Identification of P4502C18 and P4502C19 as LowKM Diazepam N-Demethylases
Abstract
The present study provides a detailed kinetic analysis of diazepam metabolism by all four known members of the human P4502C subfamily expressed from their cDNAs in Escherichia coli. Both P4502C18 and P4502C19 were found to be low KM diazepam N-demethylases with apparentKM values of 24 ± 4 μM and 21 ± 3 μM, respectively. These values closely resemble the lowKM component of diazepamN-demethylase activity exhibited by human liver microsomes. In addition, P4502C19 also catalyzed diazepam 3-hydroxylation with aKM value of 21 ± 9 μM. Although P4502C8 was essentially inactive in catalyzing diazepam metabolism, P4502C9 catalyzed the N-demethylation with a relatively highKM of 80 ± 15 μM and an overall 3- to 6-fold lower catalytic efficiency, compared with P4502C18 and P4502C19, respectively. At a substrate concentration of 10 μM, diazepamN-demethylation in a panel of human liver microsomes was inhibited 42 ± 12% (mean ± SD, N = 6) by a polyclonal anti-CYP2C antibody. In the same experiment, 3-hydroxylation remained unaffected (<10% inhibition). 1 μM of the CYP3A inhibitor ketoconazole inhibited 37 ± 19% of theN-demethylation and 86 ± 5% of 3-hydroxylation. Estimates of relative contributions to diazepamN-demethylation of P4502C9 (8 ± 4%), P4502C18 (<2%), and P4502C19 (33 ± 14%) and to diazepam 3-hydroxylation of P4502C19 (9 ± 3%) based on the kinetic parameters of the recombinant enzymes and on specific contents of the individual 2C P450s determined in immunoblots are consistent with the inhibition data. In conclusion, these data confirm that both P4502C19 and P4503A are major contributors to human liver microsomal diazepamN-demethylation at low substrate concentrations, whereas P4503A is the major enzyme responsible for 3-hydroxylation.
The benzodiazepine diazepam (Valium) is widely used as a muscle relaxant, anxiolytic, sedative, and anticonvulsant. It is highly lipophilic and extensively bound to proteins in plasma (1). Diazepam is metabolized in the liver by P4501-mediated reactions (fig.1). About 60% of a given dose isN-demethylated to yield nordazepam, the major metabolite found in plasma (2, 3). C3-hydroxylation of diazepam yields temazepam. Both metabolites can be further converted to oxazepam. The 3-hydroxylated metabolites (temazepam and oxazepam) are conjugated with glucuronic acid before being excreted via the kidneys (4,5). There is a large interindividual variation in the clearance of diazepam, showing a dependence on age, sex, liver disease (6), and concomitant exposure to P450 inducers or inhibitors (7-9). In vivo studies suggest a role of polymorphic (S)-mephenytoin 4′-hydroxylase in diazepam metabolism, because poor metabolizers of (S)-mephenytoin were also found to be slow metabolizers of diazepam (2, 10).
Diazepam metabolism in humans.
Diazepam undergoes either N-demethylation or C3-hydroxylation to yield nordazepam or temazepam, respectively. Each of these metabolites can be further converted to oxazepam. The 3-hydroxylated metabolites (temazepam and oxazepam) are conjugated with glucuronic acid before being excreted viathe kidneys.
Recent in vitro studies with P450 isoform-specific inhibitors and with inhibitory anti-CYP2C and anti-CYP3A antibodies provide evidence that P450s of the 2C and 3A subfamilies are involved in human liver microsomal diazepam metabolism. These studies indicate that diazepam 3-hydroxylation is mainly catalyzed by 3A P450s at both high and low substrate concentrations, whereas theN-demethylation seems to be at least partially mediated by 2C P450s at therapeutically relevant substrate concentrations (11, 12). However, the identity and relative contribution of the participating 2C enzymes have not been fully elucidated. In humans, there are four closely related 2C enzymes: 2C8, 2C9, 2C18, and 2C19 (13, 14). P4502C19 has recently been identified as the (S)-mephenytoin 4′-hydroxylase (15, 16). To date, it has not been possible to make a direct comparison of diazepam metabolism by all four members of the human P4502C subfamily, due to the limited availability of the 2C P450s. We have recently expressed the human cytochrome P450s 2C8, 2C9, 2C18, and 2C19 to a high level in Escherichia coli as chimeric enzymes, in which a portion of the N-terminal membrane anchor sequence was replaced with a modified sequence derived from P45017A (17).
