception of CYP2D6, which remained low whatever the culture conditions, a trend toward either close or higher (CYP2B6 and CYP3A4) levels of other transcripts was observed in HepaRG cells. As expected, the greatest values found in human hepatocytes were obtained after 3 to 5 days of culture. Compared to HepG2 cells, major differences were noticed: indeed, some transcripts were not detected in these cells (CYP2C9, CYP2E1, CYP3A4, aldolase B), whereas the levels of others, with the exception of CYP2D6 and especially α-fetoprotein, showed a trend toward a decrease (CYP1A2, CYP2B6, CAR, UGT1A1, GSTA1/2, GSTA4, GSTM1, haptoglobin, and albumin) or were close (AhR, PXR, PPARα) compared to HepaRG cells. Whatever the cell type and the culture conditions, no variations were observed in thioredoxin mRNAs as well as in transcripts of glutathione-related enzymes involved in glutathione metabolism (data not shown).
Comparative expression of P450s and nuclear receptors mRNA in HepaRG cells, freshly isolated human hepatocytes, and HepG2 cells
Results are expressed as percentage compared to freshly isolated hepatocytes (FIH) arbitrarily set at 100%. FIH correspond to a pool of three different cell populations. Results are the mean of two independent experiments in duplicate.
Comparative expression of phase 2 enzymes and liver-specific protein mRNA in HepaRG cells, freshly isolated human hepatocytes, and HepG2 cells
Results are expressed as percentage compared to freshly isolated human hepatocytes (FIH) arbitrarily set at 100%. FIH correspond to a pool of three different cell populations. Results are the mean of two independent experiments in duplicate.
Expression of P450s and nuclear receptor mRNA in primary human hepatocytes
RT-qPCR analysis was performed on hepatocyte RNA samples prepared from primary hepatocytes from day 0 (freshly isolated hepatocytes) to day 5 of culture in the absence of DMSO. The experiment was performed on one cell population. Results are expressed as percentage compared to freshly isolated hepatocytes (arbitrarily set at 100%).
Phase-contrast micrographs of HepaRG cells. Cells were seeded at low density and cultured for either 10 days without DMSO (the cells exhibited variable shapes) (A), 30 days without DMSO (B), or 15 days without DMSO, and then exposed to 2% DMSO for 15 days (C). Differentiated HepaRG cells were seeded at high density and maintained in culture for 72 h in a medium deprived of 2% DMSO (D), or supplemented with 2% DMSO (E). H, hepatocyte-like cells; EP, epithelial-like cells; BC, bile canaliculus. Original magnification 150×.
mRNA analysis by RT-qPCR. A, HepaRG cells seeded at low density and cultured for 5, 8, 15, and 30 days in the absence or presence of 2% DMSO between days 15 and 30. B, HepaRG cells cultured for 35 or 37 days. At day 15, 2% DMSO was added to the culture medium until day 34. Then, the cells were cultured the last 24 h (day 35) or 72 h (day 37) without 2% DMSO. C, HepaRG cells seeded at high density and cultured for 24 or 72 h with or without 2% DMSO.
