Abstract
Interindividual variability in protein expression of organic anion-transporting polypeptides (OATPs) OATP1B1, OATP1B3, OATP2B1, and multidrug resistance-linked P-glycoprotein (P-gp) or ABCB1 was quantified in frozen human livers (n = 64) and cryopreserved human hepatocytes (n = 12) by a validated liquid chromatography tandem mass spectroscopy (LC-MS/MS) method. Membrane isolation, sample workup, and LC-MS/MS analyses were as described before by our laboratory. Briefly, total native membrane proteins, isolated from the liver tissue and cryopreserved hepatocytes, were trypsin digested and quantified by LC-MS/MS using signature peptide(s) unique to each transporter. The mean ± S.D. (maximum/minimum range in parentheses) protein expression (fmol/µg of membrane protein) in human liver tissue was OATP1B1- 2.0 ± 0.9 (7), OATP1B3- 1.1 ± 0.5 (8), OATP2B1- 1 1.7 ± 0.6 (5), and P-gp- 0.4 ± 0.2 (8). Transporter expression in the liver tissue was comparable to that in the cryopreserved hepatocytes. Most important is that livers with SLCO1B1 (encoding OATP1B1) haplotypes *14/*14 and *14/*1a [i.e., representing single nucleotide polymorphisms (SNPs), c.388A > G, and c.463C > A] had significantly higher (P < 0.0001) protein expression than the reference haplotype (*1a/*1a). Based on these genotype-dependent protein expression data, we predicted (using Simcyp) an up to ∼40% decrease in the mean area under the curve of rosuvastatin or repaglinide in subjects harboring these variant alleles compared with those harboring the reference alleles. SLCO1B3 (encoding OATP1B3) SNPs did not significantly affect protein expression. Age and sex were not associated with transporter protein expression. These data will facilitate the prediction of population-based human transporter-mediated drug disposition, drug-drug interactions, and interindividual variability through physiologically based pharmacokinetic modeling.
Introduction
Hepatic transporters, present at the sinusoidal or canalicular membrane, can determine the plasma concentration of drugs by affecting their metabolic or biliary clearance (Backman et al., 2002; Schneck et al., 2004; Shitara et al., 2004, 2006; Giacomini et al., 2010; Schipani et al., 2012). Consequently, these transporters can affect the efficacy (Bailey et al., 2010; Tomlinson et al., 2010) and toxicity (Alexandridis et al., 2000; Bosch Rovira et al., 2001; Marsa Carretero et al., 2002) of drugs by modulating their exposure to the target sites (Harwood et al., 2013). Hence, it is important to delineate the role of hepatic transporters in drug disposition and local tissue drug exposure, particularly because plasma drug concentrations are generally used as a surrogate measure of tissue concentrations to describe pharmacokinetic-pharmacodynamic relationships and to predict drug-drug interactions (DDIs) or drug-gene interactions (Lon et al., 2012; Harwood et al., 2013). To achieve these goals on a population basis, physiologically based pharmacokinetic (PBPK) models (e.g., Simcyp) are increasingly being used in drug development and pharmaceutical research (Varma et al., 2012, 2013). For drugs where transporters are involved in their disposition, successful use of PBPK models requires critical information on the tissue localization and expression of the transporters, including the effect of covariates, like genotype, age, and sex, on transporter expression (Deo et al., 2012; Chu et al., 2013; Harwood et al., 2013; Prasad et al., 2013). However, such data are currently not available. Here we report protein quantification data on the hepatic transporters as a start to fill this crucial knowledge gap.
Recent US Food and Drug Administration draft guidance on pharmacokinetic DDIs (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf) has highlighted the clinical importance of hepatic organic anion-transporting polypeptide transporters (OATPs), ABC drug transporter ABCB1 or P-glycoprotein (P-gp), and breast cancer resistant protein (BCRP or ABCG2) because of their broad substrate specificity and the potential to be involved in DDIs. We (Deo et al., 2012; Prasad et al., 2013) and others (Balogh et al., 2012; Bi et al., 2012; Kimoto et al., 2012; Ohtsuki et al., 2012; Schaefer et al., 2012; Tucker et al., 2012) have reported data on the expression of some of these hepatic transporters. Here we extended these studies to determine 1) the interindividual variability in expression of OATP1B1 (SLCO1B1), OATP1B3 (SLCO1B3), OATP2B1 (SLCO2B1), and P-gp (ABCB1) in a large set (n = 64) of human liver samples; and 2) the influence of genotype, age, and sex on such expression.
Materials and Methods
Chemicals and Reagents.