The present study compares catalytic properties of the purified recombinantly expressed human P450s 2C8, 2C9, 2C18, and 2C19 for diazepam metabolism. Detailed kinetic analysis reveals that P450s 2C18 and 2C19 are efficient low KM diazepamN-demethylases. In addition, P4502C19 showed a lowKM , diazepam and nordazepam 3-hydroxylase activity. Estimates for the relative contribution of P450s 2C9, 2C18, and 2C19 to diazepam metabolism in a panel of human liver microsomes are provided based on the kinetic data obtained for the recombinant enzymes and on the specific contents of the individual 2C enzymes determined in immunoblots. These estimates are compared with inhibition data obtained with an anti-CYP2C antibody raised against purified P4502C19 from human liver.
Materials and Methods
Chemicals and Reagents.
Diazepam, nordazepam, temazepam, oxazepam, and all routine chemicals were obtained from Sigma Chemical Co. (St. Louis, MO). Ketoconazole was purchased from ICN (Aurora, OH). HPLC solvents were obtained from Fisher Scientific (Malvern, PA).
Human Liver Microsomes.
Human liver samples were obtained from the Liver Transplant Procurement and Distribution System (Minneapolis, MN). Microsomes were prepared by differential centrifugation as described previously (18).
Expression of Human 2C P450s in E. coli.
The expression and partial purification of P450s 2C8, 2C9, 2C18, and 2C19 from the bacterium E. coli have been described in detail previously (17). The amino acid sequences of the recombinant 2C8, 2C9, and 2C18 correspond to those derived from previously published cDNA sequences designated IIC2 (19), 65 (20), and 29c (20), respectively. The 2C19 sequence differed from the reported sequence (20) by a single amino acid substitution I330V (A → G substitution at nucleotide 991 of the cDNA). This substitution was seen in several independent PCR products, suggesting that it was not an artifact of the PCR procedure and thus likely represented a natural variant of 2C19. The expressed human 2C P450s recapitulated their specificity toward the marker substrates taxol (2C8), tolbutamide (2C9), and (S)-mephenytoin (2C19) (17). Rabbit liver NADPH-P450 reductase was purified to homogeneity as described (21) with minor modifications (22).
Reconstitution of Catalytic Activity.
To analyze the kinetic parameters for diazepam, nordazepam, and temazepam metabolism, the four partially purified human 2C P450s (10 pmol) were each reconstituted with 0.3 units (1 unit = 1 μmol of reduced cytochrome c/min at 30°C) of rabbit liver NADPH-P450 reductase; 30 μg/ml DLPC; 1 mM NADPH; 5 mM isocitrate; 0.5 units/ml isocitrate dehydrogenase; 5 mM MgCl2; and 5–500 μM diazepam, nordazepam, or temazepam in 50 mM potassium phosphate buffer (pH 7.4; total volume of 250 μl). This amount of reductase gave maximum activity in preliminary reconstitution experiments (data not shown). The DLPC, P450, and reductase were initially mixed together and allowed to preincubate for 10 min on ice before the addition of the other components. Substrates were added to the reaction mixtures in methanol (final methanol concentration: 1% v/v). Reactions were started by the addition of NADPH and were incubated for up to 30 min at 37°C. Metabolite formation was linear over this period of time (data not shown). Control incubations were run without either NADPH, reductase, P450 or substrate, respectively. After HPLC analysis, none of the control incubations revealed background peaks that would interfere with the quantification of metabolites (data not shown).
Sample Preparation and HPLC Analysis.
