Abstract
Effects of freezing, thawing, and storage at room temperature of human liver samples on the contents and catalytic activities of individual forms of cytochrome P450 (P450 or CYP) were examined. The stability of liver genomic DNA was also investigated. There were no significant decreases in microsomal levels or catalytic activities of P450 enzymes by storage at −80°C for 5 years. We then examined the effects of freezing/thawing of liver samples. Levels of P450 forms were determined immunochemically and by the activities of typical drug oxidation reactions in liver microsomes of human samples, which were divided into two groups. One group of samples was thawed and kept at 25°C for 6 hr and then frozen again and kept for 1 week at −80°C. In the other group, liver microsomes were prepared at the same time (as those from the other group), but not thawed and refrozen. Thawing the liver samples and storage for 6 hr at 25°C decreased contents of total P450 by about 90% and activities of both NADH-ferricyanide and NADPH-cytochrome c reductases by about 80%. However, the decrease in b5 levels was only about 30%. Spectral studies of P450 suggested that thawing the liver samples and holding them at 25°C produced inactive form P420. P450 proteins were detected by immunoblot analysis with or without thawing, but catalytic activities for individual P450s were decreased drastically by thawing and holding samples for 6 hr at 25°C. Only 10% of tolbutamide methyl hydroxylation activity was present, and there was no detectable ethoxyresorufin O-deethylation activity in such microsomes after thawing and holding samples for 6 hr at 25°C. Genomic DNA from human livers was also found to be degraded after the samples were thawing. These results suggested that thawing and holding the liver samples at 25°C decreased the levels and activities of P450s in microsomes and that there are differences in stabilities in individual forms of P450 proteins. P450 proteins determined immunochemically do not always reflect P450-catalytic functions in human liver microsomes because of difficulties in obtaining fresh liver samples.
Liver microsomal drug-metabolizing enzymes contribute significantly to the biotransformation of xenobiotic chemicals such as drugs, toxic chemicals, and carcinogens as well as of endobiotic chemicals including steroids, fatty acids, prostaglandins, and vitamins (1, 2). Among various liver enzymes examined, P450 is most important in determining pharmacokinetic and toxicokinetic properties of drugs (3,4). P450 comprises a superfamily of the enzymes (5), and the catalytic roles of P450 enzymes in the same gene families have been suggested to be generally similar among animal species examined (6, 7). However, recent studies have indicated numerous cases in which species-related differences exist in the expression and catalytic roles of individual forms of P450 in experimental animals and humans, and it is important to identify which human P450 enzymes contribute to the oxidation of drugs to define the safety of clinically used drugs (8, 9).
Roles of human P450 enzymes in the oxidation of a variety of xenobiotic chemicals have been determined recently, using recombinant P450 proteins in the chimeric organisms into which respective human P450 cDNAs had been introduced (10-13). However, caveats must be taken if two or more P450 enzymes are involved in (i) oxidation at the same site of a drug substrate with different affinities or (ii) at the different sites of drug molecules forming different metabolites (14-17). Use of human liver microsomes is, therefore, of great use in characterizing drug oxidation reactions catalyzed by different forms of P450 (3, 18). Owing to the difficulties in obtaining fresh liver samples, investigators are studying human P450 research using surgical liver samples or liver tissues from organ donors (18). However, only limited information is available as to whether each of the P450 forms in human livers degrade at the same rates when human samples were kept for few hours without freezing or were stored for long time in freezer (19).
In this study, we examined the effects of freezing and thawing of human liver samples and handling them at room temperature on the contents and catalytic activities of individual forms of P450. Two types of experiments were carried out. One of the experiments was designed to see if the microsomal P450 enzymes are degraded when human liver samples were kept at −80°C for long time (5 years). The other experiments were carried out to compare the stabilities of individual forms of P450 when frozen human liver samples were thawed and kept at room temperature (25°C) for 6 hr. The 6 hr at 25°C conditions was selected to approximate conditions in which surgical and organ donor samples are not properly handled before freezing for long term storage.
Materials and Methods
Chemicals.
Coumarin, tolbutamide, and ethoxyresorufin were purchased from Sigma Chemical Co. (St. Louis, MO). Other drug substrates, their metabolites, and reagents used in this study were obtained from sources as described previously or of highest qualities commercially available (14, 18,20-22).
Enzyme Preparation.
