Abstract
The metabolic pathways of clozapine (CZ, Clozaril (Novartis Pharmaceuticals Corporation, East Hanover, NJ), 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine, a tricylic benzodiazepine neuroleptic which has a reduced risk of unwanted neurological effects, were determined in normal male volunteers after a single oral dose of 50 mg of [14C]CZ. There was no radioactivity in exhaled breath, and excretion of total radioactivity was approximately 50% in urine and 30% in feces; parent CZ was a minor component in the excreta. The metabolic profiles were determined in urine and feces using HPLC coupled with radioactivity monitoring. The major metabolic pathways were demethylation, oxidation of the aromatic ring in the 7- and 8-positions, and conjugation. The major urinary components were 8-hydroxy-deschloro-DCZ (desmethylCZ) and its glucuronide, 7-hydroxy-8-chloro-DCZ sulfate and CZ-NO (clozapine N-oxide). Minor amounts of CZ, 7-hydroxy-8-chloro-CZ glucuronide and DCZ were also present. In feces the major component was CZ-N-glucuronide. Urinary excretion of CZ-NO was more rapid than the products of aromatic ring hydroxylation and conjugation.
Clozapine, CZ1, (Clozaril), 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine, is a tricyclic benzodiazepine neuroleptic which has a reduced risk of unwanted neurological effects in comparison with other drugs (1) and is efficacious in treatment-resistant schizophrenics (2). To date, metabolism studies with CZ have been conducted in patient populations using HPLC with UV detection. The availability ofCZ and two metabolites, DCZ and CZ-NO,has enabled investigators to quantitate these analytes in plasma and urine (3-9). In addition, it has been reported that after a single oral dose of CZ-NO to one patient, CZ was present in plasma, which indicated that the interconversion of CZand CZ-NO in vivo was possible (10). Where new urinary metabolites (conjugates, hydroxylated derivatives, and products of the glutathione pathway) have been characterized and identified, no definitive quantitative data has been reported (9, 11-13). This study presents the results of the first investigation into the identification of the urinary and fecal metabolites of CZ in humans after a single oral dose with a carbon-14 labeled modification. In addition to evaluating the relative importance of the metabolites of CZin the excreta, quantitative data regarding the contributions of the routes of excretion of CZ and its metabolites are presented.
Materials and Methods
Chemicals.
The [14C]CZ (fig. 1) was prepared by the Radiosynthesis Laboratory of Novartis Pharmaceuticals Corporation, East Hanover, NJ. The 14C-label was introduced specifically into the 11-position of the tricyclic moiety (specific activity of 5.50 μCi/mg [Batch No. H-02835]). Radiochemical purity (>95%) was determined by inverse isotope dilution, and the chemical purity of the product was demonstrated by comparison of its melting point and infrared spectrum with those of an authentic sample. Synthetic reference compounds, CZ, CZ-NO, andDCZ, were provided by Novartis Pharmaceuticals, Inc., Basel, Switzerland. HPLC-grade solvents were used throughout. Metabolites were derivatized with TFAA (Aldrich); metabolites derivatized with TFAA were also silylated using Regisil (Regis Chemical Co., Martin Grove, IL).
Structure of [14C]CZ.
Radioactivity Counting.
The radioactivity in all samples was measured by scintillation counting in a Packard Tri-Carb Liquid Scintillation Spectrometer, Model 3320 or 460 (Packard Instrument Company, Downers Grove, IL). Aliquots (0.1 ml) of urine, urine and fecal extracts, and column fractions were counted directly by adding the sample to 10 ml of ACS (Amersham, Arlington Heights, IL) liquid scintillation cocktail. Fecal residues were combusted in a Packard Tri-Carb Sample Oxidizer, Model 306, prior to counting using Carbosorb and Permafluor (Packard Instrument Company). Aliquots of the breath sample (1 ml) were prepared for direct counting using 10 ml of ACS (Amersham). Feces samples were freeze dried followed by homogenization in a blender; aliquots (20–25 mg) were weighed onto filter paper for combustion. The residue from the methanol extraction of feces was analyzed similarly. Samples were corrected for quenching using the internal standard method.
Dosing and Sample Collection.