Sample preparation and HPLC analysis of diazepam and its metabolites were performed essentially as described by Reilly et al.(23), with the following modifications. Reactions were terminated by three extractions with 500 μl of ethylacetate. Extracts were evaporated to dryness, and the residues were dissolved in 200 μl of the mobile phase [methanol/water/triethylamine, 55:45:0.02 (v/v/v), adjusted to pH 5.0 with phosphoric acid]. The mobile phase was delivered at a flow rate of 1.0 ml/min with a Beckman 127 HPLC pump (Beckman, Fullerton, CA). The 50-μl sample aliquots were applied to a Waters Nova-Pak C18 column (150 mm length, 3.9 mm i.d., particle size 5 μm), using a Beckman 507e autosampler. Metabolites were detected by UV absorption at 236 nm using a Beckman 166 UV detector and identified by coelution experiments with the authentic standards. Absorbance data were collected and analyzed in terms of peak areas using a HP 3395 integrator (Hewlett-Packard, Palo Alto, CA). Retention times were 12.4 min for diazepam, 10.0 min for nordazepam, 7.4 min for temazepam, and 6.3 min for oxazepam. To determine the recovery rate and the quantification limit, incubation mixtures (without NADPH) were spiked with defined amounts of the authentic reference standards (5–200 pmol). These mixtures were then incubated and worked up under the same conditions as the experimental samples. The signals obtained (peak areas) were compared with those of the equivalent amount of reference standards dissolved in the mobile phase. Both the calibration and the recovery function were linear in the range from 10 to 200 pmol with correlation coefficients of r > 0.998. Recovery rates were 87% for nordazepam, 92% for temazepam, and 94% for oxazepam. Unknown concentrations of metabolites in experimental samples were determined by comparing the area of metabolite peaks with the data of the recovery curves. Under these conditions, 10 pmol of metabolite per incubation mixture could be easily quantified. This corresponds to a formation rate of 0.03 nmol/min/nmol P450 in incubations with reconstituted P450 systems and 7 pmol/min/mg protein in incubations with microsomal samples.
Anti-CYP2C Antibody.
A polyclonal anti-CYP2C antibody was raised against P4502C19 purified from human liver (18). Antibody production and IgG purification were performed as described for P4502E1 (24). One hundred micrograms of the antibody completely inhibited diazepam metabolism catalyzed by each of the recombinantly expressed P450s 2C9, 2C18, and 2C19 in reconstitution assays. The antibody also inhibited P4502C19-dependent (S)-mephenytoin 4′-hydroxylase activity in human liver microsomes (90% inhibition in liver sample UM517 at a ratio of 1.5 mg total IgG/mg of microsomal protein).2 The specificity of the antibody for human 2C enzymes was checked in immunoblots with P450s purified from human liver and with human liver microsomes. In addition to strong binding to P4502C19, cross-reactivity with P4502C8 and P4502C9 was observed. However, the antibody did not bind to P4502E1 or P4503A4.2 Strong binding to P4502C19 and cross-reactivity with 2C8, 2C9, and 2C18 were also seen in immunoblots of the recombinantly expressed human 2C P450s.3
Inhibition Studies.
Immunoinhibition experiments were conducted by preincubating the anti-CYP2C antibody with 50–100 μg of microsomal protein at room temperature for 15 min. The reactions were started by the addition of 10 μM of diazepam and 1 mM of NADPH, and were incubated at 37°C for 15 min. Other conditions were as described previously. Product formation was directly proportional to both the reaction time and the amount of microsomal protein (data not shown). Control incubations were performed without antibodies, as well as with antibodies purified from the serum of non-immunized animals. The CYP3A inhibitor ketoconazole (25) was added to the respective incubations to a final concentration of 1 μM. All incubations were performed at least in duplicate.
Additional Analytical Procedures.
P450 concentrations were determined using the method of Omura and Sato (26). NADPH-P450 reductase activities were determined by measuring cytochrome c reduction (21). Protein concentrations were measured by a bicinchoninic acid method (reagent kit; Pierce, Rockford, IL).
Data Analysis.
Kinetic parameters were estimated by fitting the original velocityvs. substrate concentration data with the Michaelis-Menten equation using the iterative nonweighted nonlinear least squares regression option of Slidewrite Plus 3.0 (Advanced Graphics Software, Inc., Carlsbad, CA). Results are reported as mean ± SD of three independent experiments.
