Abstract
The relationship between biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats was examined. The biliary excretion of seven model substrates in 96-h sandwich-cultured rat hepatocytes was determined by differential cumulative uptake of substrate in the monolayers preincubated in standard buffer (intact bile canaliculi) and Ca2+-free buffer (disrupted bile canaliculi). Biliary excretion in vivo was quantitated in bile duct-cannulated rats. The biliary excretion index of model substrates, equivalent to the percentage of retained substrate in the canalicular networks, was consistent with the percentage of the dose excreted in bile from in vivo experiments. The in vitro biliary clearance of inulin, salicylate, methotrexate, [d-pen2,5]enkephalin, and taurocholate, calculated as the ratio of the amount excreted into the bile canalicular networks and the area under the incubation medium concentration-time profile (∼0, ∼0, 4.1 ± 1.0, 12.6 ± 2.2, and 56.2 ± 6.0 ml/min/kg, respectively), correlated with their intrinsic in vivo biliary clearance (0.04, 0, 17.3, 34.4, and 116.9 ml/min/kg, respectively; r2 = 0.99). The model compound 264W94 was not excreted in bile either in vivo or in vitro. The glucuronide conjugate of 2169W94, theO-demethylated metabolite of 264W94, was excreted into bile in vitro when 2169W94, but not 264W94, was incubated with the monolayers; 2169W94 glucuronide undergoes extensive biliary excretion after administration of 264W94 or 2169W94 in vivo. Biliary excretion in long-term sandwich-cultured rat hepatocytes correlates with in vivo biliary excretion. The study of biliary excretion of metabolites in the hepatocyte monolayers requires consideration of the status of metabolic activities.
Many drugs undergo biliary excretion, although the extent of secretion in bile often is difficult to quantitate, particularly in humans. Alterations in biliary excretion due to disease states or drug interactions may have important pharmacologic and/or toxicologic implications. For example, coadministration of antibiotics with drugs that undergo enterohepatic recirculation may substantially inhibit enterohepatic recycling and result in lower plasma concentrations and pharmacologic effects of the affected drug (Parker et al., 1980). This type of drug interaction has been implicated in the failure of the oral contraceptive ethinylestradiol (Shenfield, 1993). Interactions in biliary excretion are difficult to predict due to the lack of an optimal in vitro model to evaluate and study biliary excretion.
The elucidation of biliary excretion properties of drug candidates is also a critical issue in the drug discovery and development process. Drug candidates that are extensively excreted into bile may never achieve adequate concentrations in vivo. For example, many metabolically stable peptides exhibit short residence times in the systemic circulation (Greenfield et al., 1989; Chen and Pollack, 1997) and low bioavailability after oral administration due to rapid and extensive biliary excretion (Ziegler et al., 1985; 1991). Therefore, knowledge of the extent of biliary excretion of drug candidates in the early stages of drug development may be as important as absorption and metabolic properties when selecting drug candidates.
Numerous in vivo (e.g., bile duct-cannulated animals) and in vitro preparations (e.g., isolated perfused livers, isolated hepatocytes, hepatocyte couplets, liver plasma membrane vesicles, and expressed transport proteins) have been used to investigate biliary excretion processes (Oude Elferink et al., 1995). However, existing methods may not always be applied to investigate human biliary excretion. In addition, current approaches cannot be used to efficiently examine biliary excretion processes for a large number of drug candidates. Therefore, there is a tremendous need for a rapid and inexpensive in vitro screening method that is predictive of hepatobiliary disposition in animals and humans, especially in this modern era of high synthetic capabilities (e.g., combinatorial chemistry approaches).
Long-term (more than 24 h) sandwich-cultured hepatocytes represent a potential in vitro model to study biliary excretion. Previous work has demonstrated that maintenance of hepatocytes in a collagen-sandwich configuration prolongs cell viability and preserves liver-specific protein synthesis (Dunn et al., 1989; 1991). Further studies showed that long-term sandwich-cultured hepatocytes reestablish a structurally and functionally normal bile canalicular network and show better maintenance of drug uptake and enzyme-induction potential (Sidhu et al., 1993; Musat et al., 1993; LeCluyse et al., 1996). Recently, it has been demonstrated that the expression and function of primary active transporters, such as the sinusoidal Na+/taurocholate-cotransporting polypeptide, the canalicular bile acid transporter, and the canalicular-multispecific organic anion transporter, were maintained in hepatocytes cultured in a collagen-sandwich configuration for 96 to 120 h (Liu et al., 1998;1999a).