Human liver samples were obtained from organ donors as described previously (18, 21). These samples were removed within 20 min of death, placed in ice cold 1.15% KCl (w/v), and packed in for delivery to the laboratory, at which time the samples were cut into small pieces (ca. 5–10 cm3), frozen by immersion in liquid nitrogen, and stored in small aliquots at −80°C (19, 23). Liver microsomes were prepared as described and suspended in 10 mM Tris-Cl buffer (pH 7.4) containing 1.0 mM EDTA and 20% glycerol (v/v) (24).
Genomic DNA was isolated from human livers. Briefly, about 2 g of liver was suspended in 10 mM Tris-Cl buffer (pH 7.5) containing 10 mM EDTA and 100 mM NaCl and homogenized with a Teflon-pestle (Dupont, Wilmington, DE) homogenizer. The nuclear fractions, collected by centrifugation at 3,500 × g for 5 min at 4°C, were washed twice with the same buffer by centrifugation at 9,000 × g for 15 min. Genomic DNA was isolated from the nuclear fraction by a commercial Nucleic Acid Purification System (Perkin Elmer ABI 341, Norwalk, CT).
Recombinant human CYP1A2 was purified to homogeneity from membranes ofEscherichia coli in which the modified cDNA had been introduced (25). CYP2A6, 2C9, 2E1, and 3A4 were purified to electrophoretic homogeneity from human liver as described (26-29). Microsomes of human lymphoblast cells expressing CYP2D6 were obtained from Gentest Co. (Woburn, MA). Rabbit anti-P450 antibodies were prepared and the IgG fractions were obtained as described (30). Anti-rat CYP2D1 was a generous gift from Dr. Y. Funae of Osaka City University Medical School.
Freezing/Thawing of Human Liver Samples.
Effects of freezing and thawing of human liver samples and holding at room temperature on the contents and catalytic activities of individual forms of cytochrome P450 were examined. The experiments were carried out by comparing the levels of P450 forms determined immunochemically and the activities of typical drug oxidation reactions in liver microsomes of human samples that were divided into two groups. One group of liver samples was thawed and kept at room temperature (25°C) for 6 hr and then refrozen (for 1 week at −80°C). Liver microsomes of these samples were prepared at the same time as those of the other group, which were not thawed and refrozen.
Enzyme Assays.
Liver microsomal incubations included microsomes (0.5 mg protein/ml) in 100 mM potassium phosphate buffer (pH 7.4) containing an NADPH-generating system consisting of 0.5 mM NADP+, 5 mM glucose 6-phosphate, and 0.5 unit of glucose 6-phosphate dehydrogenase/ml, and various concentrations of drug substrates (18,20, 31). For the assay of nifedipine oxidation activities, 30 mM MgCl2 was included and the buffer was replaced by 50 mM potassium HEPES buffer (pH 7.4)(32, 33).
Activities of ethoxyresorufin O-deethylation (substrate concentration of 10 μM) and coumarin 7-hydroxylation (50 μM) were assayed fluorometrically according to the methods as described (24, 34,35). Methyl hydroxylation of tolbutamide (substrate concentration of 2.5 mM) and 4′-hydroxylation of mephenytoin (0.4 mM) were determined using high-performance liquid chromatography as described (18, 28, 36,37). The methods used for bufuralol 1′-hydroxylation (substrate concentration, 0.2 mM), chlorzoxazone 6-hydroxylation (0.5 mM), and nifedipine oxidation (0.2 mM) were described previously (14, 22, 27,38).
P450 and b5 were estimated spectrally by the methods of Omura and Sato (39). NADH-ferricyanide and NADPH-cytochromec reductase activities were determined by the methods described (40-42). The contents of human P450 proteins in liver microsomes were estimated by coupled sodium dodecyl sulfate-polyacrylamide gel electrophoresis/immunochemical development (“Western-blotting”) (43). The intensities of the immunoblots were measured with an Epson GT-8000 Scanner equipped with NIH Image/Gel Analysis Program adapted for Macintosh computers. Protein concentrations were estimated by the method of Lowry et al.(44).
Electrophoretic Analysis of Genomic DNA of Human Livers.
Genomic DNA of human liver samples was analyzed on a 1% agarose (w/v) gel electrophoresis. In some experiments, exon 3 of CYP2C9was amplified using the forward primer 5′-GGATATGAAGCAGTGAAGGAA-3′ and the reverse primer 5′-GGCCTTGGTTTTTCTCAACTC-3′ (45). Genomic DNA (25 ng) was amplified for 35 cycles with a Perkin Elmer thermocycler (Gene Amp PCR System 2400) using conditions recommended by the manufacture. Amplification was performed with cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 2 min. An initial denaturation at 94°C for 5 min and a final extension at 72°C for 10 min were also performed. The PCR products were analyzed on 10% polyacrylamide gel electrophoresis.