Drug-Metabolizing Enzyme Activities. Four enzyme activities, namely, 7-ethoxyresorufin O-deethylation, 6β-hydroxylation of testosterone, tolbutamide 4-hydroxylation, and chlorzoxazone 6-hydroxylation, were analyzed in HepaRG cells cultured in five different experimental conditions (Table 5). Incubations with specific inducers were performed in media free of FCS and in the presence or absence of DMSO for either 24 h (ethoxyresorufin ± 3-methylcholanthrene) or 72 h (other substrates ± inducers). With the four substrates, the lowest values were obtained with 15-day cultures from low density cell seeding. Whatever the culture condition, only traces of 7-ethoxyresorufin O-deethylation were detected in the absence of inducer. By contrast, after a 24-h treatment with 5 μM 3-methylcholanthrene, a strong induction of this activity was observed. Furthermore, the highest values were found in DMSO-exposed cultures (more than a 2-fold increase) compared to corresponding DMSO-free cultures. Tolbutamide 4-hydroxylase activity (CYP2C9) was detected in all culture conditions. However, in basal conditions, this activity was increased around 6- to 10-fold in differentiated HepaRG cells whether seeded at low or high density and exposed or not to DMSO compared with 15-day cultures. A 72-h treatment with 25 μM rifampicin resulted in a nonsignificant increase in 15-day cultures and no more than around 2-fold increase in differentiated cells exposed or not to DMSO. Chlorzoxazone 6-hydroxylase activity (CYP2E1) was also much greater in differentiated than in 15-day HepaRG cell cultures. The highest values were obtained with cells seeded at high density. A 3-day exposure to 50 μM isoniazid had limited effects, if any. As with tolbutamide 4-hydroxylase and chlorzoxazone 6-hydroxylase activities, 6β-hydroxytestosterone formation (CYP3A4) was augmented in DMSO-free differentiated HepaRG cell cultures compared with 15-day cultures (3.5- to 5.0-fold) and was further strongly increased in differentiated cells when maintained in the presence of DMSO (around 14-fold more) whether the cells were seeded at low or high density. These activities represented around 10 and 500 to 800 pmol/mg protein/min in 15-day HepaRG cells and DMSO-treated differentiated HepaRG cells, respectively. A 72-h rifampicin treatment also resulted in a huge significant increase both in 15-day cells and in differentiated cells seeded at low and high density and exposed in the absence of DMSO (11.8-, 5.3-, and 10-fold, respectively). By contrast, when differentiated cells seeded at high density were treated with rifampicin in the presence of DMSO, no significant increase of testosterone 6β-hydroxylase activity was evidenced.
Drug-metabolizing enzyme activities in HepaRG cells
Determinations of testosterone 6β-hydroxylation (CYP3A4), O-dealkylation of 7-ethoxyresorufin (CYP1A2), chlorzoxazone 6-hydroxylation (CYP2E1), and tolbutamide 4-hydroxylation (CYP2C9) activities were performed on HepaRG cells seeded at low density and cultured for 15 days or 30 days in the presence of 2% DMSO between days 15 and 30, and on differentiated HepaRG cells seeded at high density. Pretreatment with inducers or their vehicle was performed using FCS-free medium with or without added 2% DMSO. The cultures were exposed either 24 h [3-methylcholantrene (3-MC)] or 72 h [rifampicin (RIF), isoniazid (ISO)] to the inducers or their vehicle [untreated (UT)]. Results are expressed as pmol/mg protein/min. Student's t test was applied for statistical studies between UT cells and cells treated with inducer: * p < 0.05, ** p < 0.01, and *** p < 0.001 and between HepaRG cells exposed or not to 2% DMSO during pretreatment with inducers or vehicle: §p < 0.05, §§p < 0.01 and §§§p < 0.001 (GraphPad Prism software).
Metabolic Profiles. AFB1 is the most powerful hepatocarcinogen compound in humans. Its action is mediated by the formation of AFB1-8,9-epoxide, which can covalently bind DNA to form AFB1-DNA adducts. CYP1A2 and CYP3A4 are involved in its biotransformation (Langouet et al., 1996). Metabolism of AFB1 leads to the formation of several metabolites: AFM1 and AFP1, which are hydroxylated products of AFB1, and aflatoxin B1-dialcohol and AFB1-GSH, which are detoxified products of AFB1-8,9-epoxyde. The metabolite profiles of AFB1 after incubation with differentiated HepaRG cells and 3-day primary human hepatocytes are displayed in Fig. 3A. AFM1, AFP1, and aflatoxin B1-dialcohol were identified, and the same range of values was obtained in both cell cultures (AFM1, 129.6 and 192; AFP1, 3160 and 44,160; and AFB1-diol, 44 and 44 pmol/mg protein/min in HepaRG cells and human hepatocytes, respectively). AFB1-GSH conjugates were detected only after a 3-methylcholanthrene induction in HepaR cells and were found in higher amounts in human hepatocyte cultures (<50 and 153.6 pmol/mg protein/min, respectively).