The ProteoExtract native membrane protein extraction kit was procured from Calbiochem (Temecula, CA). The protein quantification bicinchoninic acid (BCA) kit and the in-solution trypsin digestion kit were purchased from Pierce Biotechnology (Rockford, IL). Synthetic signature peptides (Table 1) for OATP1B1, OATP1B3, OATP2B1, and P-gp were obtained from New England Peptides (Boston, MA). The corresponding stable isotope-labeled (SIL) internal standards were obtained from Thermo Fisher Scientific (Rockford, IL). High-performance liquid chromatography–grade acetonitrile was purchased from Fischer Scientific (Fair Lawn, NJ), and formic acid was purchased from Sigma-Aldrich (St. Louis, MO). All reagents were analytical grade.
Multiple reaction monitoring parameters of peptides selected for targeted analysis of OATP1B1, OATP1B3, OATP2B1, and P-gp
Labeled amino acid residues are shown in bold and italic.
Human Liver Samples and Hepatocytes.
Sixty-four liver tissue samples from the human liver bank of the School of Pharmacy, University of Washington (UW), were used. Most of the subjects were Caucasian [except one Asian male (HL165) and three Black males not of Hispanic origin (HL104, 105, and 137)]; the subjects’ ages ranged from 7 to 70 years and comprised 30 females and 34 males. Procurement (approved by the UW Human Subjects Division), characteristics, and storage of these samples have been previously described (Paine et al., 1997), and additional details are provided in Supplemental Table 1. Most of these livers were obtained from organ donors who met with accidental death (e.g., trauma from vehicular accidents, subarachnoid hemorrhage, cerebrovascular accident) and were harvested from breathing donors who were perfused with UW solution for organ transplant purposes. Most of the subjects were not taking any chronic medication but did receive medication in the intensive care unit before tissue harvest. Cryopreserved human hepatocytes (seven batches of individual and five batches of pooled hepatocytes) (Table 2) were procured commercially from Celsis IVT (London, UK), Xenotech (Lenexa, KS), Life Technologies (Carlsbad, CA), and BD Gentest (San Jose, CA).
Characteristics of cryopreserved hepatocytes purchased from commercial sources
Membrane Protein Extraction and Total Protein Quantification.
The liver tissue (∼100 mg) was processed, as we have described before, to isolate total membrane proteins (Calbiochem) (Deo et al., 2012; Prasad et al., 2013). Briefly, the tissue was homogenized in 2 ml of extraction buffer I (ProteoExtract native membrane protein extraction kit) containing protease inhibitor cocktail (10 µl) of the kit and incubated with gentle shaking for 10 minutes. The resultant homogenate was centrifuged at 16,000g for 15 minutes, and the supernatant was discarded. The pellet was resuspended in 1 ml of extraction buffer II of the kit with 10 µl of protease inhibitor cocktail. The latter was incubated with gentle shaking for 30 minutes at 4°C followed by centrifugation at 16,000g for 15 minutes at 4°C. Total isolated membrane protein concentration (i.e., the supernatant) was determined using the BCA protein assay kit. The supernatant was diluted to a working concentration of 2 µg of protein per microliter or lower. Similar to the tissues, the pellet of cryopreserved hepatocytes (2–5 × 106 cells) were processed as discussed except the cells were washed twice with washing solution of the kit before the addition of 2 ml of extraction buffer I. The remaining procedure was the same as described for the liver tissue.
Purification of Human Pgp.
Human Pgp from crude membranes of High Five insect cells was purified as described previously (Sauna et al., 2006) with some modifications. Briefly, the crude membranes were solubilized with n-dodecyl-β-d-maltoside (DDM) (1.00% w/v) in the presence of 20% glycerol. Solubilized proteins were subjected to metal affinity chromatography (Ni-NTA resin; Qiagen Inc, Valencia, CA) in the presence of 0.51 mM DDM; purified P-gp was eluted with 200 mM imidazole. The eluate was further purified by gel filtration chromatography using Superdex S-200 column to remove imidazole. The fractions containing P-gp were then concentrated using Amicon ultrafiltration concentrators with 100 KDa cutoff (EMD Millipore, Billerica, MA) to ∼0.5–1.5 mg/ml and stored at −70°C. The protein concentration was quantified using the BCA protein assay kit as per the manufacturer’s instructions.
Peptide Selection, Trypsin Digestion of Membrane Proteins, and Sample Preparation.