Statistical Analysis.
Statistical analysis was analyzed by Student’s t-test.
Results
Effects of Storage of Human Liver Samples on the P450 Contents and Activities in Microsomal Fractions.
Frozen liver samples from six organ donors were divided into two groups. One group of samples was used to prepare liver microsomes one month later after freezing the livers at −80°C, and the microsomes thus obtained were stored at −80°C for 5 years. Other group of liver samples was kept at −80°C for 5 years and then liver microsomes were prepared. P450 contents and P450-dependent drug oxidation activities in liver microsomes of both groups were determined and compared (table1). Total P450 contents determined spectrally were not different in the two groups; the relative levels of P450 and P420 by spectral analysis were essentially the same in both groups (results not shown). We also determined P450-dependent drug oxidation activities with the substrates ethoxyresorufin, coumarin, tolbutamide, mephenytoin, bufuralol, chlorzoxazone, and nifedipine (shown to be prototypic substrates for CYP1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4, respectively, in human liver microsomes (3, 7, 18, 46)). There were no significant differences in liver microsomal drug oxidation activities between the two groups.
Effects of storage of liver samples at −80°C for 1 month or 5 years on the microsomal P450 contents and drug oxidation activities
Effects of Thawing of Liver Samples on the Contents and Catalytic Activities of P450 in Microsomal Fractions.
Liver samples from six organ donors were divided into two groups. One group of liver samples was thawed and kept at room temperature (25°C) for 6 hr and then frozen again and kept at −80°C for 1 week. This treatment was selected to reflect poor conditions for determining samples prior to long term storage. Other samples were kept at −80°C without thawing. Liver microsomes of these samples were prepared at the same time as those from the other group, which were not thawed and refrozen.
Effects of thawing and holding the samples at 25°C for 6 hr on the microsomal protein and P450 levels in 16 human liver samples were examined (table 2). Protein levels were not changed, but P450 levels in liver microsomes were decreased by about 90%.
Effects of thawing of human samples and storage at 25°C for 6 hr on the contents of microsomal proteins and P450
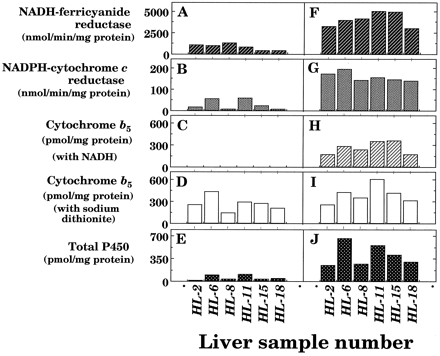
We also determined the effects on other microsomal electron transfer proteins as well as P450 in 6 of 16 human subjects (fig.1). About 20% of NADH-ferricyanide and NADPH-cytochrome creductase activities were remained in the microsomal fractions of liver samples with thawing and storage at 25°C for 6 hr.b5 levels were rather stable when the hemoprotein was determined using sodium dithionite as a reducing equivalent. However, no spectral changes of b5were detected in thawed samples when NADH was used to supply reducing equivalents, probably owing to the loss of NADH-b5 reductase activities in the microsomal fractions (data not shown).
NADH-ferricyanide reductase (A and F) and NADPH-cytochrome c reductase (B and G) activities and contents of b 5 (C, D, H, and I) and P450 (E and J) in liver microsomes from six human samples with (A, B, C, D, and E) or without (F, G, H, I, and J) thawing and storage at 25°C for 6 hr.
Contents of b5 were estimated with NADH (C and H) or sodium dithionite (D and I) as reducing equivalents. Experimental details were described in Materials and Methods.
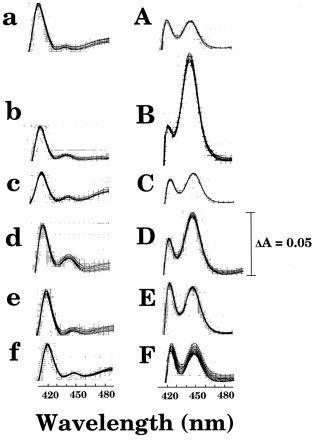
Spectral properties of P450 in microsomal fractions of 6 human livers with or without thawing and holding at 25°C for 6 hr are presented in fig. 2. Spectral characteristics indicated that most of the P450 protein was denatured to inactive form of P420.