Acetaminophen is one of the most highly used nonprescription analgesic and antipyretic drugs. At therapeutic doses, this compound is detoxified through the formation of glucuronides and sulfoconjugates that are rapidly excreted in urine. At toxic doses, a depletion in glutathione occurred and an electrophilic metabolite, the N-acetyl-p-benzoquinoneimine, is formed by CYP2E1, CYP3A4, and CYP1A2. Figure 3B shows that differentiated HepaRG cells seeded at low density were able to metabolize acetaminophen in sulfate and glucuronide metabolites. The same profile was observed when acetaminophen was incubated with human hepatocytes after 5 days in primary culture. The values were 280.2 ± 37.0 and 347.9 ± 70.2 pmol/mg protein/min for glucuronides and 51.5 ± 6.4 and 132.6 ± 11.7 pmol/mg protein/min for sulfoconjugates in HepaRG cells and human hepatocytes, respectively.
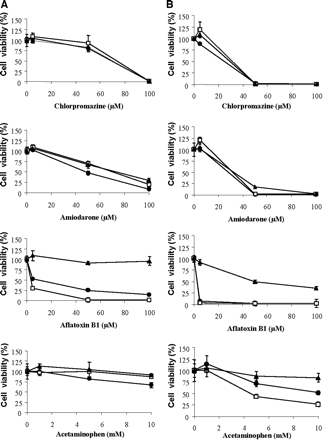
Evaluation of Xenobiotic Cytotoxicity. Toxicity of four compounds, namely acetaminophen, AFB1, amiodarone, and chlorpromazine, was estimated in differentiated HepaRG cells from low or high density cultures and in HepG2 cells using the MTT test (Fig. 4). Whatever the compound, similar data were obtained with differentiated HepaRG cells whether they were seeded at low or high density, and differences between HepaRG and HepG2 cells were observed only for the two compounds, acetaminophen and AFB1, the toxicity of which is mediated by formation of toxic metabolites by P450s. Indeed, as expected, their toxicity, especially that of the most toxic one, AFB1, was much greater in HepaRG than in HepG2 cells after either 24 or 72 h of treatment. No toxicity of AFB1 was even observed in HepG2 cells after a 24-h exposure to the highest dose tested (100 μM), whereas the IC50 was around 5 μM in HepaRG cell cultures at the same time. At 72 h, no viable HepaRG cells were observed with AFB1 concentrations greater than 5 μM, whereas around 50% of HepG2 cells were still alive with a 100 μM concentration. Similar but much less marked differences were observed with acetaminophen. The slightly more intense toxicity observed in HepaRG cells seeded at high density than in HepaRG cells seeded at low density after a 24- or 72-h incubation with AFB1 or acetaminophen was probably due to a higher proportion of hepatocyte-like cells after seeding at high density.
HPLC metabolic profiles of AFB1 and acetaminophen in primary human hepatocyte cultures and 30-day differentiated DMSO-treated HepaRG cells. A-1, aflatoxin B1-dialcohol; A-2, aflatoxin M1 (AFM1); A-3, aflatoxin P1 (AFP1); and A-4, AFB1. B-1, glucuronide acetaminophen; B-2, sulfate acetaminophen; and B-3, acetaminophen. Incubations lasted 8 h with 5 μM AFB1 and 10 h with 2 mM acetaminophen; human hepatocyte primary cultures were used 5 and 3 days after seeding, respectively. Detection was performed at λ = 250 nm for acetaminophen and by fluorescence (λex = 430 nm and λem = 370 nm) for AFB1.