Two unique signature peptides (not present in any other known protein) were selected for quantification of each transporter (Table 1) based on previously reported criteria (Kamiie et al., 2008) and literature reports (Zhang et al., 2011; Balogh et al., 2012). Briefly, peptides with transmembrane regions, single nucleotide polymorphisms (SNPs), posttranslational modifications, or those susceptible to degradation were not selected. Continuous R and K sequences (RR, RK, KR, and KK) were excluded to avoid the miscleavages by trypsin. The length of selected peptides was between 9 and 16 amino acid residues. Selected signature peptides were NVTGFFQSFK/YVEQQYGQPSSK (OATP1B1), NVTGFFQSLK/ IYNSVFFGR (OATP1B3), VLAVTDSPAR/SSPAVEQQLLVSGPGK (OATP2B1), and NTTGALTTR/IATEAIENFR (P-gp). The corresponding peptides containing labeled [13C615N2]-lysine and [13C615N4]-arginine residues were used as the internal standards.
Trypsin digestion conditions were optimized for time (24 hours) and protein:trypsin ratio (25:1; w/w), and 20 µl of 2.0 µg/µl (or lower concentration) of tissue membrane preparation was incubated with 4 µl of dithiothreitol (100 mM) and 4 µl of iodoacetamide (200 mM) in 10 µl of ammonium bicarbonate digestion buffer (50 mM, pH 7.8). The protein samples were digested by trypsin in a final volume of 60 µl at 37°C for 24 hours, and the reaction was quenched by 20 µl of SIL peptide internal standard cocktail (prepared in 70% acetonitrile in water containing 0.1% formic acid) and 10 µl of the neat quenching solvent. The samples were centrifuged at 4000g for 5 minutes. For calibration standards, the working solution (10 µl) of the standard cocktail was added in the last step instead of the neat quenching solvent.
Analytical Method Parameters.
Agilent 6460A triple-quadrupole mass spectrometer coupled to Agilent 1290 Infinity LC system (Agilent Technologies, Santa Clara, CA) operated in electrospray ionization positive ionization mode was used for liquid chromatography-tandem mass spectroscopy (LC-MS/MS) analysis of the signature peptides. Approximately 2 µg or less of the trypsin digest (5 µl) was injected onto the column (Kinetex 2.6 µm, C18, 100 × 3 mm, Phenomenex, Torrance, CA) and eluted at 0.4 ml/min. The mobile-phase gradient conditions were 97% A (water containing 0.1% v/v formic acid) and 3% B (acetonitrile containing 0.1% v/v formic acid) held for 4 minutes, followed by seven steps of linear gradient of mobile phase B concentration of 3%–12.5%, 12.5%–18%, 18%–19.5%, 19.5% –20%, 20%–35%, 35%–50%, and 50%–90% over 4–8 minutes, 8–11 minutes, 11–13.5 minutes, 13.5–16 minutes, 16–18 minutes, 18–18.4 minutes, and 18.4–18.6 minutes, followed by the washing step using 90% mobile phase B for 1.6 minutes, and re-equilibration for 4.8 minutes. The doubly charged parent to singly charged product transitions for the analyte peptides and their respective SIL peptides were monitored using optimized LC-MS/MS parameters (Table 1).
The calibration curve standards were prepared by spiking peptide standards into the extraction buffer II of the membrane protein extraction kit. Seven calibration concentrations ranging from ∼0.2 to 20.0 fmol (on column) were used. The analytical method was validated for accuracy, interday and intraday precision, and stability (freeze and thaw, bench-top, and autosampler conditions) as we have described previously (Prasad et al., 2013). The quality control samples prepared by spiking extraction buffer II or pooled human liver membrane were quantified after every 12–15 samples. In addition, the reliability of surrogate peptide-based LC-MS/MS protein quantification was validated using the only purified transporter available to us, namely P-gp. Four different concentrations of P-gp protein standard (2.2, 4.4, 17.7, and 35.3 fmol, on-column) were prepared as quality control samples and were then processed, as were the membrane extracts of the liver tissues. The data were processed by integrating the peak areas generated from the reconstructed ion chromatograms for the analyte peptides and their respective internal standards using the MassHunter software (Agilent Technologies). For quantification of samples or standards, peak response from the two transitions from each peptide was averaged.
Genotyping and Genotype-Dependent Changes on Protein Expression.
Genotyping data were kindly provided by Dr. Yvonne Lin, Department of Pharmaceutics, University of Washington. Briefly, genomic DNA was extracted from liver tissues. The genotype analysis was done using Affymetrix DMET Plus Array (Santa Clara, CA) according to the manufacturer’s protocol. Individual genotypes were resolved, and the minor allele frequencies were determined using the Affymetrix DMET Plus console (version 1.1) using the Dynamic Genotype Boundaries algorithm. Unpaired t test was used to compare protein expression observed in two genotypic groups. When comparing protein expression in three or more groups, the Bonferroni multiple comparison correction was applied.