Six healthy male volunteers (19–35 years old, weight 58.5–73.6 kg) gave written informed consent following protocol approval by the local institutional review board. Their vital signs and electrocardiograms were within normal limits, and blood and urine laboratory values were within 10% of the normal range. The subjects received no radioactivity in any form within one year prior to the study, nor concomitant medication during the study. All subjects fasted for 8 hr before and 2 hr after drug administration. At 8:00 a.m. on the treatment day, each subject received orally 50 mg of [14C]CZdissolved in 2 oz. of water; this was followed by ∼4 oz. of water. At 96 hr post-dosing, each subject received a capsule containing 250 mg of carmine red which served as a stool dye marker. Quantitative urine collections were obtained for each subject for the 0–3, 3–6, 6–9, 9–12, 12–24, 24–36, 36–48, 48–72, 72–96, 96–120, and 120–144 hr intervals. All feces passed during the 0–24, 24–48, 48–72, 72–96, 96–120, and 120–144 hr intervals were collected in separate plastic bags and immediately frozen. Breath samples (10 liters) from each subject were collected immediately before and at 0.5, 1, 2, 3, 4, 6, 9, 12, 24, 36, 48, 72, 96, 120, and 144 hr after dosing. All samples were stored frozen until analysis. To assist in structure elucidation, overnight urine samples were collected from male psychiatric patients that received 400–600 mg/day of CZ.
Metabolite Profiles.
Metabolite profiles in the pooled urine of the six subjects and preparative separations were obtained by HPLC using a Perkin Elmer (Norwalk, CT) Series 3 liquid chromatograph. Solvent composition was changed using linear gradient elution with 0.075M NH4HCO3 (Solvent A) and acetonitrile (Solvent B) as the mobile phases. Separations were performed using Waters μBondapak C18 columns. Analytical separations used a 3.9 mm × 30 cm, 10 mm particle size and a flow rate of 2 ml/min; semi-preparative separations used a 7.8 μm × 30 cm column and a flow rate of 4 ml/min. The column effluent was monitored using a Perkin-Elmer LC-15 UV detector (254 nm). Synthetic standards (CZ, CZ-NO, and DCZ) were added to biological samples and co-elution of peaks was used to assist in the assignment of structures. For the analysis of urine and fecal extracts, the following conditions were employed. After the column was equilibrated with 99.9% solvent A for 10 min, the solvent composition was changed to 70% A in 25 min after which the composition was isocratic for 10 min. The composition was changed to 40% A in 30 min after which the composition was changed to 0.1% A and held constant for 10 min. The column effluent was collected in 0.8 min intervals, mixed with 10 ml of scintillation cocktail, and counted for radioactivity.
Urinary Metabolite Profiles.
The metabolite profile was evaluated in the 0–72 hr time interval; this sample represented an average of 92% of the urinary excretion. The pooled urine from the 0–3 hr and 48–72 hr intervals from these subjects was also prepared to investigate the composition of rapidly excreted metabolites (early time interval) and the more slowly excreted metabolites (late time interval).
Characterization of Metabolites in Solvent Extracts of Urine.
The urine of the six subjects (0–72 hr time interval) was extracted with Amberlite XAD-2 resin (Rohm & Haas, Philadelphia, PA). After the metabolites were recovered from the resin by extraction with methanol, the extract was evaporated to dryness. The residue was dissolved in water and extracted at basic and acid pHs using chloroform-IPA as previously described (9). The two organic extracts and unextractable metabolites in the aqueous remainder were reduced in volume for analysis by HPLC.
Preparation of Fecal Samples.
An aliquot (20% by weight) of the 24-hr fecal collections from each subject was combined to give a sample that represented 95% of the total fecal excretion in the 0-144-hr interval. Time intervals that represented less than 5% of the dose of the total fecal excretion were not included. Each fecal sample was exhaustively extracted with methanol. The extracts of each subject were combined and the remaining fecal residue was dried.
Isolation of Urinary Metabolites.
The urine (0–72 hr) from four subjects and urine samples from eight psychiatric patients were combined and extracted with Amberlite XAD-2 resin. The methanol extracts of the resin were used to isolate and identify CZ and metabolites 1, 6,8, and 9. Metabolite 3 was isolated using a similar procedure (the urine of three subjects was mixed with the urine of 19 psychiatric patients). The methanol extracts were evaporated to dryness and redissolved in water. The aqueous sample was extracted with ethyl acetate (3 × 150 ml) followed by three extractions with chloroform-IPA (3 × 150 ml; 3:1;v/v), the remaining aqueous portion was used to isolate 3.