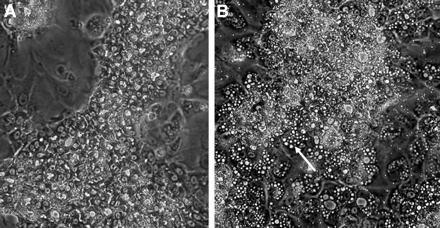
For the other two compounds, amiodarone and chlorpromazine, which do not require metabolism to exhibit toxic effects, the dose-response curves were quite similar in the two cell lines. Amiodarone, an anti-arrhythmic drug, induced phospholipidosis characterized by cytoplasmic accumulation of vesicles in hepatocytes (Poucell et al., 1984). Such vesicles were easily observed in HepaRG cells after a 24-h treatment by phase-contrast microscopy (Fig. 5); they also appeared in HepG2 cells (not shown). Similar dose-response curves were observed in HepaRG and HepG2 cells. IC50 values were around 50 μM and 20 μM after, respectively, 24 and 72 h of treatment. Chlorpromazine, a cholestatic agent, exhibited IC50 values close to those calculated in amiodarone-treated HepaRG and HepG2 cell cultures.
Discussion
Primary human hepatocytes represent the most pertinent model for in vitro drug metabolism and toxicity studies. However, they are not functionally stable with time in culture. Indeed, in agreement with previous observations (Morel et al., 1990, 1993; Abdel-Razzak et al., 1993) and as shown in this study, compared to those expressed in freshly human hepatocytes, a rapid and marked decrease of transcripts was observed for some genes, especially those encoding P450s, after cell plating, although significant levels of various phase 1 and phase 2 xenobiotic-metabolizing enzyme activities were still observed for at least a few days in standard culture conditions. Transcript levels expressed in freshly human hepatocytes are comparable to those found in human liver tissue (Rodriguez-Antona et al., 2000), and a pool of three cell populations was used to reduce interindividual variations. Several approaches have been reported to improve preservation of liver-specific functions in primary hepatocyte cultures, e.g., coculture with rat liver bilary epithelial cells (Guguen-Guillouzo et al., 1983), plating cells at different densities, and using sandwich configuration by an additional layer of extracellular matrix (Hamilton et al., 2001); however, in all cases, marked phenotypic changes have been observed, resulting, particularly, in reduced expression of several major P450s. By contrast, most human and animal hepatoma cell lines have retained little drug metabolism capacity. We show here that HepaRG cells exhibited a unique behavior in culture characterized by their ability to express various liver-specific functions after having reached confluency.
Six P450s were analyzed; three of them, CYP2C9, CYP3A4, and CYP2D6, are responsible for the metabolism of around 90% of the drugs presently in use, whereas the three others, CYP1A2, CYP2B6, and CYP2E1, also metabolize various drugs and other chemicals, including a number of carcinogens. When comparison was performed with human hepatocytes in primary culture maintained 3 to 5 days without DMSO, transcript levels of these P450s were in the same range as those found in differentiated HepaRG cells except for CYP3A4, which is particularly enhanced in DMSO-treated HepaRG cells. Furthermore, levels of P450 activities in differentiated HepaRG cells were also comparable to those usually found in primary human hepatocyte cultures (Guillouzo and Chesne, 1996; Gomez-Lechon et al., 2001). By contrast, the levels of P450s expressed in HepG2 cells (Sassa et al., 1987; Sumida et al., 2000; Ogino et al., 2002) and other transformed hepatocytes of tumoral origin (Iwahori et al., 2003), or obtained by oncogenic immortalization (Pfeifer et al., 1993; Mills et al., 2004), are quite low if detectable. Recently, Mills et al. (2004) measured conversion of testosterone to 6β-hydroxytestosterone catalyzed by CYP3A4 in an immortalized human hepatocyte cell line and found that, although responsive to the prototypical inducer rifampicin, this activity was several hundred-fold lower than that estimated in HepaRG cells. Even when compared to the levels of P450 activities determined in human hepatoma B16A2 cells (Gomez-Lechon et al., 2001), another cell line obtained in our laboratory and found to express various liver-specific functions, those found in HepaRG cells were much higher (formation of the 6β-hydroxytestosterone was around 60 pmol/mg protein/min in B16A2 cells and around 500 pmol/mg protein/min in differentiated HepaRG cells in the presence of 2% DMSO).