Results
Kinetic Studies with Recombinant Human 2C P450s.
Typical HPLC chromatograms obtained after incubation of 100 μM diazepam with reconstituted P450s 2C9, 2C18, and 2C19 are shown in fig.2. P450s 2C9, 2C18, and 2C19 were all active in diazepamN-demethylation, with 2C19 showing the highest rates, followed by 2C18 and 2C9. In addition, 2C19 also catalyzed diazepam 3-hydroxylation, and 2C18 formed an unidentified diazepam metabolite with a retention time of 5.4 min. Reconstituted P4502C8, which was previously shown to catalyze the 6α-hydroxylation of taxol (17), produced no detectable metabolites in incubations with 100 μM of diazepam (table 1). 2C9, 2C18, and 2C19 also showedN-demethylase activity in incubations with 100 μM temazepam, with 2C19 again showing the highest rates, followed by 2C18 and 2C9 (chromatograms not shown).
HPLC chromatograms of diazepam and metabolites formed by E. coli-expressed human 2C enzymes.
10 pmol of P450 reconstituted with 0.3 units of rabbit liver reductase was incubated for 30 min with 100 μM diazepam in the presence of 1 mM NADPH. Sample preparation and HPLC with UV detection set at 236 nm were performed as described in Materials and Methods. Labeled peaks: a, diazepam, b, nordazepam, c, temazepam, and d, unidentified diazepam metabolite. Unlabeled minor peaks are impurities also seen in control incubations performed without NADPH.
Kinetic characteristics for diazepam metabolism by human 2C P450s 1-a
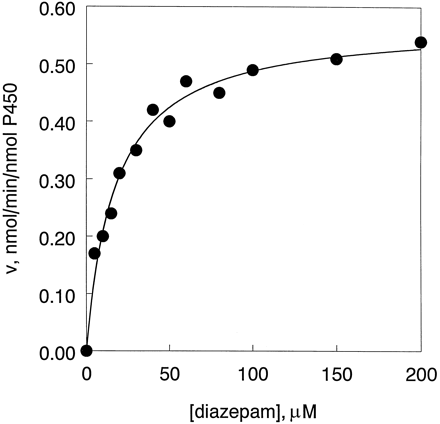
Kinetic analysis of diazepam metabolism by the human P4502C enzymes using various substrate concentrations ranging from 5 to 500 μM were performed in triplicate. Representative velocity vs.substrate concentration plots for diazepam N-demethylation catalyzed by 2C9, 2C18, and 2C19 are shown in fig. 3. A similar plot for diazepam 3-hydroxylation catalyzed by 2C19 is displayed in fig. 4. Curves constructed by nonlinear regression analysis using the Michaelis-Menten equation provided a good fit to the experimental data. Kinetic parameters derived from these plots are summarized in table 1. Both 2C18 and 2C19 are efficient lowKM diazepam N-demethylases, withKM values of 24 ± 4 μM and 21 ± 3 μM. These values closely resemble the low KM component of diazepam N-demethylase activity exhibited by human liver microsomes (11, 12). 2C9 has a roughly 3-fold higherKM and a lower Vmaxresulting in an overall 3- to 6-fold lower catalytic efficiency (Vmax/KM ) for diazepamN-demethylation, when compared with 2C18 and 2C19, respectively. Of the four human 2C P450s, only 2C19 was capable of catalyzing the 3-hydroxylation of diazepam at detectable levels with aKM value of 21 ± 9 μM. 2C19 also showed a low KM nordazepam 3-hydroxylase activity (KM = 11 μM, Vmax = 0.13 min−1, determined in a single experiment, data not shown).
Diazepam N-demethylation by human 2C P450s as a function of substrate concentration.
10 pmol of P450 was reconstituted with 0.3 units of rabbit liver reductase as described in Materials and Methods. Curves were fitted with the Michaelis-Menten equation by nonlinear regression. See table 1 for calculated values of Vmax andKM . CYP2C9 (✚), CYP2C18 (▴), and CYP2C19 (•).
Diazepam 3-hydroxylation by CYP2C19 as a function of substrate concentration.