Isolation of Fecal Metabolites.
Fecal extracts of three subjects were combined, evaporated to dryness, suspended in water, and extracted with XAD-2 resin. The methanol extract of the resin was evaporated to dryness and the residue was resuspended in water. After extraction with ethyl acetate (3 × 100 ml) and chloroform-IPA (3 × 100 ml, 3:1;v/v) the remaining aqueous fraction was separated using the following gradient program: 99.9% A for 20 min followed by a linear gradient to 70% A in 25 min and then isocratic for 1 min followed by an immediate change to 0.1% A. The major component was isolated and incubated with Glusulase (DuPont Pharmaceuticals, Wilmington, DE) after which the sample was extracted with ethyl acetate. The ethyl acetate extracts were evaporated in vacuo for analysis. HPLC was performed at a flow rate of 2 ml/min employing the following gradient: isocratic at 99.9% A for 20 min followed by a linear gradient over 25 min to 70% A. After 1 min the solvent composition was changed to 0.1% A. The column effluent from the UV detector was passed in totothrough a Berthold Model 503HS radioactivity monitor operated in the heterogeneous counting mode. Analysis by TLC was achieved on silica gel 60 using CHCl3:methanol:diethylamine (8:0.5:0.5;v/v/v). Radioactivity was located using a Berthold Linear Analyzer LB2832 (Berthold Instruments, Nashua, NH).
Combined Glucuronidase/Sulfatase Enzyme Hydrolysis.
Samples were incubated at 37°C for 2–3 hr at pH 5 with Glusulase (DuPont Pharmaceuticals) containing 90,000 units of β-glucuronidase and 10,000 units of sulfatase.
GC-RAM Analysis.
A Perkin-Elmer Sigma 3B gas chromatograph coupled to a Berthold Instruments Gas Proportional Counter equipped with a model BF 2304 rate meter and a Nuclear Chicago Combustion oven was used. Separations were achieved on a glass column (6′ × 1/4“ ID 2% OV-1 on Chromosorb WHP 100/120 mesh). The program used was 250°C for 5 min followed by 2°/min to 290°C. The carrier gas (helium) flow rate was 50 ml/min and the propane gas flow rate of the gas proportional counter was 1.2 ml/min.
Spectroscopy.
Proton and carbon-13 NMR spectra were recorded at 200 MHz in CDCl3 or CD3OD using a JEOL FX-200 Spectrometer. Low resolution EI mass spectra were recorded on a LKB-9000 by direct inlet. Samples (metabolites or standard) were also analyzed by DCI with isobutane or ammonia as the CI reagent gas and by FAB (VG 7070E) using thioglycerol or glycerol as the matrix material. GC-MS (VG 7070E) in either the EI mode or isobutane CI mode of ionization used a Hewlett-Packard (Avondale, PA) 12-m fused silica capillary column with OV-101 stationary phase. The column was directly coupled to the source without a separator.
Results
Excretion of Total Radioactivity and Parent Drug.
There was no radioactivity detected in exhaled air. Excretion of total radioactivity (table 1) was slow with 30% of the dose being recovered in the urine in the first 24 hr; a negligible portion of the dose was recovered in feces in this time interval. The overall recovery (0-144 hr) of total radioactivity was ∼50% in the urine and ∼30% in the feces. CZ was a minor component in urine (0.5% of the dose; 0–72 hr pooled samples) and feces (2% of the dose; 0–144 hr pooled samples).
Excretion of radioactivity (mean ± SD, N = 6) after a single 50 mg oral dose of [14C]CZ
Characterization and Quantitation of Metabolites.
The chromatographic profile of the metabolites of CZ in urine is shown in fig. 2, panel A. The prominent metabolites were 1, 5, 6,and 8; metabolites 3 and 9 each represented less than 3% of the dose (table 2). After extraction of the feces, 13% of the dose remained in the fecal residue. The prominent metabolites in the fecal extract (fig. 2,panel B) were 3 and 7; metabolites8 and 9 each represented less than 2% of the dose (table 2). Peak 2 in urine and 4 (urine and feces) represented very minor components, and there were no attempts to quantitate and identify them.
Metabolite profiles of CZ in urine (A) and feces (B).