PBPK Simulations.
The effect of SLCO1B1 polymorphism on the pharmacokinetics of OATP1B1 substrates rosuvastatin and repaglinide was predicted using the population-based ADME simulator of Simcyp (version 12.0, SimCYP Ltd, Sheffield, UK). The rosuvastatin and repaglinide parameters of Simcyp library file were used as such except that the relative expression factor (see Supplemental Table 2) of OATP1B1 was varied for a given genotype or haplotype compared with the default SimcyP value for wild-type (set at 1). The 90% confidence intervals (CIs) of mean relative expression factors were used to estimate population variability in each group (see Supplemental Table 2). Expression of other transporters (i.e., OATP1B3, OATP2B1, BCRP, and sodium-taurocholate cotransporting polypeptide (NTCP)) was assumed constant for all simulations. Data for 16 subjects (70 kg; age 18–65 years, proportion of females: 0.5) given a single dose of rosuvastatin (20 mg) or repaglinide (0.50 mg) were simulated (four trials × 4 subjects).
Results
Analytical Methods.
The calibration curves were linear across the calibration range ∼0.2–20.0 fmol (on column). The lower limit of quantification was between 0.1 and 0.3 fmol (on-column) for all the peptides (except YVEQQYGQPSSK, which had a lower limit of quantification of 1.2 fmol). Accuracy and precision in the quantification of the quality control samples were acceptable (CV <25%) at three different concentrations. Trypsin digestion of the transporters was maximized by monitoring the time at which there was no further increment in the yield of the peptide (data not shown). All the peptides were stable during sample preparation (i.e., when exposed to three cycles of freeze and thaw, at bench-top for 6 hours, and in an autosampler for 48 hours).
Using the purified P-gp, our peptide-based LC-MS/MS approach was able to recover the concentration of P-gp in the quality control samples with an accuracy of 124.0% ± 11.2% and 74.7% ± 4.1% using the P-gp surrogate peptides 1 and 2 (Table 1), respectively. For the remaining transporters, since purified protein standards are not available, the analytical method was based on the assumption that the proteins are completely digested by trypsin to their corresponding peptides and there is a complete extraction of membrane proteins from the tissue homogenate. Although we used two peptides to quantify each protein, only one peptide for OATP1B1, peptide 1, was detectable with acceptable sensitivity. For the remaining transporters, we observed a minor (up to 1.5-fold) but systematic difference (P < 0.05, paired t test, Table 3) in protein quantification between the two different signature peptides. We assume that this difference was due to different degrees of trypsin digestion. Therefore, the results reported here are based on the peptides that yielded higher protein expression, namely, NVTGFFQSFK (OATP1B1), NVTGFFQSLK (OATP1B3), VLAVTDSPAR (OATP2B1), and NTTGALTTR (P-gp). Nevertheless, our final conclusions about the effect of genotype, age, and sex on transporter expression were the same irrespective of the peptide used (see later).
Mean ± S.D. (range, i.e., maximum/minimum in parentheses) protein expression in human livers and human hepatocytes observed in this study (bolded) or reported by others
Interindividual Variability in Transporter Expression in Human Livers and Cryopreserved Human Hepatocytes.
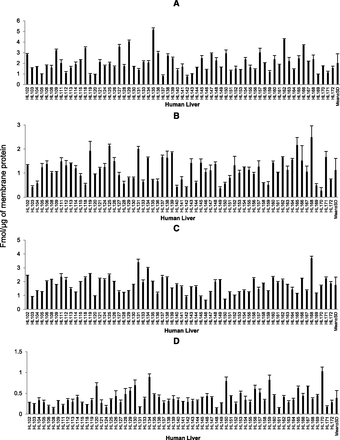
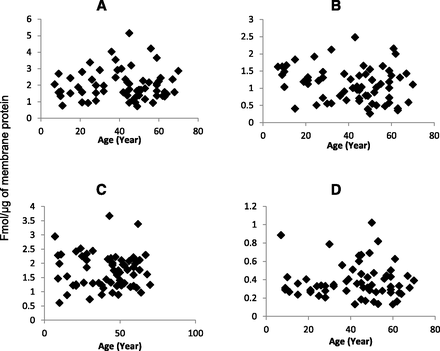
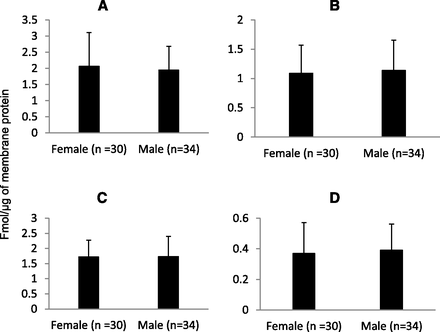
Our yield of total membrane protein was 3.7 ± 1.1 mg/100 mg tissue. The expression of OATPs and P-gp in tissue samples was comparable to that observed in the cryopreserved hepatocytes (Table 3). Similar to human liver tissue, the expression of these transporters in hepatocytes were in the order of OATP1B1 > OATP2B1 > OATP1B3 > P-gp. Interindividual variability, calculated as the -fold range in transporter expression (i.e., maximum or minimum expression) was 5- to 8-fold (see Fig. 1). Expression of all the transporters was independent of age (Fig. 2) or sex (Fig. 3).
