Identification, Excretion, and Stereochemistry of Urine Metabolites
Abstract
Nine urinary metabolites of selegiline hydrochloride [N-methyl-N-propargyl(2-phenyl-1-methyl)ethylammonium chloride], a monoamine oxidase inhibitor, after administration to humans were identified. Their identities were confirmed by comparison of the spectra from GC/MS of peaks with those of authentic compounds. The following metabolites and unchanged drug (selegiline) were detected in urine: (R)-desmethylselegiline, (R)-methamphetamine, (R)-amphetamine, (1S,2R)-norephedrine, (1R,2R)-norpseudoephedrine, (1S,2R)-ephedrine, (1R,2R)-pseudoephedrine, (R)-p-hydroxyamphetamine, and (R)-p-hydroxymethamphetamine. The metabolites excreted 2 days after administration of 2.5–10 mg of selegiline hydrochloride amounted to 44–58% of the dose. Selegiline was metabolized by three distinct pathways: N-dealkylation, β-carbon hydroxylation, and ring-hydroxylation. The major metabolite was (R)-methamphetamine. During metabolism, no racemic transformation occurred and β-carbon hydroxylation showed apparently product stereoselectivity.
Selegiline is a selective, irreversible inhibitor of monoamine oxidase that has been used in combination withl-dopamine in the treatment of parkinsonism (1, 2).
There have been several reports of studies of selegiline metabolism either in vivo or vitro. Reynolds et al. (3) showed that methamphetamine and amphetamine were excreted in human urine after oral administration of the drug. Later, Heinonenet al. (4) identified the three metabolites (methamphetamine, amphetamine, and desmethylselegiline) in human urine. Also, p-hydroxyamphetamine andp-hydroxymethamphetamine have been identified as additional urinary metabolites in the rat (5-7). However, nop-hydroxylated metabolite in humans has been identified, and neither unchanged drug nor β-hydroxylated metabolite was found in urine after administration of selegiline to humans or rats.
Selegiline has a chiral center on the α-carbon atom of the phenylethylamine moiety. There are no detailed studies on the stereoselective metabolism of selegiline in the literature (8, 9).
Ten metabolites, together with unchanged drug, were identified and quantified from human urine after administration of selegiline. Herein we also report on the stereochemical aspects of selegiline metabolism.
Materials and Methods
Chemicals.
(R)-Amphetamine sulfate, (S)-amphetamine sulfate, (S)-methamphetamine, and (1R,2R)-norpseudoephedrine · HCl1were purchased from Sigma (Deisenhofen, Germany). (1R,2S)-Norephedrine · HCl, (1S,2R)-norephedrine · HCl, (1R,2S)-ephedrine · HCl, (1S,2R)-ephedrine · HCl, and (1S,2R)-pseudoephedrine · HCl were purchased from Fluka (Neu-Ulm, Germany). Pure standards ofp-hydroxyamphetamine, p-hydroxymethamphetamine,p-hydroxynorephedrine, and p-chlorphentermine (internal standard) were provided by Tropon (Köln, Germany). Arylsulfatase/β-glucuronidase was provided by Serva (Heidelberg, Germany). Reagents were purchased from various sources: the chiral derivatizing reagent MTPA · Cl was from JPS Chimie (Bevaiz, Swiss); and diethylether, methanol, acetonitrile, TFA, sodium bicarbonate, and potassium carbonate were from Merck (Darmstadt, Germany). MSTFA, MTESTFA, and MBTFA were purchased from Sigma.
Drug Administration and Sample Collection.
10, 5, and 2.5 mg of selegiline · HCl (2, 1, and 0.5 tablets of Movergan, respectively; ASTA Pharma AG, Frankfurt, Germany) were orally administered to male volunteers. Subjects were four healthy males, aged 25–34. Urine samples were collected at various times over 72 hr and stored at 4°C.
Isolation of Unconjugated Metabolites.
To 3.0 ml of urine, ∼100 mg of sodium bicarbonate:potassium carbonate (2:1, g/g) and 50 ng of internal standard (p-chlorphentermine) were added. The metabolites were extracted with 8 ml of diethylether-tert butanol (7:1, v/v). The organic layer was transferred into a 15-ml glass centrifuge tube, with 0.4 ml of 0.06 M hydrochloric acid added. Extraction was performed by mixing for 5 min at 1200g, and the organic layer was aspirated and discarded. The aqueous layer was dried in a desiccator over phosphorus pentoxide:potassium hydroxide.