HPLC profile of pooled urine (direct injection) and fecal extract of six subjects. The pooled excreta represented 92% of the total excretion in urine and 95% of the excretion in feces. Column, Waters μBondapak C18 column, 3.9 mm × 30 cm, 10 μm particle size; Solvent A: 0.075M NH4HCO3 and Solvent B: acetonitrile; flow rate of 2 ml/min; the column was equilibrated with 99.9% A for 10 min. After injection of the sample the column was eluted as described in the experimental section until 99.9% B was attained; the metabolite pattern was obtained after collecting the column eluent in scintillation vials in 0.8 min intervals.
Estimation of metabolite recovery in urine and feces
Metabolites 8, 9, and CZ were extracted from urine at pH 8 and following acidification of the urine sample, only metabolite 6 was present in the organic extract. In the remaining aqueous portion, metabolites1, 3, 4, and 5 were present which indicated that they were most likely conjugated metabolites. The per cent composition of the metabolites in the extracts and remaining aqueous portion of the urine sample were in approximately the same ratios as found in urine (fig. 2, panel A). The metabolite patterns of the 0–3 hr (4% dose) and 48–72 hr (5.8% dose) collection intervals of urine were qualitatively similar (fig.3). However, 8 was the major component in the 0–3 hr time interval whereas in the 48–72 hr time interval1, 5, and 6 were the prominent components and 8 was minor.
Composition of metabolites in the 0–3 hr (early) and 48–72 hr (late) excretion time intervals.
Since the per cent dose of total radioactivity excreted in each time period were similar the results are presented as per cent urinary radioactivity.
Identification of Metabolites.
The basis for identification was coelution of available synthetic standards with 8 (CZ-NO), 9(DCZ), and CZ; the molecular ions (DCI) ofCZ, 8, and 9 was used for confirmation. Structures 1, 3, 5, and6 were determined from their mass spectra and, when possible, by interpretation of their NMR spectra. After extraction of urine with XAD-2 resin 91% of the radioactivity was recovered in the methanol extract; an additonal 9%, representing polar metabolites, was used to isolate and identify 3. The fecal extract contained 16.4% of the radioactvity with the remainder present in the fecal residue. Recovery of radioactivity during separations by HPLC was >90%.
Metabolite 1.
The molecular weight of 1 was 470 amu and ions at m/e of 294, 225, and 209 amu were present; the chlorine isotope cluster was not observed. The m/e of 294 amu corresponded to the loss of 176 amu which represented the loss of a glucuronic acid residue. The addition of a hydroxyl group to the benzodiazepine (209 amu) moiety accounted for the ions observed at m/e 225 and 209 amu. Treatment of 1 with Glusulase® gave a new peak with the retention time of 6which confirmed that it was a conjugate.
Metabolite 6.
The mass spectrum of 6, which showed no chlorine isotope cluster, established the molecular weight as 294 amu. Low resolution EI-MS gave two prominent fragment ions, m/e 209 and 85 amu. The ion at m/e 209 amu represented a hydroxylated benzodiazepine ring system similar to that observed for 1. The loss of m/e 85 amu from 294 amu corresponded to the loss of an unsubstituted piperazinyl ring and demonstrated that demethylation had occurred. The13C-NMR chemical shifts (table 3) for positions 1, 2, 3, 4 and 6 of metabolite 6 were almost identical to those of CZ. The chemical shifts for carbons 7and 9 were at lower field strengths indicating that substitution of a more electronegative atom for chlorine at the8-position. After treatment with TFAA and Regisil, a single component (GC-RAM) was observed; the m/e 558 (GC-MS)of this component corresponded to the introduction of two trifluoroacetyl groups and a TMS group.
13C-NMR shifts of CZ and metabolites 5 and 6
Metabolite 3.
The EI-MS of 3 established the molecular weight to be 342 amu and the chlorine isotope cluster was observed. The m/e of 342 amu corresponded to the addition of an oxygen atom to CZ however absence of m/e 326 indicated that this component was notCZ-NO which lost oxygen under the conditions of MS analysis. This indicated that 3 had a hydroxyl group most likely on an aromatic ring. FAB gave an m/e of 518 amu; the chlorine isotope cluster was observed. An ion of 342 amu was present and the loss of m/e 176 (glucuronic acid moiety) from the molecule would account for the ion observed in the EI-MS. In analogy to the substitution pattern of the chlorine atom and the hydroxyl group of 5, the structure for 3 is proposed.
Metabolite 5.