Interindividual variability and mean ± S.D. (last bar) in hepatic protein expression of OATP1B1 (A), OATP1B3 (B), OATP2B1 (C), or P-gp (D) in liver samples.
Hepatic protein expression of OATP1B1 (A), OATP1B3 (B), OATP2B1 (C), or P-gp (D) does not correlate with age (P < 0.05).
Hepatic protein expression of OATP1B1 (A), OATP1B3 (B), OATP2B1 (C), or P-gp (D) is not dependent on sex.
Effect of Genotype on Hepatic Transporter Protein Expression.
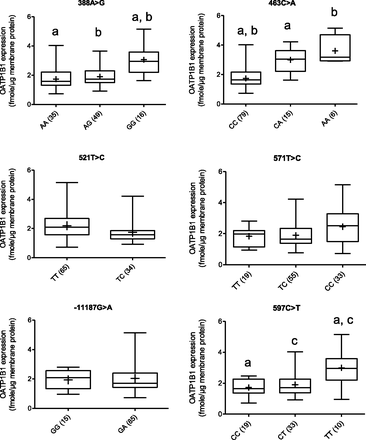
Reported SNPs of all transporters (except SLCO2B1) were observed in our liver bank (Table 4). Among the OATPs, only OATP1B1 expression was genotype-dependent. When analyzed individually (without regard to haplotypes), livers homozygous for the nonsynonymous SLCO1B1 SNPs, c.388A>G or c.463C>A, or the synonymous SNP, c.597C>T, expressed 1.8-, 2.1-, and 1.7-fold higher OATP1B1 protein than those with the corresponding wild-type allele, respectively (P < 0.0001) (Fig. 4). Moreover, livers that were heterozygous for c.463CA had higher OATP1B1 expression than the wild-type livers (P < 0.0001), and the expression in the former was comparable to that in homozygous livers, c.463AA (Fig. 4). The expression of OATP1B1 in livers carrying a single c.388AG, c.597CT, c.571TT, c.571TC, or -1187GA allele was not significantly different from the wild-type livers. Only one liver in our tissue bank was homozygous for the functionally relevant SNP c.521T>C. The expression of OATP1B1 in livers with c.521TT versus 521TC was not significantly different (Fig. 4).
Frequency of OATP1B1, OATP1B3, and P-gp SNPs detected in the University of Washington (UW) liver bank
OATP1B1 protein expression based on individual alleles (% frequencies in parentheses). Horizontal line: median; +: mean value; boxes: 25th–75th percentiles; whiskers: nonoutlier range. Same letters indicate significant difference; P < 0.0001 (a, b), P < 0.005 (c).
