Catalytic, Immunochemical, and Structural Characterization
Abstract
The baculovirus expression vector system was used to overexpress human FMO3 in insect cells for catalytic, structural, and immunochemical studies. Membranes prepared from infectedTrichoplusia ni cell suspensions catalyzed NADPH-dependent metabolism of methyl p-tolyl sulfide at rates 20 times faster than those obtained with detergent-solubilized human liver microsomes. Sulfoxidation of the methyl and ethyl p-tolyl sulfides by recombinant human FMO3 proceeded with little stereochemical preference, whereas sulfoxidation of the n-propyl andn-butyl homologs demonstrated increasing selectivity for formation of the (R)-sulfoxide. This chiral fingerprint recapitulated the metabolite profile obtained when detergent-treated human liver microsomes served as the enzyme source. Catalytically active human FMO3 was purified to apparent homogeneity by cholate solubilization and sequential column chromatography on Octyl-Sepharose, DEAE-Sepharose, and hydroxyapatite. Purified FMO3 exhibited the same electrophoretic mobility as native microsomal enzyme, and immunoquantitation showed that this isoform represents ∼0.5% of human liver microsomal protein. Therefore, FMO3 is quantitatively a major human liver monooxygenase. LC/electrospray-mass spectrometry analysis of purified FMO3 identified >70% of the tryptic peptides, including fragments containing motifs for N-linked glycosylation and O-linked glycosylation. Although insect cells have the capacity for glycan modification, MS analysis of the tryptic peptides demonstrated that these sites were not modified in the purified, recombinant enzyme. Edman degradation of the recombinant product revealed that posttranslational modification of human FMO3 by insect cells was limited to cleavage at the N-terminal methionine, a process seen in vivo with animal orthologs of FMO3. These studies demonstrate the suitability of this eukaryotic system for heterologous expression of human FMOs and future detailed analysis of their substrate specificities.
Mammalian FMO is a microsomal FAD-containing enzyme system that oxygenates a variety of soft nucleophiles. For many years, only the major hog liver (FMO1) and rabbit lung (FMO2) forms of the enzyme were recognized, and extensive studies have been conducted on their mechanism of action, toxicological importance, and divergent physical and catalytic properties (1, 2 and references therein). The full extent of the multiplicity of this enzyme system, however, has become evident only in the last 5 years, with the molecular characterization of several additional families of the enzyme that are identified as FMO3, FMO4, and FMO5 in the new nomenclature (3).
FMO3 seems, qualitatively, to be a major form of the enzyme expressed in adult human liver (4-6), in contrast to many experimental animal species where FMO1 is the dominant hepatic isoform. In humans, FMO1 expression is most marked in fetal liver and adult kidney (5), and expression levels of FMO3 protein in these tissues are very low or absent (6). Therefore, human FMO3 is subject to both developmental regulation and organ-selective expression. FMO3 orthologs have been isolated from macaque, rabbit, and rat liver (6-8), but native human liver microsomal FMO(s) have yet to be purified, and quantitative determinations of FMO isoform content in human tissues have not been performed. However, catalytic studies conducted with membrane preparations from Escherichia coli expression systems are beginning to identify substrates for the human FMOs (9-14).
Previous functional studies that we performed with human liver microsomes suggested that adult human liver expressed a form of FMO that had little stereochemical preference for the oxidation of prochiral short-chain arylalkyl sulfides (15). This adult human liver microsomal activity was functionally distinct from the FMO complement present in human kidney microsomes and human fetal liver microsomes, both of which exhibited a high degree of stereoselectivity for the formation of (R)-sulfoxides from short-chain arylalkyl sulfides, such as methyl p-tolyl sulfide (15). Subsequent immunochemical and functional studies, conducted with purified and recombinant forms of FMO from monkey and rabbit, provided indirect evidence that this human isoform belonged to the FMO3 family (6, 16,17).