Effects of thawing of six human livers and storage at 25° for 6 hr on the spectral properties of P450 in microsomal fractions (1 mg protein/ml) of samples with (a, b, c, d, e, and f) or without (A, B, C, D, E, and F) treatment.
Liver samples used were HL-2 (a, A), HL-6 (b, B), HL-8 (c, C), HL-11 (d, D), HL-15 (e, E), and HL-18 (f, F). Other details were described inMaterials and Methods.
Stabilities of Individual Forms of P450 in Microsomal Fractions of Six Human Livers.
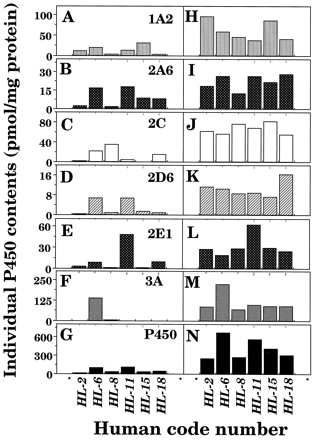
Microsomes (12 μg of protein) from 6 human livers were subjected to SDS-polyacrylamide gel electrophoresis, with or without thawing and storage at 25°C for 6 hr, and the P450 proteins were detected immunochemically using antibodies raised against CYP1A2, 2A6, 2C9, 2D1, 2E1, and 3A4 on immunoblotting analysis. Levels of individual P450 enzymes were determined using purified CYP2A6, 2C9, 2E1, and 3A4 enzymes and the recombinant CYP1A2 and 2D6 as standards. About 70% of the P450 was recovered immunochemically in liver microsomes of individual human subjects, and it was also found that there was good correlation between spectrally determined P450 and sum of the immunochemically determined P450s in microsomes of 6 human livers with and without thawing and storage at 25°C for 6 h (r = 0.92). However, it should be mentioned that in some samples (particularly sample HL-6), P450 proteins determined immunochemically were calculated higher than those determined spectrally (see below).
Levels of individual forms of P450 in microsomes of six human livers with or without thawing and storage at 25°C for 6 hr were compared (fig. 3). There were decreases in levels of individual forms of P450 in microsomal fractions after the livers were thawed and kept at 25°C for 6 hr, and the extent of P450 degradation was different with human samples and individual P450 forms examined. For example, decreases in P450 proteins were very notable in human sample HL-2 as compared with those in other human samples. Also, the sum of the contents of individual P450 proteins determined immunochemically in microsomes of sample HL-6, whose livers were kept at 25°C for 6 hr, were about 3-fold higher than the contents of total P450 determined spectrally. Samples HL-6 and HL-11 contained mainly CYP3A4 and 2E1, respectively, in liver microsomes, and these P450s were present at relatively high levels in immunoblotting analysis after the samples were thawed and kept at 25°C for 6 hr.
Interindividual variations in contents of total P450 determined spectrally and individual forms of P450 determined immunochemically in liver microsomes of six human subjects.
Individual P450 forms were determined in liver microsomes (12 μg protein) of six human samples using antibodies raised against CYP1A2 (A), CYP2A6 (B), CYP2C9 (C), CYP2D1 (D), CYP2E1 (E), and CYP3A4 (F). Microsomes with (A to G) or without (H to N) thawing and storage at 25° for 6 hr the liver samples were subjected to SDS-polyacrylamide gel electrophoresis.
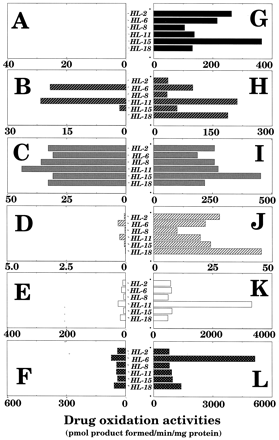
Catalytic activities for typical drug oxidations by individual P450 forms were determined in microsomes of 6 human samples with or without thawing the livers and holding samples at 25°C for 6 hr (fig. 4). In the figure, the scale of the activities (abscissa) in the samples with thawing and storage at 25°C for 6 hr (figs. 4A, 4B, 4C, 4D, 4E, and 4F) was set up by one-tenth of those that were not (figs. 4G, 4H, 4I, 4J, 4K, and 4L). Several P450 proteins were detected on immunoblotting analysis with large interindividual variations in samples in both treatments, but catalytic activities for individual P450 enzymes were decreased drastically by thawing and holding samples for 6 hr at 25°C. Among the P450-catalyzed reactions examined, about 10% of tolbutamide methyl hydroxylation activity was present in microsomes of the livers with thawing and storage at 25°C for 6 hr, while there were no detectable levels of ethoxyresorufin O-deethylation activities in such microsomes. Considerable levels of coumarin 7-hydroxylation activity were detected in microsomes of livers with thawing and standing the samples for 6 hr at 25°C, and these activities were somewhat correlated with the levels of CYP2A6 in such microsomes (see figs. 3B and 4B; r = 0.81). Similar tendencies were also observed with the catalytic activities bufuralol 1′-hydroxylation (CYP2D6, r = 0.88), chlorzoxazone 6-hydroxylation (CYP2E1, r = 0.73), and nifedipine oxidation (CYP3A4, r = 0.82).