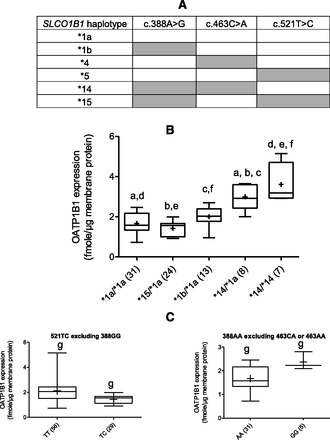
In agreement with other studies (Kalliokoski et al., 2008a; Nies et al., 2013), we observed significant linkage disequilibrium among the above SNPs. Therefore, we examined the expression of OATP1B1 based on SLCO1B1 haplotypes of the three previously described key variants (c.388A>G, c.463C>A, and 521T>C; Fig. 5A). Hepatic OATP1B1 protein expression was significantly higher (P < 0.0001) in livers with SLCO1B1 haplotypes *14/*1a and *14/*14 compared with those harboring the reference allele (i.e., *1a/*1a), *15/*1a or *1b/*1a (Fig. 5B). The carriers of haplotypes *14/*1a and *14/*14 had, respectively, 1.9- and 2.2-fold higher OATP1B1 expression than those carrying the reference allele. Furthermore, livers homozygous for c.388A>G independently increased OATP1B1 protein expression even when carriers of both 463AC and 463AA were excluded (Fig. 5C). SNPs c.388A>G and c.521T>C show the opposite effect on the pharmacokinetics of a number of drugs (Niemi et al., 2004; Kameyama et al., 2005; Hartkoorn et al., 2010; Rodrigues et al., 2011; Schipani et al., 2012; Sortica et al., 2012; Nies et al., 2013). In agreement with these observations, when homozygous alleles of 388A>G were excluded from analysis, we observed a modest but statistically significant (P < 0.05) decrease in OATP1B1 expression in individuals with c.521TC versus c.521TT (Fig. 5C). An independent effect of SNP c.521CC (*5) could not be examined because of its low frequency in our liver bank.
(A) SLCO1B1 haplotype classification based on the presence of the three previously described key variants. (B) Hepatic OATP1B1 protein expression is haplotype-dependent. SLCO1B1 haplotype (% frequencies in parentheses) are arranged by median OATP1B1 protein expression. (C) When carriers of c.388GG were excluded, OATP1B1 protein expression in livers harboring c.521TC allele was moderately but significantly lower than those harboring the TT allele. OATP1B1 protein expression in livers harboring c.388GG (but not SNP c.463CA or AA) was moderately but significantly higher than those harboring the AA allele. Horizontal line: median; +: mean; boxes: 25th–75th percentiles; whiskers: nonoutlier range. Same letters indicate significant difference; P < 0.0001 (a, b, c, d, e, f) and P < 0.05 (g).
Subjects harboring SLCO1B3 haplotype (c.334G>T, c.699A>G, c.1557G>A, and c.1833A>G) did not affect protein expression. Genotype-dependent protein expression could not be investigated for P-gp because of the large number of haplotypes, each with a limited sample size. Individually, none of the ABCB1 SNPs significantly affected protein expression (Table 4).
Quantitative Impact of OATP1B1 Polymorphism on Pharmacokinetics of Rosuvastatin and Repaglinide.
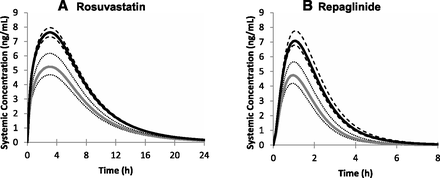
Utilizing the protein expression data (Figs. 4 and 5; see Supplemental Table 2), we predicted the impact of the SLCO1B1 genotypes on the human pharmacokinetics of OATP1B1 substrates rosuvastatin and repaglinide. In agreement with the expression data, the various genotypes and haplotypes of OATP1B1 had marked effects on the predicted plasma concentration-time profiles of the OATP1B1 substrates rosuvastatin and repaglinide (Fig. 6), translating into reductions in area under the curve (AUC) of up to 40% (Fig. 7) compared with the respective wild-type and reference allele.
Mean rosuvastatin (A) and repaglinide (B) plasma concentration-time profiles (po) in populations harboring OATP1B1 wild-type (*1a/*1a, black lines) or *14/*14 (gray lines) haplotypes. The 90% CIs around the mean are represented by the dashed and dotted lines respectively.
Simcyp predicted mean AUC of rosuvastatin (A) or repaglinide (B) in virtual populations harboring different SLCO1B1 genotypes or haplotypes. Error bars show 90% CIs around the mean.