Comparative cytotoxic effects of chlorpromazine, amiodarone, AFB1, and acetaminophen on HepaRG and HepG2 cells. Differentiated HepaRG cells from low density seeding (•) and high density seeding (□) and confluent HepG2 cells (▴) were exposed to the chemicals for 24 h (A) or 72 h (B). Cell viability was assayed using a standard MTT test. The results were normalized to control cells and expressed as means ± S.D. (n = 3 cultures).
Most of the genes belonging to the CYP1–4 families can be transcriptionally induced by xenobiotics, and specific receptor proteins are playing a key role in gene induction stimulated by each of the four mechanistically distinct classes of P450-inducing xenochemicals (Rushmore and Kong, 2002; Dickins, 2004). The first identified, the aryl hydrocarbon receptor, belongs to the bHLH/PAS (basic helix-loop-helix/Per-Arnt-Sim) family of transcription factors and stimulates transcription of CYP1A genes and various other genes by a well understood mechanism. This receptor, which is expressed in various cell types, including undifferentiated ones (van Grevenynghe et al., 2005), was found at comparable levels in HepaRG cells, whatever their stage of differentiation, and in primary human hepatocytes. The three other known xenobiotic induction mechanisms include three distinct orphan receptors, CAR, PXR, and PPARα, which are members of the hormone nuclear receptor family. They form heterodimers with 9-cis retinoid X receptor and, for CAR and PXR, regulate expression of the CYP2 and 3 families and several GSTs and UGT enzymes; they also interact with nuclear receptors controlling various pathways of endogenous metabolism. PPARα regulates expression of CYP4A in rodents and plays a role in the regulation of lipid and carbohydrate metabolism. These receptors, which are most highly expressed in the liver, were also found in high levels in differentiated HepaRG cells. Interestingly, a high expression of CAR has never been reported before in any hepatoma cell line (Zelko and Negishi, 2000). Accordingly, CYP1A2, 2C9, and 3A4, known to be transcriptionally increased by prototypical inducers in normal hepatocytes, were similarly induced in HepaRG cells.
Effects of amiodarone on HepaRG cells. Phase-contrast micrographs of confluent DMSO-treated HepaRG cells cultured for 24 h in a FCS-free medium containing either only the vehicle (0.2% DMSO) (A) or 50 μM amiodarone (B). Numerous intracytoplasmic vesicles are visible in amiodarone-treated HepaRG cells (arrow). Original magnification 300×.
For some functions, the highest values were found in HepaRG cells maintained in the presence of DMSO. This agent is recognized as a differentiation-inducing compound in many tumor cell lines. DMSO also increases albumin production in immortalized hepatocytes (Higgins and Borenfreund, 1980) and favors cell survival and maintenance of differentiated functions, such as production of plasma proteins, in primary rat hepatocytes (Isom et al., 1985; Mitaka et al., 1993). The mechanism(s) by which DMSO induces cellular differentiation is still poorly understood; it has been shown to act as a reactive oxygen species scavenger and an antiapoptotic agent (Villa et al., 1991; Gilot et al., 2002). The results reported here clearly show that DMSO induced a more differentiated state and enhanced expression of several metabolizing enzymes in HepaRG cells. However, it was ineffective on some P450s and the liver-specific proteins studied, including albumin. Interestingly, certain P450 inducers were ineffective in DMSO-exposed HepaRG cells, especially when cells were seeding at high density. Indeed, the levels of activities reached in the presence of DMSO were comparable to those observed in DMSO-free cells treated with the prototypical inducer. It may be postulated that by enhancing expression of certain nuclear receptors and liver transcription factors, DMSO allowed certain P450 genes to reach maximum transcription activity as already observed by others (Nishimura et al., 2003; Su and Waxman, 2004).