In the present studies, we have expressed human FMO3 (9) using the BEVS.1 Direct comparison of the stereochemistry of arylalkyl sulfoxides generated by baculovirus-expressed human FMO3 and solubilized human liver microsomes confirmed that FMO3 is a major FMO isoform involved in these reactions in human liver. In addition, the high levels of expression attained with the BEVS facilitated the purification of human FMO3 from infectedT. ni cells. This, in turn, permitted immunoquantitation of human liver microsomal levels of this isoform and an evaluation of the extent of posttranslational processing of human FMO3 by the eukaryotic BEVS.
Materials and Methods
Reagents.
Restriction enzymes were supplied by either Boehringer Mannheim (Mannheim, Germany), GibcoBRL (Grand Island, NY), or New England Biolabs (Beverly, MA), and were used in buffer systems provided by the manufacturers. High-Five T. ni cells were obtained from GibcoBRL. Deoxyoligonucleotides were purchased from GibcoBRL or synthesized at the University of Washington Biopolymer Facility. HyQCCM-3 media was obtained from Hyclone Laboratories (Logan, UT). Arylalkyl sulfides and their corresponding sulfoxides were synthesized as described previously (16, 17). Optima grade HPLC solvents were obtained from Fisher Scientific (Pittsburgh, PA). Emulgen 911 was obtained from Kao Corporation (Tokyo, Japan). Octyl-Sepharose, sodium cholate, and sequencing grade trypsin were obtained from Sigma Chemical Co. (St. Louis, MO). DEAE-Sepharose Fast-Flow was purchased from Pharmacia (Piscataway, NJ), and Hypatite C was obtained from Clarkson Chemical Co.
General Methods.
E. coli XL-1 Blue (GibcoBRL) was used for all subcloning transformation experiments. Bacteria were grown on tryptone/yeast extract medium (1% tryptone/0.5% yeast extract/1% NaCl) containing 100 μg/ml ampicillin and/or 15 μg/ml tetracycline. Preparation and transformation of competent cells and agarose gel electrophoreses were performed by standard procedures (18). DNA fragments were isolated from agarose gels by electroelution (International Biotechnologies Inc., New Haven, CT; protocol for Unidirectional Electroeluter Analytical component). PCRs were performed in a Perkin-Elmer DNA Thermal Cycler using Taq DNA polymerase and buffers provided by Boehringer Mannheim. The sequences of PCR primers for amplification of baculoviral DNA were identical to those provided in the BacPAK kit (Clonetech Laboratories, Inc., Palo Alto, CA). Sequencing of DNA fragments was conducted with plasmid DNA using the Sanger dideoxy method (19) using enzyme and reagents provided with the Sequenase system (United States Biochemical Corp., Cleveland, OH).
Construction of Recombinant Baculovirus.
Human FMO3, in the E. coli expression vector pJLFMO3, was digested with XbaI/HindIII to liberate a 1.7 kb fragment that was subsequently ligated intoXbaI/HindIII-cleaved pFastBac (Gibco). In this donor plasmid, pFBFMO3, the FMO3 coding sequence is placed behind the viral polyhedrin promoter. Elements of E. coli transposon 7, located on the donor plasmid, helper plasmid, and bacmid DNA direct the site-specific insertion of FMO3 into recombinant bacmid. Selection was achieved by blue/white screening of bacterial colonies due to the disruption of the lacZα peptide during the recombination process. Bacmid DNA, containing the low copy-number mini-F replicon plus a kanamycin resistance marker was propagated in E. coli. High molecular weight bacmid DNA was isolated and used to transfect insect cells for the generation of infectious viral particles. Viral stocks, amplified from the transfected cells, were used to infect fresh T. ni cultures for expression of human FMO3.
Baculovirus-Mediated Expression in Suspension Culture.
Growth and passage of T. ni cells were conducted at 27°C on 100 mm culture dishes using HyQCCM-3 media. In all cases, media were supplemented with 8–10% heat-inactivated fetal bovine serum obtained from Sigma. Antibiotic and antifungal agents were included routinely at the following final concentrations: penicillin-G, 100 μg/ml; streptomycin sulfate, 61 μg/ml; and amphotericin-B, 0.6 μg/ml. Suspension cultures were innoculated directly from culture dishes and were grown in batches of 250–300 ml in 2-liter Erlenmeyer flasks using a spin bar on a magnetic stir platform. Vigorous stirring was necessary to reduce cell clumping and to ensure an adequate oxygen supply to the cells. Infection was conducted at a cell density of 0.8–1.2 × 106 cells/ml using a high virus:cell ratio (multiplicity of infection > 10). Cells were pelleted ∼3 days postinfection, resuspended, and washed once in glycerol-containing storage buffer (50 mM potassium phosphate, 20% glycerol, 1 mM EDTA; pH 7.4), repelleted, and stored at −80°C until further use.