P450-dependent drug oxidation activities in microsomes of six human samples with (A, B, C, D, E, and F) or without (G, H, I, J, K, and L) thawing the liver samples and storage at 25° for 6 hr.
Substrate oxidations determined were ethoxyresorufin O-deethylation (A and G), coumarin 7-hydroxylation (B and H), tolbutamide hydroxylation (C and I), bufuralol 1′-hydroxylation (D and J), chlorzoxazone 6-hydroxylation (E and K), and nifedipine oxidation (F and L).
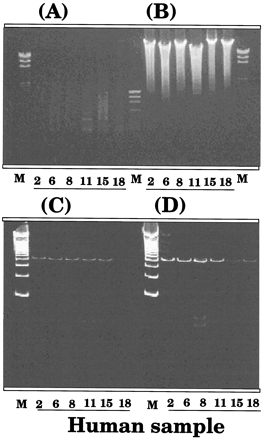
Effects of thawing of liver samples and standing at room temperature on genomic DNA and amplified DNA products with CYP2C9 DNA probe.
Genomic DNA of six human liver samples was also degraded after the samples were thawing and stored at 25°C for 6 hr when analyzed by agarose gel electrophoresis (figs. 5A and 5B). The absorbance ratio of 260 nm and 280 nm was essentially the same in genomic DNA isolated from liver samples with or without thawing and standing the samples at 25°C for 6 hr (results not shown). We also determined the amplified products of genomic DNA probed with primers of exon 3 of theCYP2C9 gene using polyacrylamide gel electrophoresis (Figs.5C and 5D). The results showed that the product with same molecular weight (420 bp) was detected in samples with thawing and holding at 25°C for 6 hr, while the intensities of the bands were weaker.
Effects of thawing liver samples and storage at 25°C for 6 hr on the agarose gel electrophoresis of isolated genomic DNA (A and B) and on a 10% polyacrylamide (w/v) gel electrophoresis of the amplified DNA in exon 3 fragment of 420 bp (C and D).
Liver DNA from six human subjects with (A and C) or without (B and D) thawing the liver samples and storage at 25°C for 6 hr was subjected to agarose (A and B) or polyacrylamide (C and D) gel electrophoresis. In A and B, the molecular weight markers (M) used were λ/Hind III and φX174/Hae III, and in part C and D were a 100 bp DNA ladder that contained 100 bp, 200 bp, 300 bp, 400 bp, 500 bp, etc.
Discussion
In this study the effects of storage and freezing/thawing/storage at room temperature of human liver samples on the contents and catalytic activities of individual forms of P450 were examined. Our results indicate that storage of human liver samples at −80°C for 5 years does not affect significantly the contents of P450 and activities of P450-dependent drug oxidations in microsomes when compared with microsomes of the same human livers to which liver microsomes were first isolated and stored at −80°C for about 5 years. Powis et al. (47) have also reported that there are no significant decreases in contents of P450 and activities of biphenyl 4-hydroxylation and halothane reduction when liver microsomes, liver homogenates, or small pieces of liver samples were stored in deep freezer for 1 month, 6 months, and 12 months. P450-dependent drug oxidation activities are reported to be very stable in liver microsomes when the microsomes are suspended in buffers containing 20% glycerol (24, 48).