The sandwich-cultured hepatocyte system is composed of two compartments: cytosol and canalicular lumen. The tight junctional complex is the diffusional barrier between the canalicular lumen and the extracellular space (LeCluyse et al., 1994; Talamini et al., 1997). In this system, Ca2+ depletion increases tight junction permeability and enables substrate translocation between the canalicular and extracellular spaces based on favorable concentration gradients (Liu et al., 1999a). During cumulative uptake studies, substrate in the medium was taken up by hepatocytes and excreted into the bile canalicular networks. In standard buffer, the barrier function of the tight junctions is intact and the excreted substrate is localized in the canalicular compartment. In Ca2+-free buffer, the barrier function of tight junctions is disrupted and the substrate in the canalicular compartment diffuses back into the incubation medium. Thus, in standard buffer, the cumulative uptake of a substrate in the long-term sandwich-cultured hepatocytes represents the amount of substrate in the cytosolic and canalicular compartments; in Ca2+-free buffer, the cumulative uptake represents substrate in the cytosolic compartment. The amount of substrate secreted in the canalicular lumen, i.e., the biliary excretion of substrates in the monolayers, can be estimated from the difference in cumulative uptake in the presence and absence of Ca2+. This method has been used to quantitatively study hepatocyte polarization during the course of culture (Liu et al., 1999a). However, it remains to be determined whether the estimates of biliary excretion based on this in vitro model are consistent with in vivo biliary excretion data.
The objective of the present study was to examine the relationship between the estimated biliary excretion in the long-term sandwich-cultured hepatocytes and the extent of biliary excretion in vivo in rats. These results indicate that biliary excretion in rat hepatocytes cultured in a collagen-sandwich configuration for 96 h correlates with in vivo biliary excretion in rats.
Materials and Methods
Chemicals.
[3H]Taurocholate (3.4 Ci/mmol; purity >97%), [14C]salicylate (55.5 mCi/mmol; purity >99%), and [3H][d-pen2,5]enkephalin (36 Ci/mmol; purity >97%) were obtained from DuPont-New England Nuclear (Boston, MA). [3H]Methotrexate (13.7 Ci/mmol; purity >99%) and [3H]inulin (1.3 Ci/mmol; purity >97%) were obtained from Amersham International plc (Buckinghamshire, England). Compounds [14C]264W94 [(3R, 5R)-3-butyl-3-ethyl-2, 3, 4, 5-tetrahydro-7, 8-dimethoxy-5-phenyl-1, 4-benzothiazepine-1, 1-dioxide; 45.5 mCi/mmol; purity >99%] and [14C]2169W94 [(3R, 5R)-3-butyl-3-ethyl-2, 3, 4, 5-tetrahydro-7-methoxy-8-hydroxy-5-phenyl-1, 4-benzothiazepine-1, 1-dioxide; 43.7 mCi/mmol; purity >99%] were obtained from Glaxo Wellcome, Inc. (Research Triangle Park, NC). Collagenase (type I, class I) was obtained from Worthington Biochemical Corp. (Freehold, NJ). Dulbecco’s modified Eagle’s medium (DMEM)1, fetal bovine serum, and insulin were purchased from Gibco (Grand Island, NY). Rat tail collagen (type I) was obtained from Collaborative Biomedical Research (Bedford, MA). All other chemicals and reagents were of analytical grade and were readily available from commercial sources.
Animals.
Male Wistar rats (250–280 g), obtained from Charles River Laboratories, Inc. (Raleigh, NC), were used as liver donors. Rats were housed individually in stainless steel cages in a constant alternating 12-h light/dark cycle at least 1 week before the study was performed and were fed ad libitum until use. Bile duct-cannulated rats (200–250 g) were obtained from Charles River Laboratories, Inc. (Raleigh, NC). All procedures were approved by the Institutional Animal Care and Use Committee.
Preparation of Culture Dishes.
Plastic culture dishes (60-mm) were precoated with rat tail collagen at least 1 day before preparing the hepatocyte cultures. To obtain a gelled collagen substratum, ice-cold neutralized collagen solution (0.1 ml, 1.5 mg/ml, pH 7.4) was spread onto each culture dish. Freshly coated dishes were placed at 37°C in a humidified incubator for approximately 1 h to allow the matrix material to gel, followed by addition of 3 ml DMEM to each dish and storage in a humidified incubator.
Culture of Rat Hepatocytes.