Preparation of Microbial Membranes.
Insect cells were disrupted by five passes in a tightly fitting glass/Teflon homogenizer in 10 mM potassium phosphate, 1.15 M KC1, 10 mM EDTA, 0.2 mM PMSF (pH 7.5). The suspension was centrifuged at 1,000g for 10 min to remove large cellular debris, and the supernatant recentrifuged at 100,000 g for 60 min to sediment insect cell membranes. This pellet was washed with 100 mM pyrophosphate, 1 mM EDTA (pH 7.5) and the membranes sequestered as a concentrated suspension (10–20 mg/ml) in storage buffer at −80°C until use. E. coli membranes containing human FMO3 were prepared as described previously (9).
Purification of Human FMO3 from Insect Cell Membranes.
Cell membranes (∼150 mg protein) were solubilized by homogenization in 10 volumes of 100 mM potassium phosphate, 20% glycerol, 1 mM EDTA, 1 mM DTT, 0.2 mM PMSF, and 1% sodium cholate (pH 7.5). This mixture was stirred for 45 min on ice and then centrifuged at 100,000g for 60 min to remove insoluble material. The supernatant was adjusted to 6% polyethylene glycol 8000, stirred on ice for a further 30 min, and precipitated material sedimented by ultracentrifugation. The supernatant was dialyzed overnight against buffer containing 10 mM potassium phosphate, 1 mM EDTA, 0.1 mM DTT, 0.2 mM PMSF, 0.5 M NaC1, and 0.5% sodium cholate [pH 7.4 (buffer B).] The dialysate was clarified by centrifugation and loaded onto a 2.5 × 20 cm Octyl-Sepharose column equilibrated with 10 column volumes of buffer B. A yellow band accumulated at the top of the column and began to migrate slowly after the addition of 0.1% Emulgen 911 to buffer B. After the yellow band had moved half-way down the column (∼2 column volumes), it was eluted with 10 mM potassium phosphate, 20% glycerol, 1 mM EDTA, 0.1 mM DTT, 0.2 mM PMSF, 0.2% sodium cholate, and 1% Emulgen 911 (pH 7.4). FMO-containing fractions were pooled, dialyzed overnight against 10 mM potassium phosphate, 20% glycerol, 0.1 mM EDTA, 0.5% Emulgen 911 [pH 7.4 (buffer C)] and loaded onto a 1.6 × 10 cm DEAE-Sepahrose Fast-Flow column equilibrated with 10 column volumes of buffer C. The unbound fraction from this column was loaded directly onto Hypatite C equilibrated in buffer C. FMO bound tightly to this hydroxyapatite column, which was washed overnight with 25 mM potassium phosphate, 20% glycerol, 0.1 mM EDTA, and 0.5% sodium cholate [pH 7.4 (buffer D)]. The UV absorbance of the column effluent was monitored at 280 nm, and when it dropped below 0.02 absorbance units, FMO was eluted with 250 mM potassium phosphate in buffer D. Concentrated fractions of human FMO3 were dialyzed against 2 × 1-liter of 100 mM potassium phosphate, 20% glycerol, and 0.1 mM EDTA (pH 7.4) and stored in 0.5 ml aliquots at −80°C until use. FAD concentrations were measured by the fluorimetric method of Faeder and Siegel (20).
Human Liver Preparations.
Microsomes were prepared from eight liver samples (HL102, HL103, HL106, HL109, HL112, HL123, and HL131) stored in the Human Liver Bank in the Departments of Medicinal Chemistry and Pharmaceutics at the University of Washington. Samples designated HL102, HL109, HL123, and HL125 were obtained from male heart/kidney transplant donors aged 21, 46, 15, and 33 years, respectively. HL112 (male, 28 years) was obtained immediately at autopsy. HL103, HL122, and HL131 were from female transplant donors aged 15, 50, and 62 years, respectively. HL122, HL123, HL125, and HL131 had been treated with the cytochrome P450 inducers dexamethasone and phenytoin. No significant drug histories were recorded for the other samples. No unusual liver pathology was associated with any of the samples.