Isolation of Conjugated Metabolites.
3.0 ml of urine was adjusted to pH 5.2, with 1 ml of 0.2 M sodium acetate buffer, and incubated with 50 μl of arylsulfatase/β-glucuronidase from Helix pomatia (Serva) at 52°C for 5 hr. After cooling, the solution was neutralized with 5 M KOH and adjusted to pH 9.6 with 200 mg of sodium bicarbonate:potassium carbonate (2:1, g/g). Fifty nanograms of internal standard (p-chlorphentermine) was added. The procedure for the extraction of the hydrolyzed metabolites from urine is identical with that for the extraction of unconjugated metabolites.
Quantification of Selegiline and Its Metabolites (10).
The dry residue was dissolved in 50 μl of a mixture of acetonitrile:TFA (60:40, v/v) that contained 200 ppm of methyl orange. The mixture was titrated with MSTFA until the color of the reaction mixture changes from red to yellow. The sample was heated for 10 min at 60°C. Two drops of MBTFA were added to the reaction mixture, and the sample was heated for an additional 10 min at 60°C. After cooling, 2 μl of the solution was injected into the GC/MS system.
Calibration curves of peak area ratios of standard to internal standard against concentrations of standards were constructed over the concentration range of 1.0–1000 ng/ml of standards in the urine. Concentrations of the unknown samples were determined by comparison to peak area ratios from the standard curve.
Identification of the Stereoisomers (11).
The dry residue was dissolved in 50 μl of a mixture of acetonitrile:TFA (60:40, v/v) that contained 200 ppm of methyl orange. The mixture was titrated with MTESTFA until the color of the reaction mixture changed from red to yellow. The sample was heated for 10 min at 60°C. MTPA · Cl (5 μl) was added to the reaction mixture, and the sample was heated for an additional 5 min at 60°C. Two microliters of the solution was injected into the GC/MS system.
GC/MS.
All mass spectra were obtained with an HP 5890/5971A instrument, including an HP 9144 disk drive and an HP Think Jet printer. Separation was achieved with an HP fused-silica capillary column with crossed-linked 5% phenylmethylsilicone (SE-54) that was ∼17 min length, with a 0.2 mm internal diameter, and a 0.33 μl film thickness. The operating parameters of GC/MS were as follows: detector, mass selective detector in scan or SIM mode; ionization, electron impact mode; ionization potential, 70 eV; injector temperature, 280°C; interface temperature, 300°C; initial oven temperature, 100°C; ramp, 15°C/min; final oven temperature, 320°C for 2 min; carrier gas, helium at a flow of 1.0 ml/min; and split ratio, 1:8. The ions m/z 140 for amphetamine-N-TFA,m/z 154 for methamphetamine-N-TFA, m/z179 for norephedrine-N-TFA-O-TMS, norpseudoephedrine-N-TMS-O-TMS, ephedrine-N-TFA-OTMS, pseudoephedrine-N-TFA-O-TMS, m/z 206 forp-hydroxyamphetamine-N-TFA-O-TMS andp-hydroxymethamphetamine-N-TFA-O-TMS,m/z 178 for desmethylselegiline, and m/z 96 for selegiline were selected for GC/MS (SIM) detection. Full-scan mass spectra (m/z 40–550) were recorded for analyte identification.
GC with NPD.
All GC experiments were performed with a HP 5890 A gas chromatograph equipped with an NPD. GC temperature and column conditions were identical with those for GC/MS.
Results and Discussion
Identification of Metabolites.
To detect the urinary metabolites of selegiline, selegiline was administered to humans. The extracts from human urine were examined by GC-NPD and GC/MS as free form or derivatives. Identification of metabolites was conducted by comparison of the EI mass spectra and the gas chromatographic retention times of the extracted metabolites with those of authentic compounds. In this study, nine urinary metabolites and unchanged drug were identified.
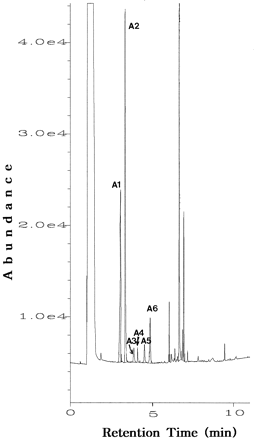
GC-NPD of the extract from human urine without derivatization gave six peaks (A1–A6) (fig. 1), that afterN-trifluoroacetylation, O-trimethylsilylation showed 10 peaks (B1–B10) as shown in fig. 2.