Hepatocytes were isolated with a two-step perfusion method as reported previously (Liu et al., 1998). Rats were anesthetized with ketamine and xylazine (60 and 12 mg/kg i.p., respectively) before portal vein cannulation. The liver was perfused in situ with oxygenated Ca2+-free Krebs-Henseleit bicarbonate buffer containing 5.5 mM glucose for 10 min at 37°C followed by perfusion with Krebs-Henseleit bicarbonate buffer containing collagenase type I (0.5 mg/ml) for 10 min. The hepatic capsule was removed with forceps. The hepatocytes were released by shaking the liver gently in 100 ml DMEM. The released cells were filtered through a sterile nylon mesh (70 μm). The hepatocyte suspensions were centrifuged at 50gfor 3 min. The cell pellet was resuspended in 25 ml DMEM and an equal volume of 90% isotonic Percoll (pH 7.4); the resulting cell suspension was centrifuged at 150g for 5 min. The pellet was resuspended in 50 ml DMEM and the cell suspensions were combined into one tube followed by centrifugation at 50g for 3 min. Hepatocyte viability was determined by trypan blue exclusion. Only those hepatocyte preparations with viability greater than 90% were used for further studies. Hepatocyte suspensions were prepared with DMEM containing 5% fetal calf serum, 1 μM dexamethasone and 4 mg/liter insulin. Hepatocyte suspensions were added to the precoated dishes at a density of 2 × 106 cells/60-mm dish. Approximately 1 h after plating the cells, the medium was aspirated and 3 ml of fresh DMEM was added. For transport studies, hepatocytes that had been seeded for 3 to 5 h without collagen overlay were defined as 3-h or short-term cultured hepatocytes.
To prepare sandwich-cultured hepatocytes, neutralized collagen solution (0.1 ml, 1.5 mg/ml, pH 7.4) was added to the monolayers 24 h after the cells were seeded. Cultures with collagen overlay were incubated for 45 min at 37°C in a humidified incubator to allow the collagen to gel before the addition of DMEM. Medium was changed on a daily basis until the fourth day after the cells were seeded. These hepatocytes were referred to as 96-h or long-term cultured hepatocytes.
Cumulative Uptake Studies in Sandwich-Cultured Hepatocytes.
Hepatocytes cultured in a collagen-sandwich configuration were incubated in 3 ml of standard buffer or Ca2+-free buffer at 37°C for 10 min. After removing the incubation buffer, uptake was initiated by addition of 3 ml of standard buffer containing substrate to each dish. Substrate concentrations were selected in the linear range based on preliminary studies or previously published data (Liu et al., 1999b). After incubation for designated times, cumulative uptake was terminated by aspirating the incubation solution and rinsing 4 times with 3 ml of ice-cold standard buffer to remove extracellular substrate. After washing, 2 ml of 1% Triton X-100 solution was added to culture dishes and the cells were lysed by shaking the dish on a shaker for 20 min at room temperature. An aliquot (1 ml) of lysate was analyzed by liquid scintillation spectrometry. Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA) was used to determine the protein concentration in the culture extracts using bovine serum albumin as standard. Triton X-100 (1%) did not interfere with the assay. All values for substrate uptake into cell monolayers were corrected for nonspecific binding to the collagen by subtracting the substrate uptake determined in the appropriate control dishes in the absence of cells as described previously (Liu et al., 1998). Nonspecific binding for a given substrate was consistent between petri dishes.
Biliary Excretion in Rats after i.v. Administration of 264W94 and Oral Administration of 2169W94.
[14C]264W94 was formulated as a solution in a mixture of sterile water/polypropylene glycol 400/ethanol (2:1:1 v/v/v) at a concentration of 0.125 mg/ml. After collection of predose bile, [14C]264W94 solution was administered by caudal vein injection (0.1 mg/kg). For the 2169W94 studies, [14C]2169W94 was prepared as a suspension at a concentration of 0.1 mg/ml in 0.5% (w/v) methylcellulose in water. After collection of predose bile, [14C]2169W94 suspension was administrated by gavage (1.0 mg/kg). All rats were placed into individual plastic metabolism cages that allowed the rats unrestrained movement. Bile was collected into polypropylene containers surrounded by ice. For the 264W94 studies, the bile container was changed at 1, 2, 3, 4, 5, 6, 12, and 24 h after the dose; for the2169W94 studies, the container was changed at 8 and 24 h after the dose. Previous studies indicated that samples were stable on ice for 24 h. Bile samples were stored at −20°C until analysis.
Analytical Procedure.
Aliquots of cell lysate or bile samples containing 264W94 or2169W94 were mixed with 2-fold volumes of ice-chilled acetonitrile and centrifuged to remove precipitated proteins. The supernatant was evaporated under nitrogen at room temperature and reconstituted in 100 μl of a 70/30 mixture of 50 mM ammonium acetate/acetonitrile/trifluoroacetic acid (95:5:0.1 v/v/v) and acetonitrile. The sample extracts were injected onto a Waters Symmetry C18 column (3.9 × 150 mm) and eluted by a 85:15 mixture of 50 mM ammonium acetate (pH 4.0) and acetonitrile; the percentage of acetonitrile was increased by a Waters 600E System Controller to 55% over a period of 20 min and then to 100% during the next 10 min. Radiocarbon that eluted from the HPLC was quantitated with an on-line radioactivity detector (Radiomatic Flo-One/Beta Radio-Chromatography Detector Series 500TR Series, Packard Instrument Co., Meriden, CT). The peaks of 264W94, 2169W94, and 2169W94 glucuronide were identified by comparing them with purified standard compound. Under these conditions, baseline separation of these three components was achieved. The concentration of the three components was determined by normalizing the eluted radioactivity in each peak to the total injected radioactivity.