Assay for Sulfoxide Formation.
Incubation mixtures contained 100 mM glycine, 25 mM potassium pyrophosphate (pH 8.5), 0.1–0.3 mg T. ni or E. coli membrane protein, and 0.5 mM NADPH in a final volume of 1.0 ml. Reactions were initiated by the addition of methyl, ethyl, propyl, or butyl p-tolyl sulfide (1 mM) in 10 μl of methanol, and terminated after a 30-min incubation at 37°C by the addition of 7 ml dichloromethane. Ethyl p-tolyl sulfoxide or propylp-tolyl sulfoxide (5 μg) was added as an internal standard, and the reactions were extracted and analyzed for rates of formation of alkyl p-tolyl sulfoxides as described previously (16). Human liver microsomal protein (3 mg/3 ml incubation)—from tissue samples HL106, HL109, and HL125—were solubilized in buffer containing 0.1% Lubrol PX and 10% glycerol as detailed previously (15), and assayed for NADPH-dependent sulfoxide formation. In some experiments, T. ni cell membranes containing human FMO3 were also treated with detergent before incubation with the sulfide substrates. The prochiral selectivity of human FMO3-dependent arylalkyl sulfoxide formation was determined by chiral-phase HPLC using a Chiralcel OB stationary phase (15, 16).
Structural Analysis of Purified Human FMO3 by LC/ES-MS.
Trypsin-generated peptides were analyzed by LC/ES-MS according to the following procedures. FMO3 (2 nmol) was digested in 1 ml of 100 mM ammonium bicarbonate (pH 8.1) with 1% w/w trypsin added in two aliquots, at 0 hr and at 1 hr, to ensure complete digestion. Digestions were allowed to proceed for 3 hr at 37°C, terminated by the addition of liquid nitrogen, and lyophilized. The digests were redissolved in 50–100 μl HPLC-grade H2O, and peptides separated by reversed-phase HPLC using a gradient of 0–90% acetonitrile in 0.05% trifluoroacetic acid. Mass analysis and peptide matching was conducted by LC/ES-MS using a Micromass Quattro II triple quadrupole fitted with a MegaFlow Electrospray source, as described previously (21). Selected-ion monitoring was performed at m/z 855 to search for the tryptic fragment corresponding to the peptide—SVFSN61SSK—that contains a consensus motif forN-glycosylation. This peptide was collected and submitted to infusion electrospray-MS/MS sequencing to confirm the identify of the peptide. A second LC/ES-MS/MS approach was also taken to monitor diagnostic oxonium ions, at m/z 204 and m/z 366, which result from glycans under CID/MS (22). Additional searches were conducted for ions at m/z 792, m/z 817, andm/z 1224, which correspond to three unmodified tryptic fragments that contain potential O-linked glycosylation sites at Thr29, Thr249, and Thr381(23).
Western Blot Analysis of Human Liver Microsomal FMO3.
Western blots of purified FMO3 standards (0.14–0.56 μg protein) and six human liver microsomal preparations [HL102, HL112, HL122, HL123, HL125, and HL131 (15 μg/lane] were probed with anti-macaque liver FMO IgG (1 μg/ml), which has been shown previously to be highly selective for FMO3 orthologs (6, 16). Anti-rabbit IgG alkaline phosphate conjugate was used as the secondary antibody (1:1,000) and immunoreactive protein visualized with the 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium phosphatase substrate system. Band intensities were quantitated by laser densitometry.
Other Methods.
SDS-PAGE (9% gels) and Coomassie staining were conducted as described previously (6). Protein determinations were made by the Lowry et al. (24) method. Edman analysis was conducted with 1 nmol of purified FMO3, using a gas-phase sequenator located in the Department of Biochemistry at the University of Washington.