GC-NPD chromatogram of the metabolites in urine 2–4 hr after administration of selegiline.
Peaks: A1, amphetamine; A2, methamphetamine;A3, desmethylselegiline; A4, selegiline;A5, nor(pseudo)ephedrine; and A6, (pseudo)ephedrine.
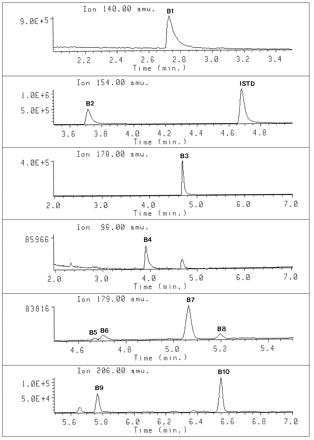
GC-SIM chromatograms of the metabolites in urine 2–4 hr after administration of selegiline with ions at B1 m/z 140, B2 m/z 154, B3 m/z 178, B4m/z 96, B5–B8 m/z 179, and B9–B10m/z 206.
Peaks: B1, amphetamine,N-TFA; B2, methamphetamine,N-TFA; B3, desmethylselegiline,N-TFA; B4, selegiline;B5, norpseudoephedrine,N-TFA,O-TMS;B6, norephedrine,N-TFA,O-TMS;B7, ephedrine,N-TFA,O-TMS;B8, pseudoephedrine,N-TFA,O-TMS;B9,p-hydroxyamphetamine,N-TFA,O-TMS; B10,p-hydroxymethamphetamine,N-TFA,O-TMS; and I, chlorpentermine,N-TFA (internal standard).
Peaks A1, A2, and A3 gave the same retention times as amphetamine, methamphetamine, and desmethylselegiline. B1, B2, and B3 gave the same retention times and diagnostic ions as the trifluoroacetylated derivatives of amphetamine, methamphetamine, and desmethylselegiline, respectively (fig. 3) From these results, A1 (B1), A2 (B2), and A3 (B3) were identified as amphetamine, methamphetamine, and desmethylselegiline.
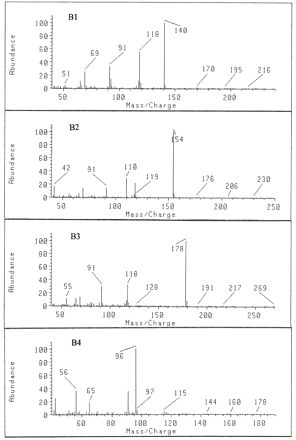
EI spectra of (B1) amphetamine,N-TFA; (B2) methamphetamine,N-TFA; (B3) desmethylselegiline,N-TFA; and (B4) selegiline.
Peaks A4 and B4 showed the same retention time and mass spectrum as authentic selegiline. Selegiline does not have any functional group to be derivatized by the described derivatization method, such as the amino, hydroxyl, or carboxylic group. The retention times and mass spectra before and after derivatization would be unchanged. The EI mass spectrum showed the base peak at m/z 96 and principal fragment ions at m/z 56, 91, 65, 97, and 115 (fig. 3).
Otherwise, peak A5 after selective derivatization gave two peaks (B5 and B6), and peak A6 gave two peaks (B7 and B8). Peak B6 had a longer retention time than peak B5, but no significant difference was between the mass spectra of the two peaks. The EI mass spectra of peaks B5 and B6 showed the base peak at m/z 179, and at the same retention times and diagnostic ions as the derivatives of norpseudoephedrine and norephedrine, respectively (fig.4). Also, peak B8 had a longer retention time than peak B7, but no significant difference was between the mass spectra of the two peaks. In EI mass spectra, peaks B7 and B8 gave the base peak atm/z 179, and at the same retention times and diagnostic ions as the derivatives of ephedrine and pseudoephedrine, respectively (fig.4).
EI spectra of (B5 and B6) nor(pseudo)ephedrine,N-TFA,O-TMS; (B7 and B8) (pseudo)ephedrine,N-TFA,O-TMS; (B9) p-hydroxyamphetamine,N-TFA,O-TMS; and (B10) p-hydroxymethamphetamine, N-TFA,O-TMS.
Peak B9 gave EI mass spectrum with a molecular ion at m/z319, and principal fragment ions at m/z 140, 179, and 206. The analytical data of B9 agreed with those ofp-hydroxyamphetamine (fig. 4).