10 pmol of P450 was reconstituted with 0.3 units of rabbit liver reductase as described in Materials and Methods. See table 1for calculated values of Vmax andKM .
Kinetic parameters obtained for the recombinant enzymes were used in combination with the specific contents of the individual 2C P450s as determined by immunoblot analysis (table 2) to predict the relative contribution of P450s 2C9, 2C18, and 2C19 to diazepamN-demethylation and 3-hydroxylation in a panel of human liver microsomes (table 3). 2C9 was easily detected in all six samples, with a mean specific content of 40 ± 11 pmol/mg protein (table 2). In contrast, 2C18 levels were below the detection limit (< 2.5 pmol/mg protein). 2C19 was present at high levels (67 pmol/mg protein) in two samples (UM517 and UM494). Three samples had intermediate 2C19 levels of ∼30 pmol/mg protein and one sample (UC9203) showed only a very weak band for 2C19 that did not allow for accurate quantification. At a substrate concentration of 10 μM, P4502C19 is predicted to provide a major contribution of 33 ± 14% to diazepam N-demethylation in all five samples where its concentration could be estimated. In each case, 2C9 is predicted to provide a lesser contribution of 8 ± 5%. 2C18 is unlikely to contribute due to low expression levels. In addition, P4502C19 may provide a small contribution of 9 ± 3% to diazepam 3-hydroxylation.
Contents of P450s 2C8, 2C9, 2C18, and 2C19 in human liver microsomal samples 2-a
Relative contribution of P450s 2C9, 2C18, 2C19, and 3A to diazepam N-demethylation in human liver microsomal samples at a substrate concentration of 10 μM
Inhibition Studies.
Inhibition experiments with a polyclonal anti-CYP2C antibody and with the CYP3A inhibitor ketoconazole were used to evaluate the relative contribution of 2C vs. 3A P450s to diazepam metabolism in human liver microsomes (table 3). The amount of anti-CYP2C IgG required for maximum inhibition was determined in two microsomal samples (fig.5). Using a substrate concentration of 10 μM, maximum inhibition of diazepam N-demethylation was achieved at a ratio of 5 mg IgG/mg of microsomal protein. This ratio was subsequently used to probe the remaining samples. The observed inhibition of diazepam N-demethylation in the microsomal panel varied from 25 to 60% with a mean of 42 ± 12% (table 3). This is in reasonable agreement with the sum of the predicted contributions of 2C19 (33 ± 14%) and 2C9 (8 ± 5%) to diazepamN-demethylation. The 3-hydroxylation remained virtually unaffected by the antibody (<10% inhibition). In the presence of 1 μM of the CYP3A inhibitor ketoconazole, diazepamN-demethylation was inhibited 25–74%, with a mean of 37 ± 19%, and the 3-hydroxylation was inhibited 86 ± 5%. At this concentration, ketoconazole had no significant effect on diazepam metabolism by reconstituted recombinant 2C9, 2C18, or 2C19 (<10% inhibition). Taken together, anti-CYP2C inhibition and ketoconazole inhibition account for 66–99% of diazepamN-demethylation and 80–100% of diazepam 3-hydroxylation in the human liver microsomal panel.
Inhibition of diazapam N-demethylation in human liver microsomes by anti-CYP2C IgG.
10 μM of diazepam and 1 mM of NADPH were incubated for 15 min with human liver microsomes previously exposed to the indicated amount of anti-CYP2C IgG. Experimental details are given in Materials and Methods. Data are expressed as the percentage of catalytic activity compared with the original activity in incubations without IgG. Control incubations with IgG from nonimmunized animals gave no inhibition. Data points represent mean values of duplicate determinations. N-demethylation: UM517 (•), JS (▴). 3-Hydroxylation: UM517 (○), JS (▵). Control activities (incubations without IgG): UM517 (N-demethylation), 75 pmol/min/mg protein; UM517 (3-hydroxylation), 90 pmol/min/mg protein; JS (N-demethylation), 37 pmol/min/mg protein; JS (3-hydroxylation), 58 pmol/min/mg protein.
