Abstract
The tricyclic antidepressant amitriptyline and the H1-receptor antagonist diphenhydramine are conjugated in human liver microsomes fortified with UDP-glucuronic acid at their tertiary amino groups with the formation of quaternary ammonium glucuronides. The kinetics of the reactions were found to be biphasic with apparentKM1 andKM2 values of 1.4 μM and 311 μM for amitriptyline and 2.6 μM and 1180 μM for diphenhydramine in four liver samples.Vmax1 values varied between 2 and 17 pmol·mg protein−1·min−1for the two substrates and Vmax2 values between 80 and 740 pmol·mg protein−1·min−1. A close correlation existed between amitriptyline and diphenhydramine glucuronidation rates in microsomes from seven livers at concentrations corresponding to 10–40% ofKM2. At low concentrations, diphenhydramine competitively inhibited the glucuronidation of amitriptyline.Vmax/KMvalues of the high-affinity UDP-glucuronosyltransferase(s) (UGTs) exceed those of the low-affinity enzyme(s) severalfold, such that the former should make the major contribution toN-glucuronidation of the drugs at therapeutic concentrations in vivo.
Attachment of a glucuronosyl residue to an aliphatic tertiary amino group is a biotransformation reaction common to many basic amphiphilic drugs. The resultant quaternary ammonium glucuronides have been detected as in vivo metabolites of H1-antagonists (1-3), tricyclic antidepressants (4-11) and neuroleptics (6, 11, 12), and structurally related drugs (13-15) in humans (1-15) and nonhuman primates (14,15). Their formation in vitro can be demonstrated using liver microsomes from man (16-20) or rabbit (6, 18, 19). Various experimental approaches have been used in order to elucidate which one of the individual isozymes belonging to two UDP-glucuronosyltransferase (UGT) families (21) catalyse the N-glucuronidation reaction in human liver. The involvement of a specific UGT isozyme was indicated by the lack of a correlation between the glucuronidation rates of imipramine and those of 1-naphthol or steroid substrates (19). While purification of an N-glucuronidating enzyme from human liver has hitherto not been achieved, human UGT1.4 expressed in human embryonic kidney cells proved able to glucuronidate a number of tertiary amine drugs in addition to primary and secondary aromatic amines (22) and numerous hydroxylated endo- and xenobiotics (23).
The present investigation aimed at elucidating, in more detail, the kinetics of conjugation of two drugs, amitriptyline and diphenhydramine, in human liver microsomes to their quaternary ammonium glucuronides (fig. 1). The tricyclic antidepressant amitriptyline is the only drug with tertiary amino group for which urinary N-glucuronide excretion has been measured in larger groups of patients and volunteers (5, 7-9), with the results varying between 2.5 and 21% of the dose (7, 8) or between 3.4 and 58% of total metabolites recovered (5, 9). Amitriptyline conjugation can be performed by expressed UGT1.4 (22, 23), and its rate in liver microsomes exhibited considerable variation among samples from different donors (17). Ingestion of the H1-antagonist diphenhydramine by two volunteers led to the recovery of 2.9 and 5%, respectively, of the dose in urine as the N-glucuronide (3). It is formed by expressed UGT1.4 at a rate similar to amitriptylineN-glucuronide (23), but its production in liver microsomes has apparently not been studied.
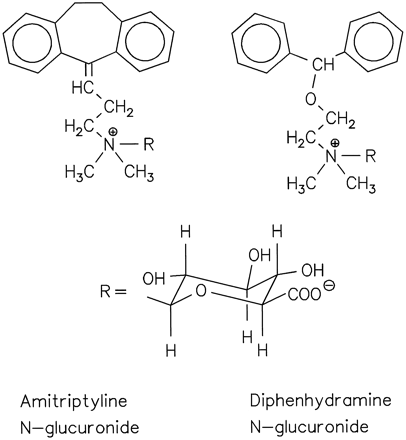
Structures of the N-glucuronides of amitriptyline and diphenhydramine.
Materials and Methods
Substances.
Amitriptyline hydrochloride was a gift from Troponwerke (Köln, Germany), and diphenhydramine hydrochloride was purchased from Sigma (Deisenhofen, Germany). Their N-glucuronides were synthesized as described previously (24) by a modification of the procedure of Luo et al. (25). UDP-glucuronic acid was purchased from Boehringer Mannheim (Mannheim, Germany), cation exchanger SCX 500-mg cartridges from Analytichem International (Harbor City, CA), Triton X-100 and nonanoyl-N-methylglucamide from Sigma and organic solvents from E. Merck (Darmstadt, Germany).