The EI mass spectrum of B10 showed a molecular ion at m/z333 and diagnostic ions m/z 154, 179, and 206, and was identical to that of the derivative ofp-hydroxymethamphetamine (fig. 4).
Identification of the Stereoisomers of the Selegiline Metabolites.
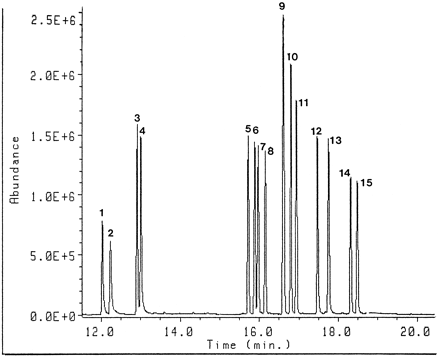
For the identification of the stereoisomers of the selegiline metabolites, the method of Shin and Donike (11)—applicable to the resolution and quantification of trace optical isomers containing more than one reactive functional group—was used. Figure5 shows the chromatogram of theN-MTPA,O-TES derivatives of the authentic standard. All enantiomers were well separated through conversion to the derivatives, but the derivatives of (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine were overlapped. The stereochemical identities of the metabolites in urinary extracts were confirmed by comparison of the gas chromatographic retention times of the diastereomeric derivatives of the extracted metabolites with those of the authentic standard. It appeared from this work that the metabolites identified—amphetamine, methamphetamine, desmethylselegiline, p-hydroxyamphetamine, andp-hydroxymethamphetamine—have the (R)-absolute configuration. We also identified the absolute configurations of the four β-hydroxylated metabolites. Their absolute configurations were (1S,2R)-norephedrine, (1R,2R)-norpseudoephedrine, (1S,2R)-ephedrine and (1R,2R)-pseudoephedrine. No peak of (1R,2S)-ephedrine or (1S,2S)-pseudoephedrine, which could not be separated by this method, was found in the extracted metabolites. These facts indicated that it was during metabolism of selegiline that no racemic transformation at the 2-carbon position occurred.
Gas chromatographic separation of N-MTPA(+),O-TES derivatives of the authentic standards on capillary column.
Key to peak numbering: 1, (+)-amphetamine; 2, (−)-amphetamine; 3, (+)-methamphetamine; 4, (−)-methamphetamine; 5, (+)-norpseudoephedrine;6, (−)-norephedrine; 7, (−)-norpseudoephedrine;8, (+)-norephedrine; 9, (−)-ephedrine and (+)-pseudoephedrine; 10, (+)-ephedrine; 11, (−)-pseudoephedrine; 12, (+)-p-hydroxyamphetamine; 13, (−)-p-hydroxyamphetamine; 14, (+)-p-hydroxymethamphetamine; and 15, (−)-p-hydroxymethamphetamine.
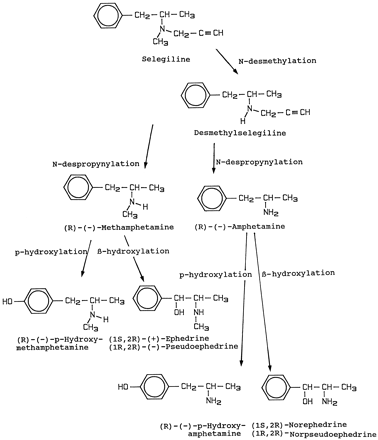
The main routes of metabolism of selegiline in humans are shown in fig.6.
Metabolic pathways of selegiline in humans.
Urinary Excretion of Metabolites.
The urinary excretion of the metabolites after oral administration of selegiline · HCl for the time period of collection (0–48 hr) is summarized in table 1. Selegiline was detected as free form. Determination of amphetamine, methamphetamine, and desmethylselegiline was conducted as trifluoroacetylated forms, whereas norephedrine, norpseudoephedrine, ephedrine, pseudoephedrine,p-hydroxyamphetamine, andp-hydroxymethamphetamine were determined asN-trifluoroacetylation-O-trimethylsilylation derivatives. From our present study, the detection limit of selegiline was ∼0.3 ng/ml, and those of the other metabolites were ∼0.1 ng/ml. There was a good linear relationship between the injection amounts and the detector response (r = 0.999). Amounts of conjugated forms were calculated from the difference in the amounts of metabolites present in the urine extract before and after hydrolysis with β-glucuronidase/arylsulfatase.