Mass spectral analysis (FAB) established the m/e of 408 amu as the molecular weight; the chlorine isotope cluster was observed. Also present in the mass spectrum was m/e 328 amu which corresponded to the loss of a methyl group from CZ (326 amu) and the addition of an oxygen atom. Enzymatic hydrolysis gave a new component with m/e of 328 (DCI); the chlorine isotope cluster was also present. The difference between m/e 408 and 328 represents the loss of SO3and indicated that the metabolite was present in urine as a sulfate conjugate. The 13C-NMR shifts (table 3) of the carbon atoms in positions 1, 2, 3, 4, and 9 of 5 were almost identical to that of CZ and indicated the presence of an electronegative atom at position 7.
Metabolite 7.7 ,
similar to 3, could not be extracted into organic solvents prior to treatment with Glusulase. After enzyme treatment of 7, CZ was isolated by HPLC and characterized by radio-TLC which suggested that it was most likely a conjugate and may have been theN+-glucuronide of CZ.
Discussion
In normal subjects a single oral dose of [14C]CZ was rapidly absorbed and, prior to excretion, extensively metabolized. The time to peak of parent drug was 2–3 hr with a peak concentration of 55 ng/ml; CZaccounted for ∼1/3 of the total radioactivity in blood at almost all time points and exhibited a half-life of 5.1 hr. This relatively short terminal half-life was confirmed in a subsequent study in which psychiatric patients received 75 mg of CZ (14). However, the t1/2 after multiple oral dosing was found to be 15.8 hr and the shorter half-life determined earlier was not predictive of the levels found in this study (15). Although the half-life after a single dose increased upon multiple dosing, suggesting the possibility of concentration dependent pharmacokinetics, it has been found that at steady state, linearly dose-proportional changes with respect to area under the curve, peak, and minimum clozapine plasma concentrations were observed after administration of 37.5 mg, 75 mg, and 150 mg b.i.d. which indicates that there is no concern (15). Since Michaelis-Menton kinetics were not followed, the difference may be a result of a pharmacokinetic compartment not apparent from the single dose data which has been reported for other antipsychotic agents. Alternatively, metabolites present in the systemic circulation may inhibit the biotransformation of CZ, giving rise to slower elimination at steady state (16).
In the earliest investigation of the metabolism of CZ in a patient population (9), urinary metabolites were identified by comparisons (TLC) with standards and characterized by their extraction at basic and acidic pHs. CZ, DCZ, andCZ-NO were extracted from urine at pH 8. Extraction of urine at an acidic pH yielded a single metabolite which was proposed to be a phenol derivative of DCZ based on its infrared spectrum and extraction characteristics. The remaining unextractable metabolites were conjugates since drug related material was extracted after treating the urine with β-glucuronidase and arylsulfatase. In this present study with [14C]CZ, these findings were confirmed. CZ, DCZ, and CZ-NOwere the only components in the basic extract of urine and 6was recovered from urine after acidification. The major unextractable metabolites were conjugates (1, 3, and5).
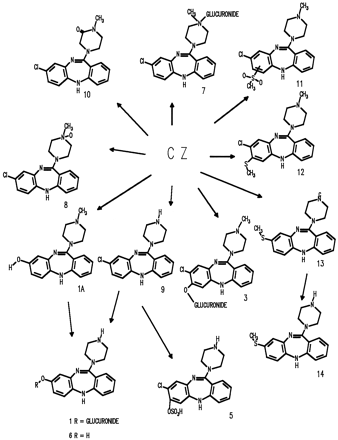
Mass spectrometry and NMR have been used to show that one of the metabolic pathways involved the loss of the 8-chloro substituent leading to 6, 8-hydroxy-11-(1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine (11). Metabolite 1A (fig. 4) was also identified and represents a minor component and can be considered as the precursor to 6. In addition, analysis of human urine by MS has demonstrated that pathways leading to products of the glutathione pathway were also present (11) but were not detected in this present study and are presumably minor components in urine and feces. Metabolites 3, 8, and 9 have been reported to be present in human urine (12, 17). TheN+-glucuronide of CZ, 7, was detected in human urine (13) and was not specifically detected in urine in this work.
The metabolites of CZ identified and characterized in humans to date are shown in fig. 4. The major phase I pathways were demethylation and hydroxylation of the benzodiazepine ring system primarily in the 7 and 8 positions; conjugation with both glucuronic acid and sulfate represented important pathways. Excretion ofCZ-NO occurred rapidly and was the major component in the earliest excretion time interval, whereas in the later time interval the hydroxylated metabolites and their conjugates predominated.