Discussion
Previous in vivo studies have reported reduced plasma clearances and increased plasma half-lives of both diazepam and nordazepam in Caucasian and Korean slow metabolizers of (S)-mephenytoin. It was suggested that the polymorphically expressed (S)-mephenytoin 4′-hydroxylase may participate in the biotransformation of these drugs (2, 10). Premedication with rifampicin, a well-known inducer of subfamily 3A P450s, has been shown to increase both the 3-hydroxylation and the N-demethylation of diazepam by >400%, indicating a possible participation of P4503A (8). In a separate in vivo study, it was reported that a 40–180% increase of (S)-mephenytoin 4′-hydroxylation in extensive metabolizers was observed after rifampicin premedication (27). This data suggest that rifampicin might also induce P4502C19, which was recently identified as the (S)-mephenytoin 4′-hydroxylase (15, 16).
Previous in vitro studies indicated that more than one enzyme is likely to participate in human diazepamN-demethylation and provided additional evidence for a possible link between interindividual differences in the metabolism of diazepam and (S)-mephenytoin (11, 28, 29). More recently,in vitro studies with P450 isoform-specific inhibitors and with anti-CYP2C and anti-CYP3A antibodies indicated that diazepam 3-hydroxylation in human liver microsomes is mainly catalyzed by 3A P450s, whereas the N-demethylation seems to be mediated by both 3A and 2C P450s. The relative contribution of 2C enzymes to theN-demethylation was found to be increased at low substrate concentrations (11, 30). Kinetic studies revealed the presence of two distinct catalytic activities for the N-demethylation in human liver microsomes; one with a low apparentKM of 20 ± 3 μM and one with a high apparent KM of 686 ± 245 μM. The former activity was initially assigned to a 2C enzyme, based on a 70–80% inhibition observed with an anti-CYP2C antibody at low substrate concentrations (11). Interestingly, the low KM diazepam N-demethylase activity was not present in liver microsomes of poor metabolizers for (S)-mephenytoin 4′-hydroxylation, suggesting that P4502C19 might be the lowKM diazepam N-demethylase (12).
In the present study, the successful expression of a full set of all four known members of the human P4502C subfamily to high levels inE. coli (17) has enabled a detailed in vitrokinetic comparison of these enzymes for diazepam metabolism. Both P450s 2C18 and 2C19 were found to be efficient diazepamN-demethylases with apparent KM values of 24 ± 4 μM and 21 ± 3 μM that closely resemble the low KM component of diazepamN-demethylase activity observed in human liver microsomes by Yasumori et al. (11, 12). 2C9 was also active in diazepamN-demethylation, but displayed a 3-fold higherKM and a 3- to 6-fold lower catalytic efficiency (Vmax/KM ), compared with 2C18 and 2C19, respectively. 2C8 was essentially inactive toward this substrate.
In combination with the specific contents of the 2C P450s determined in immunoblots, the kinetic data obtained for the recombinant enzymes allowed us to estimate the relative contribution of the individual 2C P450s to diazepam metabolism in a panel of human liver microsomes. At a substrate concentration of 10 μM, 2C19 was estimated to contribute 33 ± 14% to the N-demethylation (table 3), but only 9 ± 3% to the 3-hydroxylation. 2C9 was predicted to provide only a minor contribution of 8 ± 5% to theN-demethylation. This is consistent with a recent in vitro study (30) that reported only small effects on microsomal diazepam metabolism (<15% inhibition) after addition of 25 μM of sulfaphenazole, a potent specific inhibitor of 2C9 with aKi of 0.12 μM (25, 31). However, the relative contribution of 2C9 to diazepam N-demethylation may be considerably higher in 2C19-deficient samples.
Presently, P4502C18 has not been isolated from human liver, and little is known about the expression level and the catalytic properties of this enzyme. To date, the only metabolic reactions known to be catalyzed by cDNA-expressed P4502C18 are the 4′-hydroxylation of (R)- and (S)-warfarin (32) and the hydroxylation of lansoprazole (33). We report herein that 2C18 also catalyzes theN-demethylation of diazepam, as well as the formation of an as yet unidentified diazepam metabolite (fig. 2). 2C18 mRNA levels were estimated to be ∼8-fold lower than 2C8 and 2C9 mRNA levels, and a >10-fold interindividual variation was detected in samples from 17 human livers (34). The specific content of P4502C18 in the panel of six human liver microsomal samples used in the present study was below the limit of detection (<2.5 pmol/mg protein) (table 2). Based on the low expression levels, we conclude that 2C18 does not provide a significant contribution to microsomal diazepam metabolism.