Data Analysis.
Uptake data were normalized to the protein content and expressed as mean ± S.D. from three to four separate preparations of hepatocytes. Statistical differences between mean values for the 10-min cumulative substrate uptake in the presence and absence of Ca2+ were determined by Student’s ttest. A P value of < .05 was considered significant.
In vivo biliary clearance, ClB (ml/min/kg body weight), was calculated according to eq. 1:
The in vivo intrinsic biliary clearance (ClBin, ml/min/kg body weight) was estimated according to eq. 2 based on the well-stirred model of hepatic disposition assuming biliary excretion is the predominant elimination pathway (Pang and Rowland, 1977).
Biliary excretion of substrates in the monolayers was quantitatively assessed by the biliary excretion index (Liu et al., 1999a) based on eq. 3:
Biliary clearance in the sandwich-cultured hepatocytes, ClB(culture) (ml/min/kg body weight), was calculated according to eq. 4:
Results
Cumulative Uptake in Cultured Hepatocytes.
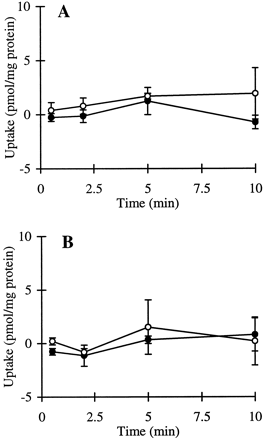
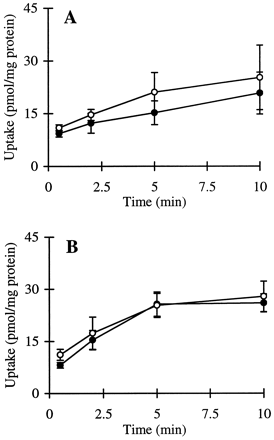
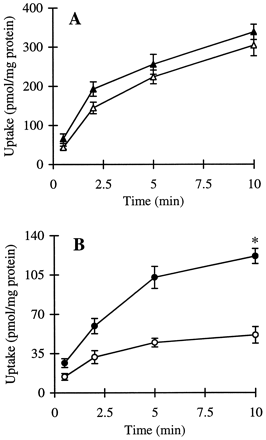
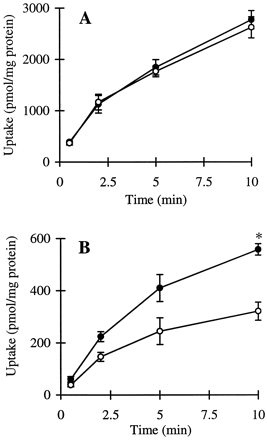
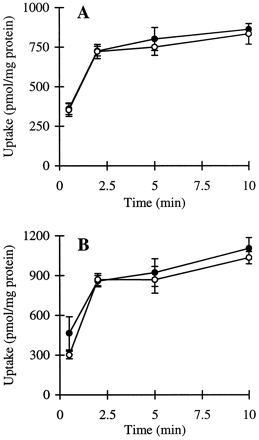
The cumulative uptake of inulin was negligible (less than 0.01% of initial added substrate) at all incubation times in either short- or long-term cultured hepatocytes (Fig. 1A and B). In the 3-h cultured hepatocytes, the cumulative uptake of salicylate, methotrexate, and [d-pen2,5]enkephalin was not significantly different in standard buffer and in Ca2+-free buffer (Figs.2A, 3A, and 4A; p > .05). However, slightly higher cumulative uptake of taurocholate in standard buffer compared with Ca2+-free buffer was observed (Fig.5A); at 10 min, the cumulative uptake in standard buffer was approximately 10% higher than in Ca2+-free buffer (p = .04). In 96-h cultured hepatocytes, extracellular Ca2+ had no effect on the cumulative uptake of salicylate (Fig. 2B, p > .05). However, the uptake of methotrexate, [d-pen2,5]enkephalin, and taurocholate in long-term cultured hepatocytes in standard buffer was significantly higher than in Ca2+-free buffer (Fig. 3B, 4B, and 5B; p < .05).