We then examined the effects of freezing/thawing of liver samples on the microsomal contents and activities of individual forms of P450. Thawing the liver samples and holding them at 25°C for 6 hr decreased contents of total P450 by about 90% and activities of both NADH-ferricyanide and NADPH-cytochrome c reductases by about 80%. However, the decrease in b5 levels was only about 30% when the hemoprotein was determined with sodium dithionite as a reducing equivalent. When NADH was used for the determination of b5 in microsomes with thawing the livers, no spectral changes were observed, indicating that electrons were probably not supplied as efficiently due to inactivation of NADH-b5 reductase. These results suggest that among microsomal electron transfer proteins, b5is relatively stable to degradation. It is interesting in this connection to note that there are different rates of biological half lives in the membrane-bound proteins in liver microsomes and that among various membrane proteins examined b5 has longer half lives than P450 enzymes and NADPH-P450 reductase in rat liver microsomes (49, 50).
Spectral determination of P450 suggested that thawing and standing the liver samples at 25°C for 6 hr produced the inactive form, P420, in microsomes. The degradation of P450 to P420 caused decreases in drug oxidation activities by liver microsomes. The decreases in levels and activities of individual forms of P450 in microsomal fractions varied with human samples and individual P450 forms examined. For example, decreases in P450 proteins in a human sample HL-2 were very notable when compared with those in other human samples. In contrast, the sum of the contents of individual P450 proteins determined immunochemically in a human sample HL-6 was significantly (about 3-fold) higher than those of total P450 determined spectrally when livers were thawed and kept at 25°C for 6 hr. These results suggest that the extents of P450 degradation are not always the same in different human samples examined, and that the levels of P450 proteins detected immunochemically do not always match those of active P450 proteins.
Despite the fact that several P450 proteins were detected on immunoblotting analysis with large interindividual variations in samples with or without thawing and storage at 25°C for 6 hr, catalytic activities for individual P450 enzymes were decreased drastically by the procedure. Among the P450-catalyzed reactions examined, tolbutamide methyl hydroxylation was more stable (but still dropped to 10%), while ethoxyresorufin O-deethylation activity was very sensitive in microsomes when livers were thawed and stored at 25°C for 6 hr. Coumarin 7-hydroxylation activities were also detected in microsomes of livers with thawing/holding at 25°C for 6 hr, and these activities were somewhat correlated with the levels of CYP2A6 determined immunochemically with some exception. Such relations in activities and contents of P450s were also observed with bufuralol 1′-hydroxylation and CYP2D6, chlorzoxazone 6-hydroxylation and CYP2E1, and nifedipine oxidation and CYP3A4. The different stabilities among individual P450 enzymes examined may be a result of the different susceptibilities of these P450 enzymes against proteolytic damage by intracellular proteases.
Agarose gel-electrophoresis of genomic DNA isolated from six human liver samples suggested that thawing/freezing and storage the livers at 25°C for 6 hr caused degradation of DNA. However, analysis with polyacrylamide gel-electrophoresis of the PCR products amplified with primers of CYP2C9 cDNA in these DNA samples suggested that small fragments of degraded DNA were retained in the DNA fractions.
In conclusion, there were no significant decreases in microsomal levels and catalytic activities of P450 enzymes when liver samples were kept at −80°C for 5 years. The results suggest that thawing of freezed liver samples and stored at 25°C for 6 hr decreased the levels and activities of P450s in microsomes and that there are differences in stabilities in individual forms of P450 proteins. The 6 hr at 25°C conditions was selected to approximate conditions in which surgical and organ donor samples are not properly handled before freezing for long term storage. Our results also suggested that P450 proteins determined immunochemically do not always show P450-catalytic functions in human liver microsomes because of difficulties in obtaining fresh liver samples.
Note added in proof
After completing to write this manuscript, we became aware of a paper of Pearce et al. (51) who reported their studies on the effects of freezing, thawing, and storing human liver microsomes. However, it should be mentioned that the present paper deals with freezing, thawing, and storage of human liver samples on the effects of P450 contents and catalytic activities in liver microsomes.
Footnotes
-
Send reprint requests to: Dr. T. Shimada, Osaka Prefectural Institute of Public Health, 3–69 Nakamichi 1-chome, Higashinari-ku, Osaka 537, Japan.
-
This work was supported in part by Grants from the Ministry of Education, Science, and Culture of Japan, the Ministry of Health and Welfare of Japan, and the Developmental and Creative Studies from Osaka Prefectural Government, and by United States Public Health Service Grants CA44353 and ES00267.
- Abbreviations used are::
- P450 or CYP
- cytochrome P450
- P420
- cytochrome P420
- b5
- cytochromeb5
- SDS
- sodium dodecyl sulfate
- Received August 26, 1996.
- Accepted October 18, 1996.
- The American Society for Pharmacology and Experimental Therapeutics