Discussion
We quantified the interindividual variability in hepatic expression of OATP1B1, OATP1B3, OATP2B1, and P-gp in human livers (n = 64) and cryopreserved hepatocytes (n = 12) by LC-MS/MS. OATP and P-gp expression was measureable in all samples. Consistent with data published by others (Nies et al., 2013), the expression of none of the studied transporters in our liver bank was age (7–70 years) or sex dependent. Since primary human hepatocyte suspension is used to predict the contribution of OATPs to hepatic uptake of drugs in humans, it is interesting to note that the expression of OATPs in human livers was similar to that in cryopreserved hepatocytes (Table 3). However, the expression of MRP2 and BCRP was significantly different (1.54 ± 0.64 vs. 0.56 ± 0.21 and 0.14 ± 0.04 vs. 0.70 ± 0.22 fmol/µg membrane protein, respectively; P < 0.0001) (Deo et al., 2012; Prasad et al., 2013). Collectively, our transporter data are comparable or modestly lower than those reported previously in a much smaller sample size (Balogh et al., 2012; Bi et al., 2012; Karlgren et al., 2012; Kimoto et al., 2012; Ohtsuki et al., 2012; Tucker et al., 2012). Based on Western blotting, interindividual variability in OATP1B1, OATP1B3, OATP2B1, and P-gp expression have been reported to be 19, 85, 17 (n = 117), and 20-fold (n = 110), respectively (Meier et al., 2006; Nies et al., 2013). Possible reasons for the differences in absolute expression values are differences in the method of tissue procurement (organ donors vs. resections from diseased livers), subject demographics, the membrane fractionation method used, the protein quantification method used (LC-MS/MS vs. immunoblotting), peptides quantified, sample preparation, or variation in trypsin digestion efficiencies. Since pure protein standards of the transporters measured are not readily available, the limitation of the peptide-based LC-MS/MS quantification methods of assuming 100% trypsin digestion could be an additional factor. However, since we validated our P-gp expression values using purified human P-gp, these values are not confounded by any issues regarding methods.
In agreement with clinical data on the effect of genotypes on in vivo activity of OATP1B1 (Mwinyi et al., 2004; Niemi et al., 2004; Voora et al., 2009; Rodrigues et al., 2011; Schipani et al., 2012; Sortica et al., 2012), we observed that protein expression of OATP1B1 was genotype dependent. SNP c.463C>A is reported to increase lopinavir clearance by 2-fold, possibly as a result of increased OATP1B1 activity (Hartkoorn et al., 2010). Consistent with this observation, we noted that livers homozygous or heterozygous for this variant contained 2-fold higher amounts of OATP1B1 than livers with wild-type alleles. Similarly, SNP c.388A>G increased OATP1B1 expression in human livers compared with the wild-type alleles. Clinically, this variant not only increases the clearance of OATP1B1 substrate drugs (e.g., statins) (Mwinyi et al., 2004; Nies et al., 2013) but also increases the efficacy (Rodrigues et al., 2011; Sortica et al., 2012) and reduces the toxicity of statins (Donnelly et al., 2011).
The preceding analysis did not take into consideration the high degree of linkage disequilibrium between OATP1B1 SNPs. Nies and colleagues recently reported that SLCO1B1 haplotype *14/*14 is correlated with 28% decrease in atorvastatin AUC relative to the reference allele (*1a/*1a); OATP1B1 protein levels were 2-fold higher in individuals with the *14/*1a haplotype (Nies et al., 2013). Consistent with this report, we observed that OATP1B1 expression in livers with the *14/*14 haplotype was 2.2-fold higher than that in livers with the reference haplotype (*1a/*1a). Similarly, consistent with previous data (Nies et al., 2013), livers with haplotype *14/*1a resulted in 1.9-fold higher protein expression. We found that the change in OATP1B1 expression was more pronounced with the 388GG allele versus the AG allele (Figs. 4 and 5). The presence of c.388GG, but not c.463AA or AC, resulted in a modestly higher OATP1B1 expression than the reference allele (*1a/*1a) despite the presence of c.521TC allele, which is known to reduce OATP activity and expression (Fig. 5).
OATP1B1 SNP c.521T>C variant is linked to decreased clearance of OATP1B1 substrates (Niemi et al., 2004; Kalliokoski et al., 2008b; Hartkoorn et al., 2010) and is associated with statin-mediated myopathy (Link et al., 2008; Voora et al., 2009). In vitro cell surface biotinylation experiments by Tirona et al. (2001) showed that the altered transport activity of the c.521T>C variant, for estrone-3-sulfate or estradiol-17-d-glucuronide, was due to decreased plasma membrane expression of the transporter. Although all livers in our liver bank harboring individual SNP c.521TC were not different from wild-type, expression of OATP1B1 was modestly decreased in carriers of SNP c.521TC versus c.521TT when livers harboring c.388GG were excluded (Fig. 5C).
We observed that the synonymous SLCO1B1 SNP c.597C>T was associated with an increase of ∼1.7-fold in hepatic protein expression. However, it is important to note here that SNPs c.388A>G and c.597C>T showed a high degree of linkage disequilibrium; thus, this may not be an independent effect of the c.597C>T variant. Nevertheless, this observation highlights the fact that quantification of tissue transporter expression can result in the discovery of novel variants resulting in hypothesis-based clinical studies to determine the clinical significance of these variants.