In agreement with the active expression of phase 1 and phase 2 xenobiotic-metabolizing enzymes, the suitability of HepaRG cells for the determination of chemical metabolism profiles was supported by analyzing metabolic profiles of acetaminophen and AFB1 and the cytotoxicity effects of several hepatotoxicants. The same metabolites as those obtained with primary human hepatocyte cultures were observed with the two compounds, indicating that HepaRG cells expressed the different enzymes involved in their biotransformation at suitable levels. This conclusion is supported by the toxicity of reference hepatotoxicants. Their cytotoxicity was dependent on the compound, dose, and duration of exposure. As expected, AFB1 and acetaminophen, which are hepatotoxic via the formation of electrophilic metabolites by P450-dependent reactions, were more cytotoxic to HepaRG than to HepG2 cells. By contrast, amiodarone and chlorpromazine induced similar toxicity in both cells. Characteristic accumulation of cytoplasmic vesicles corresponding to secondary lysosomes loaded with phospholipids (Poucell et al., 1984) was observed under light microscopy after treatment with amiodarone in both types of HepaRG cells and in HepG2 cells.
Why are HepaRG cells much more differentiated than any other human hepatocyte cell line already described? It may be noticed that they are derived from a hepatocarcinoma and not, like HepG2 cells, from a hepatoblastoma. Second, they show only limited chromosomal rearrangements (Gripon et al., 2002; Parent et al., 2004). Third, and certainly the most original feature, when passaged at low density, they recover characteristic features of progenitor cells able to differentiate in both hepatocytes and biliary epithelial cells (Parent et al., 2004) and able to form a coculture system that strongly resembles that which we have, a long time ago, described by associating primary normal hepatocytes with rat liver epithelial cells and resulting in long-term maintenance of liver-specific functions at high levels (Guguen-Guillouzo et al., 1983). Indeed, several studies have demonstrated that various functions, including phase 1 and phase 2 xenobiotic enzyme activities, were better and longer preserved in cocultures versus pure cultures of human and animal hepatocytes (Begue et al., 1983; Fraslin et al., 1985; Niemann et al., 1991). Such a model should represent a unique tool to distinguish the liver epithelial cell-type target of hepatotoxicants.
Human hepatoma cell lines have also been used for the design of bioartificial livers, although their detoxifying capacity was limited even when cultured in a three-dimensional perfusion system that led to an increased expression of CYP3A4 and inducibility by rifampicin (Iwahori et al., 2003). HepaRG cells could represent a very good alternative to either primary hepatocytes or other human hepatoma cell lines; not only do they exhibit a large set of liver-specific functions but, in addition, although they derive from a hepatitis C virus-infected liver, they do not express viral markers.
In conclusion, for the first time we report that a human hepatoma cell line is able to express the major P450-related activities as well as other liver-specific functions. The fact that when seeded at high density, hepatocyte-like clusters retain their differentiated state and can immediately be used after seeding makes them suitable for high throughput screening as well as studies on metabolism and hepatotoxicity of chemicals.
Acknowledgments
We thank the Biological Resources Centre of Rennes for the supply of isolated human hepatocytes.
Footnotes
-
This work was supported in part by the Institut National de la Santé et de la Recherche Medicale and the Association pour la Recherche sur le Cancer. Amélie Piton is the recipient of a fellowship from the Conseil Regional de Bretagne.
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.006759.
-
ABBREVIATIONS: P450, cytochrome P450; AhR, aryl hydrocarbon receptor; PXR, pregnane X receptor; CAR, constitutive androstane receptor; PPARα, peroxisome proliferator-activated receptor α; UGT, UDP-glucuronosyl transferase; GST, glutathione S-transferase; RT-qPCR, reverse transcriptase-quantitative polymerase chain reaction; FCS, fetal calf serum; AFB1, aflatoxin B1; DMSO, dimethyl sulfoxide; MTT, methylthiazoletetrazolium; AFM1, aflatoxin M1; AFP1, aflatoxin P1; GSH, glutathione; HPLC, high-performance liquid chromatography.
-
↵1 C.A. and A.P. contributed equally to this study.
- Received July 29, 2005.
- Accepted September 30, 2005.
- The American Society for Pharmacology and Experimental Therapeutics