Human Liver Microsomes.
Human liver (HL)1 samples were obtained from organ donors or as excess material on partial hepatectomy (26). Pieces 5–10 g in size were stored at −80°C until microsomes were prepared in tris-buffered isotonic sucrose containing 2 mM EDTA (26). Pellets of washed microsomes were stored at −80°C for up to 6 months. Before use they were suspended in 0.25 M tris-HCl buffer, pH 8.0, to a concentration of about 10 mg protein/ml (1 ml buffer per gram of liver). Protein was measured (27) using bovine serum albumin as the standard.
Incubation and N-glucuronide Isolation.
The standard incubation mixture of 1 or 2 ml contained 57 mM tris-HCl, pH 8.0, 5 mM MgCl2, 2 mM UDP-glucuronic acid, 1.2–1000 μM substrate, 0.5 mg/ml microsomal protein, and 0.2 mg/ml Triton X-100. Amitriptyline concentrations did not exceed 700 μM because samples started to be turbid at 700 μM and, in some cases, reaction rates were lower than at 500 μM. After a standard incubation time of 30 min at 37°C, the reaction was stopped by two extractions with 1 mltert-butyl methyl ether, which removed excess substrate. The aqueous phase was washed with 1 ml n-hexane, and the protein interphase was removed together with the organic phase. Eighty per cent of the aqueous phase was processed to purify theN-glucuronide by solid-phase extraction on a SCX cartridge essentially as described previously (24). Briefly, after sample application, the cartridge was washed with 3 ml water and 3 ml methanol and eluted with 6 ml acetonitrile/methanol/0.2 M ammonium acetate (8:2:1, v/v/v) that was evaporated under a stream of nitrogen at 40°C. Regeneration of the cartridge required washing with 3 ml methanol, 3 ml water, and 8 ml 1 M acetic acid.N-glucuronide recoveries on SCX cartridge extraction were determined in duplicate in each series of incubations and found to vary between 75% and 100% for added quantities of 3 and 4 nmol amitriptyline N-glucuronide and between 63% and 98% for 3 and 4 nmol diphenhydramine N-glucuronide. The recoveries decreased on repeated use of the cartridges (12 to 15 times); within series, they showed coefficients of variation of 6% (N= 19) and 5% (N = 15), respectively. Quantities of 0.5–2 nmol were recovered by 54–65%. Values measured in incubations were corrected for recoveries in the same series.
HPLC.
Extract residues were dissolved in 150–600 μl water, and 100 μl was injected directly for HPLC on a 4.6 × 250 mm C18-silica column; the solvent was 10 mM perchloric acid adjusted to pH 2.5/acetonitrile (70:30, v/v, for amitriptylineN-glucuronide, 71:29 for diphenhydramineN-glucuronide) (24). Quantification was based on peak heights measured at 254 nm (amitriptyline N-glucuronide) or 220 nm (diphenhydramine N-glucuronide) relative to those of external standards.
To verify the identity of the products, one sample each with 9 μM or 250 μM amitriptyline or diphenhydramine was divided before extraction, and a 50% aliquot was incubated at pH 6.8 with β-glucuronidase from Escherichia coli for 16 hr at 37°C (24). The hydrolyzed samples then were processed in parallel with the unhydrolyzed aliquots.
Calculations.
Kinetic data were plotted according to Eadie and Hofstee to obtain first estimates of kinetic parameters. Since the plots were suggestive of the involvement of two enzymes with Michaelis-Menten kinetics, the respective equation was fitted to the experimental data after logarithmic transformation (to stabilize the variance) (28, p. 237). Nonlinear regression analysis (28, p. 458) started from the first estimates and used the substrate concentration S as the independent variable and the reaction velocity V as the dependent variable. The unknown variables were common high- and low-affinity Michaelis-Menten constantsKM1 andKM2 and individual maximal reaction velocities Vmax1 andVmax2 for each liver. The goodness-of-fit is expressed by the multiple correlation coefficientr2 = 1 - (residual sum of squares/total sum of squares) (28, p. 90).
Results
Microsomes from all seven liver samples conjugated amitriptyline and diphenhydramine at measurable rates. The identity of the products was confirmed by hydrolysis of incubated samples with β-glucuronidase before extraction that led to complete disappearance of theN-glucuronide peaks in HPLC.