Results
Membrane fractions were prepared from five separate T. ni cell cultures infected with the recombinant baculovirus containing the sequence for human FMO3 described recently by Itakagiet al. (9). Enzyme activity was determined toward the substrate probe, methyl p-tolyl sulfide, and the rate and stereochemistry of methyl p-tolyl sulfoxide generated from cDNA-expressed human FMO3 is shown in Table 1. Insect cell membranes containing recombinant human FMO3 catalyzed sulfoxidation at a rate of 66 ± 20 nmol product/mg membrane protein/min. This rate exceeds that observed in detergent-solubilized human liver microsomes by 20-fold and in transformed E. coliby 2- to 3-fold. Each enzyme source, however, formed methylp-tolyl sulfoxide with essentially identical stereoselectivity.
NADPH-dependent formation of methyl p-tolyl sulfoxide by human FMO3 expressed in insect cells, in E. coli, and by detergent-solubilized human liver microsomes
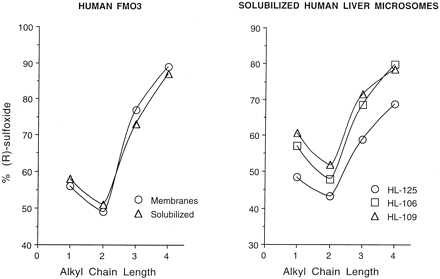
The chiral metabolite profile for baculovirus-expressed human FMO3-catalyzed sulfoxidation of a series of arylalkyl sulfides is shown in fig. 1 ((left panel). Incubations were conducted with untreated T. ni cell membranes and with detergent-solubilized membranes containing FMO3. The rate of arylalkyl sulfoxide formation decreased by 38 ± 6% in the presence of 0.1% Lubrol PX, but product stereochemistry was unaffected. Therefore, low concentrations of the nonionic detergent, Lubrol PX, do not significantly alter the stereochemistry of human FMO3-dependent sulfoxidation. Ethyl p-tolyl sulfoxide, like the methyl homolog, was formed with little stereochemical preference; but, the propyl and butyl sulfides were metabolized with increasing prochiral selectivity for formation of the (R)-sulfoxides. This chiral fingerprint is very similarly to that observed when detergent-solubilized human liver microsomes serve as the enzyme source (fig. 1, right panel), which suggests that the human FMO3 isoform expressed here in insect cells, or a functionally identical variant, is present in human liver microsomes.
Chiral metabolite profiles generated from a series of n-alkyl p-tolyl sulfides by human FMO3 expressed either in insect cells or present in detergent-solubilized human liver microsomes.
Metabolism of methyl, ethyl, n-propyl, andn-butyl p-tolyl sulfide was conducted either with untreated T. ni cell membranes or Lubrol PX-solubilized membranes (left), or with three different human liver microsomes, also solubilized with detergent (right). The NADPH-dependent rate of sulfoxide formation was determined by silica HPLC as described in Materials and Methods. Sulfoxide peaks were collected and stereochemistry determined by chiral-phase HPLC.
Preliminary studies revealed that solubilization of human liver microsomes with 1% w/v sodium cholate extracted 41–50% of the dimethylaniline N-oxidase activity (data not shown). Therefore, because substantial activity was maintained with this ionic detergent (in contrast to FMO1 forms), infected insect cell membranes were solubilized with 1% sodium cholate and FMO3 bound to Octyl-Sepharose. Although a very highly purified preparation was obtained after the first chromatography step (fig. 2,lane 2), further steps were used to remove minor contaminants and detergent to obtain preparations suitable for sequence analysis by LC/ES-MS. Purified FMO3 was obtained in 8% yield and exhibited a 4-fold enhancement in sulfoxidase activity/milligram protein, relative to cell membranes (table 2). The final preparation had a specific content of 6 nmol FAD/mg protein, which is indicative of substantial flavin loss during purification. Nonetheless, purified human FMO3 was highly active in the sulfoxidation of methylp-tolyl sulfide, exhibiting a turnover number of 34 min−1 based on holoprotein.
SDS-PAGE of human FMO3 isolated at different stages of purification.