The 25–60% inhibition of diazepam N-demethylation in a panel of human liver microsomes by the anti-CYP2C antibody observed at a relatively low diazepam concentration of 10 μM in the present study (table 3) reflects the contributions of both P450s 2C19 and 2C9. With the exception of one sample (FCD870), the degree of inhibition by the anti-2C antibody is in reasonable agreement with the sum of the relative contributions of 2C19 and 2C9 calculated using the kinetic data of the recombinant enzymes and specific contents determined in immunoblots. The remaining portion of diazepam N-demethylase activity can be largely attributed to P4503A, as shown by the ketoconazole inhibition experiments. Interestingly, the anti-CYP2C antibody had no significant effect on microsomal diazepamN-demethylation at a relatively high substrate concentration of 100 μM (data not shown). This observation is in agreement with recent in vitro studies (11, 12, 30) and can be explained by the dominance of a high-capacity, high-KM diazepam N-demethylase (probably P4503A) at higher substrate concentrations.
Even at the low substrate concentration of 10 μM, diazepam 3-hydroxylation was not greatly affected by the anti-CYP2C antibody (<10% inhibition). This is consistent with an estimated 2C19 contribution of 9 ± 3% calculated from the kinetic data and the specific 2C19 contents determined in immunoblots. The extensive inhibition (86 ± 5%) of diazepam 3-hydroxylation in the presence of 1 μM of the CYP3A inhibitor ketoconazole suggests that this reaction is mainly catalyzed by 3A P450s. This observation is consistent with previous in vitro studies that used the P4503A inhibitor troleandomycin and anti-CYP3A antibodies (11, 12, 30).
In summary, we have shown that P450s 2C18 and 2C19 are efficient lowKM diazepam N-demethylases. In addition, P4502C19 also efficiently catalyzes the 3-hydroxylation of both diazepam and nordazepam. Based on the kinetic data obtained for the recombinant enzymes and on the specific contents of the individual 2C P450s determined in a panel of human liver microsomes, it is estimated that 2C19 can provide a major contribution to diazepamN-demethylation at low substrate concentrations (33 ± 14%), but only a minor contribution to the 3-hydroxylation (9 ± 3%). Whereas 2C9 may provide up to 8 ± 5% to theN-demethylation, 2C18 is unlikely to contribute, due to low expression levels. Inhibition experiments performed with an anti-CYP2C IgG and with the CYP3A inhibitor ketoconazole are consistent with the predicted relative contributions of CYP2C19 and CYP2C9 vs.CYP3A. In conclusion, data confirm that both P450s 2C19 and 3A provide a major contribution to human liver microsomal diazepamN-demethylation at low substrate concentrations, whereas P4503A is the major enzyme responsible for the 3-hydroxylation.
Acknowledgments
We thank Keith Griffin for helpful comments during the preparation of this manuscript.
Footnotes
-
Send reprint requests to: Dr. Eric F. Johnson, The Scripps Research Institute, Division of Biochemistry/NX 4, 10550 North Torrey Pines Road, La Jolla, CA 92037.
-
This work was supported by the Deutsche Forschungsgemeinschaft (postdoctoral fellowship to F.J.) and by U.S. Public Health Service Grants GM31001 (to E.F.J.) and GM49511 (to J.L.R.).
-
↵2 J. L. Raucy et al., manuscript in preparation.
-
↵3 T. H. Richardson et al., Arch. Biochem. Biophys., in press.
- Abbreviations used are::
- P450
- cytochrome P450
- CYP
- cytochrome P450
- PCR
- polymerase chain reaction
- DLPC
- dilauroyl-l-α-lecithin
- IgG
- immunoglobulin
- Received June 10, 1996.
- Accepted November 4, 1996.
- The American Society for Pharmacology and Experimental Therapeutics