Cumulative uptake of [3H]inulin (1 μM) in standard buffer (●) and Ca2+-free buffer (○) in hepatocyte monolayers cultured for 3 h (A) and hepatocytes cultured in a sandwich configuration for 96 h (B).
Cumulative uptake of [14C]salicylate (1 μM) in standard buffer (●) and Ca2+-free buffer (○) in hepatocyte monolayers cultured for 3 h (A) and hepatocytes cultured in a sandwich configuration for 96 h (B).
Cumulative uptake of [3H]taurocholate (1 μM) in standard buffer (closed symbols) and Ca2+-free buffer (open symbols) in hepatocyte monolayers cultured for 3 h (A) and hepatocytes cultured in a sandwich configuration for 96 h (B).
*Cumulative uptake at 10 min in standard buffer was significantly greater than the cumulative uptake in Ca2+-free buffer (p < .05).
Cumulative uptake of [3H]methotrexate (1 μM) in standard buffer (●) and Ca2+-free buffer (○) in hepatocyte monolayers cultured for 3 h (A) and hepatocytes cultured in a sandwich configuration for 96 h (B).
*Cumulative uptake at 10 min in standard buffer was significantly greater than the cumulative uptake in Ca2+-free buffer (p < .05).
Cumulative uptake of [3H][d-pen2,5]enkephalin (15 μM) in standard buffer (●) and Ca2+-free buffer (○) in hepatocyte monolayers cultured for 3 h (A) and hepatocytes cultured in a sandwich configuration for 96 h (B).
*Cumulative uptake at 10 min in standard buffer was significantly greater than the cumulative uptake in Ca2+-free buffer (p < .05).
Relationship Between the Percentage of Dose Excreted in Bile in Rats and Biliary Excretion Index in Cultured Hepatocytes.
Five model substrates representing a diverse spectrum of biliary excretion properties were selected to examine the relationship between the percentage of the dose excreted in bile in vivo in rats and the biliary excretion index in sandwich-cultured hepatocytes. Information regarding the percentage of the dose excreted in rat bile after i.v. administration was obtained from the literature. The extent of inulin and salicylate secretion into bile was negligible (Eriksson et al., 1975; Laznicek and and Laznickova, 1994). Approximately 50 to 60% of a 22 μmol/kg methotrexate dose (Bremnes et al., 1989; Masuda et al., 1997) and 70% of a 14.5 μmol/kg [d-pen2,5]enkephalin dose (Chen and Pollack, 1997) were excreted into rat bile as unchanged drug in 1 h. Taurocholate biliary excretion was more rapid and extensive than methotrexate and [d-pen2,5]enkephalin. In 1 h, virtually 100% of the dose (8.0 μmol/kg) was recovered in rat bile (Inoue et al., 1985).
Biliary excretion in the sandwich-cultured hepatocytes can be expressed quantitatively as the biliary excretion index calculated from eq. 3based on the 10-min cumulative uptake data in Figs. 3B-5B. The biliary excretion index of inulin and salicylate was assumed to be negligible because no statistically significant differences in the cumulative uptake of inulin or salicylate were observed between standard buffer and Ca2+-free buffer (p> .05). The biliary excretion index of methotrexate, [d-pen2,5]enkephalin, and taurocholate was 55.4 ± 18.3%, 42.4 ± 6.5%, and 56.4 ± 5.2%, respectively. The relationship between the percentage of the dose excreted in rat bile in vivo and the biliary excretion index measured in the in vitro system is depicted in Fig.6A. The biliary excretion index was very low for compounds undergoing negligible biliary excretion in vivo (e.g., inulin and salicylate). In contrast, the biliary excretion index was moderately high for compounds that are excreted in bile in vivo (e.g., methotrexate, [d-pen2,5]enkephalin, and taurocholate).
Relationship between the percentage of the dose excreted in rat bile in vivo and the biliary excretion index (A), and in vivo intrinsic biliary clearance and in vitro biliary clearance (B), in 96-h sandwich-cultured hepatocytes for the following model substrates: inulin (■), salicylate (♦), methotrexate (○), [d-pen2,5]enkephalin (▴), and taurocholate (●).
The biliary excretion index was calculated from the 10-min cumulative uptake data (Figs. 1-5) based on eq. 3. The in vivo intrinsic biliary clearance was calculated from eq. 2 based on in vivo biliary clearance values from the literature. The in vitro biliary clearance was calculated from eq. 4. The broken line is the fit of a linear regression equation to the data (r2 = 0.99).
Correlation of In Vitro and In Vivo Biliary Clearance.