Proteins were separated on a 9% SDS-PAGE gel and stained with Coomasie Blue. Lane 1, 10 μg of T. ni cell membrane protein; lane 2, Octyl-Sepharose pool; lane 3, DEAE-Sepharose pool; lanes 4 and 5, final detergent-free preparations: Lanes 2–5 contained 6–10 pmol of holo-FMO3, (∼1–1.5 μg protein).
Purification of human FMO3 from transfected T. ni cells
Western blot analysis demonstrated that human FMO3 purified from insect cells migrated with identical electrophoretic mobility to that of the native human liver microsomal isoform (fig. 3). Densitometric quantitation of band intensities indicated that the mean concentration of FMO3 in adult human liver microsomes was 5 ± 2.5 μg/mg. This corresponds to 80 ± 40 pmol/mg if it is assumed that all of the microsomal FMO3 is in the form of holoprotein.
Immunoblot of human liver microsomes probed with anti-FMO3 IgG.
Proteins were separated on a 9% SDS-PAGE gel, transferred to nitrocellulose, and probed with anti-macaque liver FMO IgG as described in Materials and Methods. Lanes 1, 2,3, and 10 contain purified human FMO3 standards.Lanes 4–9 contain 15 μg each of six different human liver microsomal samples.
Microsomal FMOs can be subject to a variety of postranslational modifications (reviewed in 25). Examination of the predicted amino acid sequence for human FMO3 (9) reveals a site for N-linked glycosylation at Asn61 [consensus motif; Asn—X—Ser/Thr] and at least three potential sites for O-linked glycosylation [putative consensus motif; X—Pro—X—X—, where at least one X = Thr] at Thr29, Thr249, and Thr381 (23). Because the BEVS is known to be capable of expressing O- and N-glycosylated forms of a wide variety of recombinant proteins (26, 27), we examined purified FMO3 for glycan modification by LC/ES-MS.
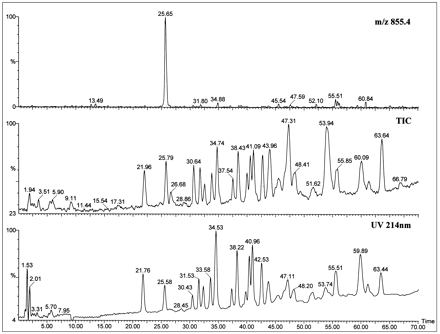
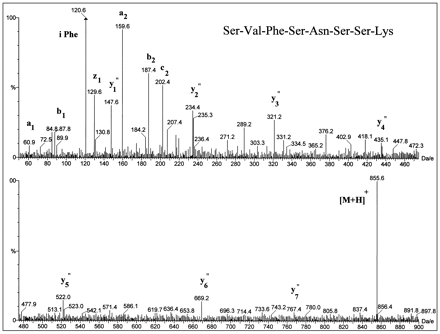
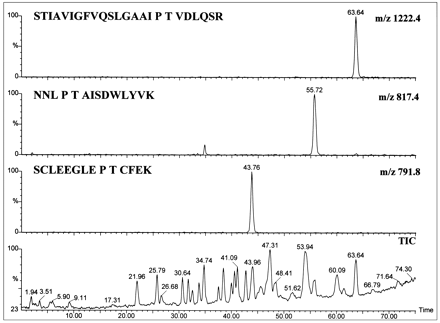
FMO3 was dialyzed, treated with sequencing grade trypsin, and the peptides separated by reversed-phase HPLC. After UV detection at 214 nm (fig. 4, bottom panel), 90% of the column effluent was diverted to a fraction collector and 10% to the mass analyzer (fig. 4, middle panel shows the total ion chromatogram). Digestion of the detergent-free protein with trypsin, which cleaves at the C-terminal side of lysine and arginine residues, furnished the—SVFSNSSK—fragment that has a mass of 854 amu, for theunmodified peptide, and contains the peptide motif forN-linked glycosylation at Asn61. Selected-ion monitoring of the protonated molecular ion identified a single fragment of the correct mass that eluted at 25.65 min (fig. 4, top panel). This peptide was sequenced by tandem MS, and the entirey′-series of predicted fragments from the octapeptide were identified (fig. 5). In addition, the protonated doubly charged molecular ions, [M + 2H]2+, of three tryptic fragments that eluted at 43.8, 55.7, and 63.6 min, respectively, (fig.6) corresponded to unmodified peptides containing a —Pro—Thr—motif for O-linked glycosylation at Thr29, Thr249, and Thr381. Finally, no evidence could be found for diagnostic oxonium ions that result from glycans under CID/MS conditions (22). These data demonstrate that the catalytically active FMO3 protein product derived from the baculovirus expression system is neither N-glycosylated, norO-glycosylated.