The rate of amitriptyline N-glucuronidation in human liver microsomes increased with increasing pH. Relative to the rate at pH 7.4, it was 125–150% at pH 7.7 and 158–167% at pH 8.0. This pH was chosen as the standard condition.
Activation of latent enzyme was examined at pH 8.0 with Triton X-100 and nonanoyl-N-methylglucamide. Whereas the latter at 0.1 and 0.2 mg/ml stimulated the N-glucuronidation of amitriptyline and diphenhydramine by less than 30%, Triton produced a concentration-dependent increase of the conjugation rates. At 9 and 250 μM amitriptyline, maximal rates of 240 and 180% of basal rates, respectively, were achieved with 0.2 mg/ml Triton corresponding to 0.4 mg/mg protein. The conjugation of 9 and 250 μM diphenhydramine was enhanced to 150 and 130%, respectively. No or only minimal further stimulation was produced with 0.3 mg/ml Triton.
Under standard conditions, N-glucuronide formation from both substrates was linear with time up to 60 min (r = 0.99,N = 6, p < 0.001). Variation of the concentration of UDP-glucuronic acid revealed KMvalues of 0.57 mM in the conjugation of 9 and 250 μM amitriptyline and 0.46 mM with 250 μM diphenhydramine as substrate. Standard incubations were carried out with 2 mM cosubstrate.
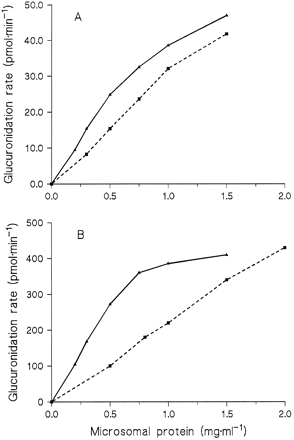
The dependence of N-glucuronidation rates on protein concentration was investigated in incubations with Triton concentrations of 0.4 mg/mg protein, but not below 0.2 mg/ml when amitriptyline was the substrate. With 9 and 250 μM amitriptyline, theN-glucuronide formation rate increased linearly with the protein concentration up to 0.5 mg/ml, whereas the increase was clearly less between 0.5 and 1.5 mg/ml (fig. 2). In contrast, the diphenhydramine N-glucuronidation rate was proportional to the protein concentration up to 1 mg/ml at 9 μM of substrate and up to 2 mg/ml at 250 μM (fig. 2).
Dependence of the glucuronidation rates of amitriptyline (solid lines) and diphenhydramine (dashed lines) on the protein concentration in microsomes from HL 17.
Substrate concentrations were 9 μM (A) or 250 μM (B).
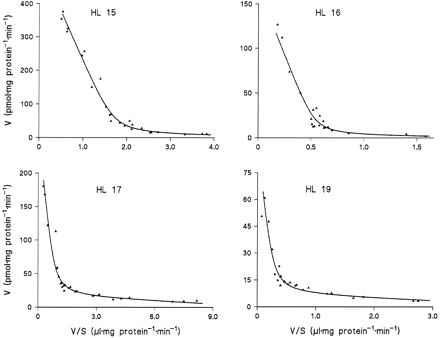
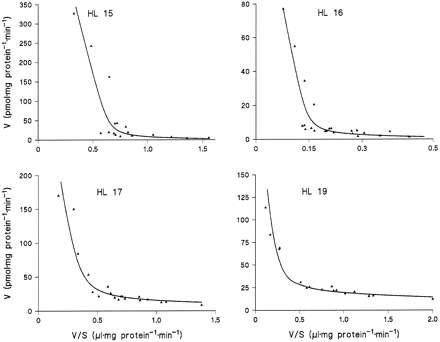
Microsomes from HL 15, 16, 17, and 19 were incubated in duplicate or triplicate with 10 to 13 different concentrations of amitriptyline and diphenhydramine and the data subjected to model analysis for two-enzyme Michaelis-Menten kinetics. With the assumption of common apparentKM values and differentVmax values for the four livers, the kinetic parameters listed in table 1 were obtained (goodness-of-fit:r2 = 0.995 with amitriptyline as substrate and 0.989 with diphenhydramine). Regression lines calculated from the kinetic parameters for Eadie-Hofstee plots fitted the experimental data very well (figs. 3 and 4).