The in vivo biliary clearance (ml/min/kg body weight) of inulin, salicylate, methotrexate, and taurocholate was 0.035 (Eriksson et al., 1975), ∼0 (Laznicek and Laznickova, 1994), 12.1 (Masuda et al., 1997), and 29.8 (Inoue et al., 1985), respectively. In vivo biliary clearance of [d-pen2,5]enkephalin, 18.5 ml/min/kg, was calculated based on eq. 1 from the data reported byChen and Pollack (1997). Based on these in vivo biliary clearance values, the intrinsic biliary clearance of inulin, salicylate, methotrexate, [d-pen2,5]enkephalin, and taurocholate was calculated from eq. 2 (0.04, 0, 17.3, 34.4, and 116.9 ml/min/kg, respectively). The in vitro biliary clearances of inulin, salicylate, methotrexate, [d-pen2,5]enkephalin, and taurocholate, calculated from eq. 4 based on the 10-min cumulative uptake data (Figs. 1B-5B) were ∼0, ∼0, 4.1 ± 1.0, 12.6 ± 2.2, and 56.2 ± 6.0 ml/min/kg, respectively. The in vivo intrinsic biliary clearance correlated well with the in vitro biliary clearance (r2 = 0.99) for the five model compounds (Fig. 6B).
Comparison of In Vivo and In Vitro Biliary Excretion of 264W94 and Its Metabolites.
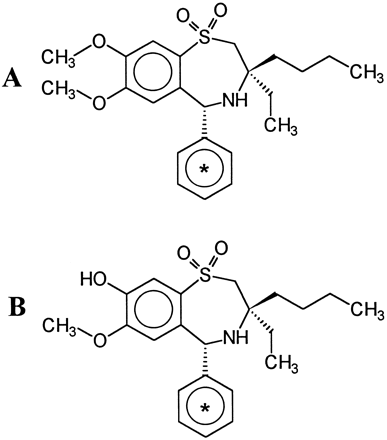
The structures of compounds 264W94 and 2169W94 are presented in Fig. 7. Compound 2169W94 is the O-demethylated metabolite of 264W94 in rats and humans, and can undergo further conjugation with urindine-5′-diphosphoglucuronic acid to form a glucuronide conjugate (Silver et al., 1996).
Chemical structure of 264W94 (A) and2169W94 (B).
*indicates position of 14C incorporated uniformly.
After i.v. administration of [14C]264W94 to rats (0.24 μmol/kg), neither 264W94 nor 2169W94 was detected in bile. However, 35.4% (n = 2) of the total administered radioactivity was recovered in bile in the first hour. Approximately, 30.0% of the radioactivity recovered in bile was the 2169W94 glucuronide; the remaining 70% of radioactivity in bile represented unidentified metabolites. After oral administration of [14C]2169W94 to rats (2.4 μmol/kg), 2169W94 was not detected in the bile in 24 h. However, 66.4% (n = 2) of the total administered radioactivity was recovered in bile in 8 h. Approximately 88.7% of the radioactivity in bile was in the form of the 2169W94glucuronide conjugate. These in vivo results demonstrate that 264W94 and its O-demethylated product, 2169W94, undergo negligible biliary excretion but the glucuronide conjugate of2169W94 undergoes extensive biliary excretion in rats.
To determine the biliary excretion of 264W94 and metabolites in 3-h and 96-h cultured hepatocytes, hepatocyte monolayers were incubated in standard or Ca2+-free buffer before cumulative uptake was conducted in standard buffer containing 3 μM [14C]264W94 or [14C]2169W94 (Figs.8 and 9). In 3-h cultured hepatocytes, the cumulative uptake measured by total radioactivity of 264W94 or 2169W94 was similar in the hepatocytes preincubated in standard buffer or Ca2+-free buffer (p > .05), suggesting that the uptake of 264W94 and 2169W94 in short-term cultured hepatocytes was not affected by preincubation of the monolayers in Ca2+-free buffer. In 96-h cultured hepatocytes, the 10-min cumulative uptake of 264W94 measured by total radioactivity was not significantly different in the monolayers preincubated in standard buffer or Ca2+-free buffer (p > .05). HPLC analysis of the cell lysate at 10 min revealed that 73.0% of the total radioactivity was in the form of 264W94 and 3.3% was the2169W94 glucuronide conjugate; 2169W94 was not detected in the lysate. In 96-h sandwich-cultured hepatocytes, 10-min cumulative uptake of 2169W94 was approximately 70% greater in the presence of Ca2+ than in the absence of Ca2+ (p < .05). In the 10-min cell lysate, approximately 16.7% of total radioactivity was in the form of 2169W94 and approximately 58.5% was the2169W94 glucuronide conjugate. Compound 2169W94forms the glucuronide conjugate that is excreted into bile canalicular networks in long-term cultured hepatocytes.