LC/ES-MS analysis of a tryptic digest of recombinant human FMO3.
Purified recombinant human FMO3 was digested with trypsin and the peptides separated by HPLC as described in Materials and Methods. (Bottom) UV detection at 214 nm. (Middle) Total ion chromatogram (TIC). (Top) Selected ion chromatogram for m/z 855. This mass corresponds to the protonated molecular ion of the peptide —SVFSN61SSK—. Therefore, the peak eluting at 25.65 min likely contains this unmodified octapeptide.
CID mass spectrum of the human FMO3 tryptic fragment corresponding to the unmodified octapeptide, —SVFSN61SSK—.
The 25.65 min peak from fig. 4 was collected and reanalyzed by tandem MS as described in Materials and Methods to determine its sequence. The complete y′-series of ions is depicted, confirming the identity of the octapeptide and the absence of glycan modification.
Identification of potential O-glycosylation sites in human FMO3 by LC/ES-MS.
A tryptic digest of recombinant FMO3 was analyzed as in fig. 4, except that searches were conducted for ions with m/z ratios corresponding to the tryptic peptides containing potential threonine glycosylation sites. (Bottom) Total ion chromatogram (TIC). (Top three panels) Selected ion chromatograms form/z 791, m/z 817 and m/z 1222 that correspond to the FMO3 tryptic fragments containing unmodified Thr29, Thr249, and Thr381, respectively.
Finally, Edman degradation analysis revealed the following N-terminal sequence —GKKVAIIGA—, which indicates loss of the initiator methionine for the purified recombinant product, and an openN-terminus. In total, 77% of the sequence of purified human FMO3 could be identified by a combination of peptide matching andN-terminal sequence analysis.
Discussion
The main objectives of the studies described herein were to achieve sufficiently high overexpression of human FMO3 to permit purification of this isoform, and its subsequent immunochemical, catalytic, and structural characterization. Because three human FMO3 sequences that differ at between 2 and 20 amino acid positions have been reported (4, 5, 9), a full characterization of this recombinant gene product is particularly important if valid conclusions are to be made regarding its substrate specificity and physiological role.
We have recently used the BEVS for the high level expression of wild-type and variant forms of the human liver cytochrome P450 isoform, CYP2C9 (21). Recombinant CYP2C9 variants were readily purified by a single hydrophobic chromatography step, therefore, we explored the use of this methodology for the expression of human FMO3. Cell membranes prepared from serum-supplemented T. ni cells infected with a recombinant baculovirus expressed significant quantities of human FMO3. These membranes also catalyzed the sulfoxidation of the FMO substrate probe, methyl p-tolyl sulfide, at an average rate of 66 nmol/mg/min. Because the purified preparation had a turnover number of 34 min−1, it can be calculated that expression levels of FMO3 as high as 1.5–2 nmol/mg membrane protein were attained routinely with the BEVS.
Sulfoxide metabolite profiling studies performed previously with microsomes prepared from human liver suggested that adult human liver selectively expressed a functionally distinctive form of FMO, which had little stereochemical preference for the oxidation of prochiral short-chain arylalkyl sulfides (15). Subsequent chiral fingerprinting with recombinant forms of rabbit FMO provided indirect evidence that this human isoform belonged to the FMO3 family, because only rabbit FMO3 exhibited little or no stereoselectivity in the formation of short-chain aralkyl sulfoxides (16). The present studies demonstrate that human FMO3 also lacks appreciable stereoselectivity in the formation of these sulfoxides and show clearly that recombinant human FMO3 recapitulates the chiral fingerprint for arylalkyl sulfoxidation evidenced by detergent-solubilized adult human liver microsomes. Therefore, these data strongly support the view that human FMO3 is a dominant FMO arylalkyl sulfoxidase present in human liver. Moreover, a close functional similarity can now be seen to exist between the human and rabbit FMO3 orthologs, although further studies will be required to determine if this conservation of function extends across species lines for the other FMO isoforms.