Apparent kinetic parameters for the N-glucuronidation of amitriptyline and diphenhydramine in microsomes from four human liver samples (mean ± SD)
Eadie-Hofstee plots for the kinetics of amitriptyline N-glucuronidation in microsomes from four human liver samples.
The lines were calculated from the kinetic parameters listed in Table1.
Eadie-Hofstee plots for the kinetics of diphenhydramine N-glucuronidation in microsomes from four human liver samples.
The lines were calculated from the kinetic parameters listed in Table1.
The high-affinity component exhibited higherVmax/KM values than the low-affinity enzyme(s) in all cases (table 1), and its contribution to totalVmax/KM ranged between 72 and 97%. Calculated glucuronidation rates by the high-affinity enzyme exceeded those by the low-affinity enzyme at amitriptyline concentrations below 3 μM (HL 15)to 27 μM (HL 19) and at diphenhydramine concentrations below 4 μM (HL 15) to 36 μM (HL 17).
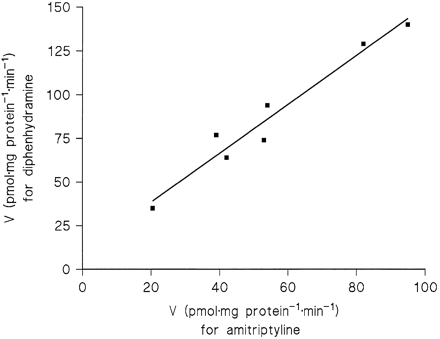
A significant correlation (r = 0.95, p= 0.05) between the Vmax2 values for amitriptyline and diphenhydramine across the four liver samples pointed to conjugation of the two substrates by the same enzyme. Measurements including three additional liver samples (HL 18, 20, and 21) at substrate concentrations of 31–500 μM were in accordance with this conclusion, because at equal fractions of theirKM2 values, the two substrates exhibited close correlations of their reaction rates. Since theKM2 value of diphenhydramine was 4-fold that of amitriptyline, correlations were calculated betweenN-glucuronidation rates in the seven liver samples for the following substrate concentrations (corresponding to 10%, 20%, or 40% of KM2): amitriptyline 31 μMvs. diphenhydramine 125 μM (r = 0.99,p < 0.001), 62.5 μM vs. 250 μM (r = 0.98, p < 0.001, fig.5), and 125 μM vs. 500 μM (r = 0.94, p < 0.002).
Correlation of the N-glucuronidation rates of 62.5 μM amitriptyline and 250 μM diphenhydramine in microsomes from seven human liver samples (r = 0.98, p < 0.001).
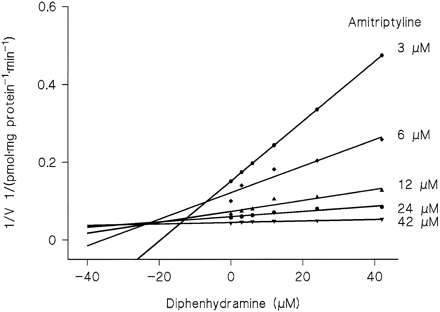
Although the Vmax1 values for amitriptyline listed in table 1 failed to correlate with those for diphenhydramine, cross-inhibition of N-glucuronidation took place when both substrates were present at low concentrations. Since the Dixon plot for the inhibition of amitriptyline conjugation by diphenhydramine in HL 19 microsomes (fig. 6) was suggestive of competitive inhibition, the data were tentatively analyzed by a two-enzyme model with competitive inhibition using the parametersKM2 and Vmax2of HL 19 (table 1) for the low-affinity enzyme. Calculated values wereKi1 6.3 ± 1.2 μM,KM1 3.7 ± 0.7 μM, andVmax1 13.4 ± 0.9 pmol·mg protein−1·min−1.
Dixon plot showing the inhibition of amitriptyline glucuronidation by diphenhydramine in microsomes from HL 19.
Discussion
In accordance with previous investigations (16-20),N-glucuronidation activity toward tertiary amine drugs was detected in microsomes from each one of the seven human liver samples examined. Some discrepancies became apparent with regard to published kinetic data for amitriptyline glucuronidation. Whereas a constant reaction rate up to 60 min incubation (17) could be confirmed now, the rate measured at pH 8.0 was linear neither with protein concentration beyond 0.5 mg/ml nor with substrate concentration up to 1 mM as has been reported for experiments at pH 8.4 (17). The present findings on optimal incubation conditions are in accordance with those for imipramine glucuronidation (18). Marked variations in amitriptyline conjugation rates among liver samples can be seen in fig.5 and from theVmax values in table 1. They are in accordance with a previous investigation (17) and correspond to pronounced interindividual differences in amitriptyline N-glucuronide excretion in patients with reported minimal and maximal values of 2.5% (7) and 21% of the dose (8).