Genetic polymorphism can affect the affinity of a substrate for a transporter (Km) or its maximal transport capacity (Vmax) or both. One factor that can affect Vmax is the magnitude of plasma membrane expression of the transporter. Although we measured total membrane expression of OATP1B1, we asked whether we could quantitatively predict the in vivo consequence of changes in the expression of the transporter using PBPK models. We assumed that OATP1B1 expression data for the various genotypes reflected a proportional change in the plasma membrane expression of the transporter. We predicted up to 40% decrease in the mean AUC of OATP1B1 substrates, rosuvastatin, and repaglinide for the various SLCO1B1 genotypes (Figs. 6 and 7; see Supplemental Table 2). Although the effect of most of these genotypes on the pharmacokinetics of these drugs remains to be tested in the clinic, where available, our predictions agreed with the observed data. For example, in agreement with the reported 32% decrease (P = 0.007) in mean AUC0-∞ (Kalliokoski et al., 2008a) of repaglinide in carriers of 388GG versus wild-type, we predicted that AUC changes from 17.2 (wild-type) to 11.8 ng/ml ⋅ h (388GG) (Fig. 7). The latter suggests that despite the fact that we measured the expression of the transporters in total membrane protein (and not specifically in the plasma membrane), we can predict the impact of OATP1B1 genotype on the in vivo disposition of OATP1B1 substrate drugs where the effect appears to occur primarily through change in protein expression. Our predictions also suggest that individuals with *14/*14 haplotypes will demonstrate the largest change in the pharmacokinetics of OATP1B1 substrate drugs.
In summary, LC-MS/MS is a sensitive, specific, simple, multiplex approach to measure simultaneously the interindividual variability in tissue expression of drug transporters. The data presented here, as well as those published previously (MRP2 and BCRP) (Deo et al., 2012; Prasad et al., 2013) in the same set of livers indicate that the interindividual variability in the expression of the major drug hepatic transporters is modest (4- to 8-fold). In addition, expression of these transporters is not associated with age (age 7–70 years) or sex. However, the expression of OATP1B1 was genotype-dependent. Our data indicate that measured expression of OATP1B1 in total membranes isolated from liver tissue (vs. plasma membrane) was predictive of the in vivo consequences of OATP1B1 genotype on drug pharmacokinetics. Collectively, the data presented here will potentially allow us to quantitatively predict transporter-based drug disposition and DDI through population PBPK modeling.
Acknowledgments
The authors thank Dr. Yvonne Lin, Department of Pharmaceutics, University of Washington, for genotyping data; Dr. Peggy Wong (Merck) for review of the statistical analysis; and Xiaoxin Cai and Dr. Xiaoyan Chu (Merck), Jen Harris and Rick Luzietti (AstraZeneca), and Johnathan Cheong (Genentech) for hepatocyte samples for several of the donors analyzed.
Authorship Contributions
Participated in research design: Prasad, Evers, Gupta, Salphati, Hop, Unadkat.
Conducted experiments: Prasad, Shukla.
Performed data analysis: Prasad, Unadkat.
Contributed new reagents or analytic tools: Evers, Gupta, Salphati, Hop, Shukla, Ambudkar.
Wrote or contributed to the writing of the manuscript: Prasad, Evers, Gupta, Salphati, Hop, Shukla, Ambudkar, Unadkat.
Footnotes
- Received July 22, 2013.
- Accepted October 11, 2013.
R.E., A.G., C.E.H., and L.S. contributed equally to the research.
This study was supported by the University of Washington Research Affiliate Program on Transporters sponsored by AstraZeneca, Genentech, and Merck & Co., Inc. (http://sop.washington.edu/uwrapt). R.E. thanks the Merck Research Laboratories New Technologies Review and Licensing Committee for funding. A.G. thanks the AstraZeneca External Science Committee and licensing group for their support. S.S. and S.V.A were supported by the Intramural Research Program of the National Institutes of Health National Cancer Institute Center for Cancer Research (grant number ZIA BC010030-13).
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
Abbreviations
- AUC
- area under the curve
- BCA
- bicinchoninic acid
- BCRP
- breast cancer resistance protein
- CL
- clearance
- DDI
- drug-drug interactions
- LC-MS/MS
- liquid chromatography tandem mass spectrometry
- MRM
- multiple reaction monitoring
- MRP-2
- multidrug resistance associated protein 2
- OATP
- organic anion-transporting polypeptide
- P-gp
- P-glycoprotein
- PBPK
- physiologically based pharmacokinetics
- SIL
- stable isotope label
- SNP
- single nucleotide polymorphism
- U.S. Government work not protected by U.S. copyright