Although several groups have expressed human FMOs in a variety of heterologous systems (9, 13, 14), functional studies have been conducted to date only with the membrane-bound forms of these recombinant enzymes. A detailed analysis of the substrate specificity of the human FMOs would be facilitated if polarographic methodology, using the purified isoforms, could be used to screen a wide variety of compounds rapidly for enzyme activity. Such an approach has allowed an enormous array of substrates to be identified for hog FMO1 (1, 2, 28), the first form of the enzyme to be purified. Therefore, we evaluated approaches to isolating human FMO3 in high purity from insect cell membranes. Cholate solubilization, together with hydrophobic and ion-exchange chromatography, provided a highly purified, catalytically active preparation, albeit in modest yield. Importantly, the availability of a primary standard permitted an immunochemical estimate of FMO3 protein levels in human liver microsomes. The mean FMO3 concentration in six human liver samples was found to be 80 ± 40 pmol/mg, which demonstrates that substantial concentrations of FMO3 are present in the average human liver. Consequently, previous attempts to isolate this isoform from human liver tissue are less likely to have been hindered by low levels of enzyme than by difficulty in removal of the enzyme from the membrane and by loss of its flavin cofactor during purification.
Posttranslational modification of FMOs seems to be relatively common. Native rabbit FMO1, hog FMO1, and rabbit FMO2 are each acetylated at anN-terminal alanine (29-31); rabbit FMO5 is acetylated at the N-terminal glycine residue (32); and native hog liver FMO1 is reported to be N-glycosylated at Asn120(25, 33). The availability of purified protein also permitted us to characterize the extent of posttranlational modification of human FMO3 isolated from transfected insect cells. Edman degradation of purified recombinant human FMO3 identified a free N-terminus and the loss of the initiator methionine residue, similar to FMO3 purified from rabbit and macaque liver (6, 7). Therefore, the BEVS processes theN-terminus of recombinant human FMO3 in a manner similar to that found with FMO3 orthologs isolated from experimental animals. LC/ES-MS analyses showed further that purified, recombinant human FMO3 contained neither N-linked nor O-linked glycans. These studies do not rule out the possibility that native human microsomal FMO3 is glycosylated. However, even if this is the case, the near identity of the chiral fingerprints obtained with arylalkyl sulfide probes from recombinant human FMO3 and by solubilized human liver microsomes suggests that glycosylation status does not have a significant influence on these catalytic functions.
In summary, we have expressed and purified human FMO3 from a baculovirus expression system. Stereoselective metabolism studies confirm that FMO3 is a functionally dominant FMO sulfoxidase present in adult human liver. Immunoquantitation demonstrates that FMO3 is a major human liver monooxygenase, and structural analysis shows that the BEVS processes human FMO3 in a manner similar to native microsomal FMO3 orthologs. Current studies underscore the suitability of the BEVS for future efforts aimed at defining physiologically relevant substrates for human FMO3.
Footnotes
-
Send reprint requests to: Dr. A. E. Rettie, Department of Medicinal Chemistry, Box 357610, University of Washington, Seattle WA 98195.
-
This work was supported in part by Grant GM43511 from the National Institutes of Health.
- Abbreviations used are::
- BEVS
- baculovirus expression vector system
- T. ni
- Trichoplusia ni
- PCR
- polymerase chain reaction
- PMSF
- phenylmethylsulfonylfluoride
- DTT
- dithiothreitol
- HL
- human liver
- LC/ES-MS
- liquid chromatography/electrospray-mass spectrometry
- CID
- collision-induced dissociation
- IgG
- immunoglobulin G
- SDS-PAGE
- sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- CYP
- cytochrome P450
- Received November 19, 1996.
- Accepted March 26, 1997.
- The American Society for Pharmacology and Experimental Therapeutics