The only KM value reported for amitriptylineN-glucuronide formation in human liver microsomes is 800 μM (20) and thus is even higher than the KMvalue of the low-affinity enzyme determined now. Biphasic kinetics have, to our knowledge, been described for ketotifen only that in incubations without Triton X-100 activation wasN-glucuronidated with apparent KMvalues of 12.5 and 100 μM, whereas in the presence of Triton a singleKM of 42 μM was measured (16). In the present series, data conforming to two-enzyme kinetics for amitriptyline and diphenhydramine N-glucuronidation were obtained in the presence of Triton. This points to the participation of more than one UGT isozyme in the conjugation of tertiary amine drugs. The high-affinity component has not been noticed in most of the previous investigations because of the use of high substrate concentrations (17,18, 20). According to the present data, this component can be expected to participate in drug conjugation, with higher efficacy at the low therapeutic concentrations prevailing in vivo. Thus, unbound amitriptyline and imipramine concentrations in plasma of patients receiving conventional doses hardly exceed 100 nM (29, 30), and these are assumed to reflect unbound concentrations in organs. The need to carry out drug metabolism studies in vitro at concentrations similar to those occurring in vivo to obtain valid information about the enzymes involved has been stressed recently by Kato and Yamazoe (31).
Whether the UGT isozymes conjugating amitriptyline and diphenhydramine are identical cannot be decided unambiguously on the basis of the present data. A common low-affinity enzyme is suggested by the significant correlation of Vmax2 values across four liver samples and by the close correlations of reaction rates at concentrations corresponding to equal fractions ofKM2. On the other hand, the reaction rate increased linearly with the protein concentration up to 2 mg/ml with diphenhydramine, but only up to 0.5 mg/ml with amitriptyline. In addition, Triton X-100 stimulated amitriptyline glucuronidation markedly more than that of diphenhydramine. This also applied to substrate concentrations of 9 μM for which the calculated contributions of the high-affinity enzyme are 66% in amitriptyline and 76% in diphenhydramine conjugation. The competitive inhibition of amitriptyline conjugation by diphenhydramine would, however, be in favor of the involvement of a common high-affinity enzyme.
One of the enzymes conjugating the two drugs is probably identical with UGT1.4, but the available data does not indicate which one. The expressed UGT1.4 conjugated imipramine, chlorpromazine, clozapine, and loxapine at their tertiary amino groups with apparentKM values of 310, 93 (22), 98, and 27 μM (23), respectively, the first three values being compatible with a low affinity towards tertiary amine drugs. Among such drugs, trifluoperazine was conjugated at the highest rate (23), whereas noN-glucuronide could be detected in urine from three patients under treatment with this drug (11). A possible explanation is that trifluoperazine is a substrate of the low-affinity UGT only that does not contribute measurably to in vivo metabolism. A remarkable feature is the strong inhibition that detergents, and particularly Triton X-100, exerted on the activity of expressed UGT1.4. The arrangement of the expressed enzyme in the membranes of the cultured cells may be different from that of native UGTs in human liver cells. Purification of active N-glucuronidating enzymes and/or expression of further isozymes will help to clarify these questions. To obtain knowledge on the UGTs relevant forN-glucuronide formation in vivo, emphasis should be placed on investigations at low substrate concentrations.
Acknowledgments
We are indebted to Prof. Dr. K.W. Bock and Prof. Dr. H. Bisswanger for valuable discussions and to Prof. Dr. W. Lauchart, Department of Surgery, University of Tübingen, for providing liver samples. Thanks are due to Mr. K. Nill for expert help with analytical techniques.
Footnotes
-
Send reprint requests to: Dr. Ursula Breyer-Pfaff, Department of Toxicology, University of Tübingen,Wilhelmstrasse 56, D-72074 Tübingen, Germany.
- Abbreviations used are::
- HL
- human liver
- UGT
- UDP-glucuronosyltransferase
- Received August 2, 1996.
- Accepted November 25, 1996.
- The American Society for Pharmacology and Experimental Therapeutics