In urine the identified metabolites accounted for 78% of the radioactivity (∼34% of the dose). There were at least five regions of radioactive materials in the chromatogram which represented minor components (∼9% of the dose). Identified fecal metabolites accounted for 9% of the dose with the remaining radioactive regions in the chromatogram accounting for an additional 6% of the dose. The balance of the radiolabel was present in the fecal residue which together with the identified metabolites accounted for 85% of the radioactivity in the fecal sample.
Excretion of the radiolabel into urine and feces was evaluated (0–144 hr) and the recovery was found to be ∼80%; there was no additional radioactivity recovered in exhaled air. In general, excretion was slow with ∼ 30% of the dose (urine + feces) recovered in the first 24 hr. Similar recoveries of the radiolabel have also been observed for compounds which are eliminated, in part, via the feces. For example, the recovery of total radioactivity into urine and feces after an oral dose of [14C]dolasetron mesylate was 83.9% with 58.6% in urine and 25.3% in feces (18). Similar recoveries (49% of the dose in urine and 24% of the dose in feces) were also observed after an oral dose of [14C]nefazodone (19). For each time interval in these studies, there was a higher SD of the per cent dose recovered in feces than in urine which is similar to that found after an oral dose of [14C]CZ. This is most likely reflective of the higher variability in the collection of feces and indicates the difficulties encountered in the homogenization of this material.
The studies investigating the isozymes involved in the metabolism ofCZ have not been extensive. Fluvoxamine, an inhibitor of CYP1A2 (20), has been associated with elevations in serum CZlevels in patients coadministered this compound (21, 22). Further evidence that CYP1A2 is involved with CZ metabolism is indicated from the finding that caffeine demethylation in vivo is correlated with the clearance of CZ (23). There is no evidence in vivo that CYP2D6 is implicated (24, 25), and ketoconazole (CYP3A4) inhibits the metabolism of CZ (26).
Drug-drug interaction studies with CZ have not been systematically evaluated. In patient populations it has been reported that the clearance of CZ is decreased when coadministered with fluoxetine (27), fluvoxamine (21, 22), and cimetidine (28). Clearance of CZ increases when coadministered with carbemazepine (21, 29) or phenytoin (30).
The metabolites of radiolabeled CZ in human urine and feces have been characterized, identified, and quantitated. In addition, the HPLC methodology coupled with radioactivity monitoring has assisted in the interpretation of the results obtained with cold methodologies applied to urine from earlier studies in patient populations. It is apparent from the adverse events reported in normal subjects who received a single oral dose of 25–50 mg of CZ (31, 32) that it is not appropriate to use this population in biopharmaceutic studies. There is a significant risk because its primary CNS effect leads to orthostatic hypotension. In addition, severe bradycardia and, in a few cases, cardiac arrest (31) have been reported. With appropriate modifications of the methodologies elaborated in this present study, metabolites in the biological fluids of patient populations should now be able to be more completely characterized and identified.
Acknowledgments
The clinical portion of this study was conducted under the supervision of Dr. G. Honigfeld and Dr. J. Kane. We thank Mr. K. C. Talbot for the synthesis of 14C-clozapine, Dr. M. Shapiro for obtaining the NMR spectra and the assistance in their interpretation, and Dr. E. Fu for providing the mass spectra and assisting in their interpretation.
Footnotes
-
Send reprint requests to: Dr. Jeremy G. Dain, Drug Metabolism and Pharmacokinetics, Novartis Pharmaceuticals Corporation, Route 10, East Hanover, NJ 07936.
- Abbreviations used are::
- CZ
- clozapine
- DCZ
- N-desmethyl-clozapine
- CZ-NO
- clozapine N-oxide
- TFAA
- trifluoroacetic anhydride
- TMS
- trimethylsilyl
- DCI
- desorption chemical ionization
- EI
- electron impact
- FAB
- fast atom bombardment
- GC-MS
- gas chromatography-mass spectrometry
- GC-RAM
- gas chromatography-radioactivity monitor
- IPA
- isopropyl alcohol
- TLC
- thin-layer chromatography
- Received October 28, 1996.
- Accepted February 4, 1997.
- The American Society for Pharmacology and Experimental Therapeutics