Urinary recovery (%) of metabolites of selegiline in humans (0–48 hr)
The major metabolite in urine was (R)-methamphetamine, accounting for ∼37% of the dose. β-Hydroxylation and aromatic hydroxylation were minor metabolic routes of selegiline in humans. Whereas the dealkylation of selegiline, as well as the β-hydroxylation of selegiline, lead to unconjugated excreted metabolites, most of the p-hydroxylated metabolites of selegiline were excreted as conjugate (57.3–77.3%).
The sum of the amounts of urinary metabolites in 2 days was 44–58% dose-independent of the amount of selegiline administered (table2). Otherwise, urinary excretion of dealkylated metabolites (48.67–48.88% of dose) by the first two subjects (Asians) was somewhat more than that (30.78–34.09% of dose) by the other two subjects (Europeans). Excretion of aromatic hydroxylated metabolites (4.84–8.77% of dose) by the first two subjects was somewhat less than that (12.42–17.54% of dose) by the latter subjects.
Urinary recovery (%) of metabolites of selegiline according to various doses
The creation of an asymmetric center by the benzyl hydroxylation reaction in this metabolism lead to the formation of two diastereoisomeric metabolites. (1S,2R)-(+)-isomers of norephedrine and ephedrine were excreted in greater amounts than (1R,2R)-(−)-isomers of norpseudoephedrine and pseudoephedrine. This metabolism showed product stereoselectivity.
Kinetic Studies.
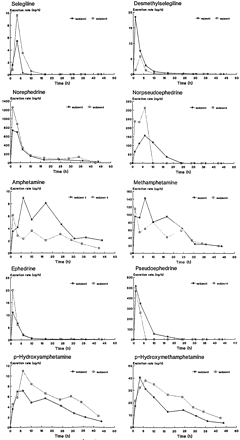
Our kinetic studies with the quantification of urine concentration of selegiline and the metabolites were performed after oral administration of 10 mg of selegiline · HCl. For selegiline, desmethylselegiline, amphetamine, methamphetamine, norpseudoephedrine, norephedrine, pseudoephedrine, and ephedrine, only the unconjugated excreted metabolites were analyzed. For the p-hydroxylated metabolites, the free and conjugated excreted metabolites were quantified. The excretion rate for selegiline and metabolites was calculated by division of the total amount via the urine collection interval. The excretion curves of the metabolites are presented in fig.7. Selegiline, desmethylselegiline, the β-hydroxylated metabolites have their highest excretion rate between 1 and 3.5 hr. Amphetamine, methamphetamine, and the p-hydroxylated metabolites have an excretion maximum between 3.5 and 6 hr. The maximal concentration of selegiline and its metabolites in urine was reached in a very short time after oral administration. This fact indicates that selegiline is readily absorbed and/or rapidly distributed and/or metabolized in urine.
Excretion of selegiline and its metabolites after an oral dose of 10 mg of selegiline · HCl.
It is important not only to identify active metabolites, especially those with a mechanism of action different from that of the parent drug, but also to detect the parent drug. Analysis of the pharmacokinetics and pharmacodynamics of selegiline to date was limited by the fact that, after oral administration of therapeutic doses, its concentration in urine is therefore too low to be detected by the available analytical method (12, 13).
Identification and detection of unchanged drug and new metabolites in this study may give especially useful new information for the studies of selegiline treatment.
Footnotes
-
Send reprint requests to: Prof. Ho-Sang Shin, Department of Environmental Education, Kongju National University, Kongju, Chungcheungnam-Do, Korea.
- Abbreviations used are::
- HCl
- hydrochloride
- MTPA · Cl
- (+-N-α-methoxy-α-(trifluoromethyl)-phenylacetyl chloride
- TFA
- trifluoroacetic acid
- MSTFA
- N-methyl-N-trimethylsilyl trifluoroacetamide
- MBTFA
- N-methyl-bis-trifluoroacetamide
- MTESTFA
- N-methyl-N-triethylsilyl trifluoroacetamide
- HP
- Hewlett-Packard
- SIM
- single ion monitoring
- TMS
- trimethylsilane
- NPD
- nitrogen-phosphorus detected
- EI
- electron impact
- TES
- triethylsilane
- Received April 23, 1996.
- Accepted February 17, 1997.
- The American Society for Pharmacology and Experimental Therapeutics