Cumulative uptake of [3H]264W94 (3 μM) in standard buffer (●) and Ca2+-free buffer (○) in hepatocyte monolayers cultured for 3 h (A) and hepatocytes cultured in a sandwich configuration for 96 h (B).
Cumulative uptake of [3H]2169W94 (3 μM) in standard buffer (●) and Ca2+-free buffer (○) in hepatocyte monolayers cultured for 3 h (A) and hepatocytes cultured in a sandwich configuration for 96 h (B).
*Cumulative uptake at 10 min in standard buffer was significantly greater than the cumulative uptake in Ca2+-free buffer (p < .05).
Discussion
Previous studies have indicated that long-term primary rat hepatocytes cultured between two layers of gelled collagen (sandwich configuration) maintain normal morphology, form extensive canalicular networks, and sustain liver-specific functions (Dunn et al., 1989;1991; LeCluyse et al., 1996). Recently, it has been demonstrated that the biliary excretion of the nonfluorescent substrate, taurocholate, in sandwich-cultured hepatocytes can be estimated as the difference in cumulative uptake of taurocholate in monolayers preincubated in standard buffer and in Ca2+-free buffer (Liu et al., 1999a).
The present study was undertaken to examine the relationship between in vitro and in vivo biliary excretion and to investigate the utility of this in vitro model to predict biliary excretion in vivo. Five nonfluorescent model substrates were used in these studies: inulin, salicylate, methotrexate, [d-pen2,5]enkephalin, and taurocholate. Inulin and salicylate were used as extracellular and simple diffusional markers, respectively. In addition, the utility of the cultured hepatocytes to predict biliary excretion of drug metabolites was assessed with the model compound 264W94 and its metabolite, 2169W94. Results indicate that the biliary excretion in sandwich-cultured hepatocytes correlates with in vivo biliary excretion. This in vitro model can be used to predict the biliary excretion of unchanged parent compound. Whether this in vitro model system can be used to predict the biliary excretion of metabolites depends upon the metabolic activity of the cultured hepatocytes.
It is well documented that Ca2+ is required to maintain the integrity of tight junctions. Previous studies (Liu et al., 1999b) have demonstrated that a 10-min incubation of hepatocyte monolayers in Ca2+-free buffer disrupted the barrier function of tight junctions in the monolayers. The disrupted tight junctions did not reseal within 10 min after replacing the Ca2+-free buffer with standard buffer. Therefore, a protocol of 10-min preincubation in Ca2+-free buffer followed by uptake in standard buffer was used in the present study.
Cumulative uptake was determined based on the total radioactivity in the cell lysate. This approach was viable because the selected model compounds are metabolically stable except salicylate. Salicylate is metabolized in rat livers and excreted extensively in urine (Laznicek and Laznickova, 1994). Thus, it was not necessary to differentiate salicylate from metabolites in the in vitro biliary excretion study.
In contrast to long-term cultured hepatocytes, intact bile canaliculi are not present in short-term cultured hepatocytes (LeCluyse et al., 1994; Talamini et al., 1997). Therefore, the cumulative uptake of compounds in short-term cultured hepatocytes only revealed the effects of Ca2+ on the transport properties. The cumulative uptake of inulin, salicylate, methotrexate, and [d-pen2,5]enkephalin did not differ in the short-term cultured hepatocytes, suggesting that extracellular Ca2+ modulation had no effect on the transport properties of these model substrates. Interestingly, the cumulative uptake of taurocholate in short-term cultured hepatocytes was slightly higher in standard buffer than in Ca2+-free buffer. This difference may be secondary to the existence of hepatocyte couplets in the short-term cultures (Graf and Boyer, 1990).
Biliary excretion of the five model substrates in long-term cultured hepatocytes was consistent with their in vivo biliary excretion properties. Quantitation of biliary excretion in the cultured hepatocytes using the biliary excretion index has been described previously (Liu et al., 1999a). The biliary excretion index represents the percentage of retained substrate in the bile canaliculi. Results indicate that compounds undergoing negligible biliary excretion in vivo based on the percentage of the dose excreted in bile (e.g., inulin, salicylate) have a low biliary excretion index (∼0) and compounds that are more extensively excreted in bile in vivo (e.g., methotrexate, [d-pen2,5]enkephalin, and taurocholate) have a high biliary excretion index (∼50%).
The relationship between the biliary excretion index and the percentage of the dose excreted in bile in vivo only reveals a categorical correlation. Methotrexate and [d-pen2,5]enkephalin represent compounds that are “highly” excreted in bile (approximately 60 and 70% of the i.v. dose was recovered in bile in 1 h, respectively). In contrast, taurocholate is “rapidly and extensively” excreted in biliary (almost all of the i.v. dose was excreted in bile in less than 1 h). The biliary excretion index can differentiate between compounds that undergo extensive versus negligible or low biliary excretion. However, the biliary excretion index does not appear to be able to differentiate between compounds that are highly excreted in bile, like methotrexate (biliary excretion index: ∼55%) or [d-pen2,5]enkephalin (biliary excretion index: ∼42%) and compounds that are “rapidly and extensively” excreted in bile, like taurocholate (biliary excretion index: ∼56%). This limitation in the biliary excretion index may be due to the fact that this index is determined predominantly by the canalicular excretory function; the percentage of the i.v.-administered substrate excreted into bile in vivo is determined by sinusoidal uptake activity, canalicular excretory activity, as well as other competitive elimination processes.
Biliary clearance may represent a more appropriate parameter for comparison of the relationship between in vivo and in vitro biliary excretion. The in vivo biliary clearance was calculated in the present study as the ratio of the amount excreted into bile at time T and the plasma AUC between time 0 and time T. Because most of the administered dose was eliminated at time T, the biliary clearance should approximate the biliary clearance calculated from time 0 to infinity. Biliary clearance calculated in this manner is a function of intrinsic biliary clearance and the hepatic plasma flow rate. To eliminate the effects of plasma flow, the intrinsic biliary clearance was calculated based on the well-stirred model of hepatic disposition (Pang and Rowland, 1977). If the red blood cell distribution of a compound is known, plasma clearance should be converted to blood clearance in eq. 1 and blood flow instead of plasma flow should be used to calculate the intrinsic clearance in eq. 2. In vitro biliary clearance was calculated as the ratio of the amount excreted into the canalicular networks in the hepatocyte monolayers and the AUC in the incubation medium. In the sandwich-cultured hepatocytes, the incubation medium was accessible to all hepatocytes on the dish at the same time. Thus, the calculated in vitro biliary clearance should represent the intrinsic biliary clearance. However, because biliary excretion involves two processes, uptake across the sinusoidal membrane and excretion across the canalicular membrane, the true intrinsic biliary clearance should be determined by transport across the canalicular membrane and calculated based on intracellular substrate concentrations. Therefore, the in vivo and in vitro “intrinsic” clearance values calculated in this study should be considered as “apparent” intrinsic biliary clearance values, which would be rate-limited by the slowest step in the process, either sinusoidal uptake or canalicular excretion.
The correlation between in vitro biliary clearance and in vivo intrinsic biliary clearance was high (r2 = 0.99) for the five model substrates. According to the in vivo intrinsic biliary clearance, the five model substrates can be classified into three groups: compounds that are not excreted in bile (inulin and salicylate; ∼0 ml/min/kg), compounds that are highly excreted in bile (methotrexate and [d-pen2,5]enkephalin; ∼17.3 and ∼34.4 ml/min/kg, respectively), and compounds that are rapidly and extensively excreted in bile (taurocholate; ∼116.9 ml/min/kg). The estimated in vitro biliary clearance adequately differentiated between these three groups of compounds (∼0, 4–13, and 56 ml/min/kg, respectively). These results suggest that the biliary clearance describes more accurately the relationship between in vivo and in vitro biliary excretion than the biliary excretion index.
To assess the utility of this in vitro model system to predict in vivo biliary excretion of drug metabolites, the in vitro and in vivo biliary excretion of 264W94, the O-demethylated metabolite (2169W94) and 2169W94 glucuronide were examined. In vitro studies conducted with rat and human liver microsomes, precision-cut liver slices, and cDNA expressed hepatic cytochrome P-450 isozymes indicated that 264W94 formed an O-demethylated metabolite at the 8-methoxy position. Among several cytochrome P-450 isozymes examined, CYP3A4 was the only one that played a major role in the metabolism of 264W94 (Silver et al., 1996). In vivo disposition studies demonstrated that neither 264W94 nor itsO-demethylated metabolite, 2169W94, were excreted in bile, but the 2169W94 glucuronide conjugate along with other unidentified metabolites were extensively excreted in bile. The lack of biliary excretion of 264W94 in long-term sandwich-cultured hepatocytes was consistent with negligible in vivo biliary excretion of 264W94. In vivo, approximately 35% of 264W94 equivalent was excreted in bile as metabolites in 1 h after i.v. administration of 264W94. In cultured hepatocytes, however, the biliary excretion of 264W94 metabolites was negligible (Fig. 8B). This apparent discrepancy between the in vivo and in vitro biliary excretion for the metabolites of 264W94 may be explained by differences in metabolic activities. In vivo, 264W94 undergoes O-demethylation to form2169W94; subsequently, 2169W94 is conjugated with uridine-5′-diphosphoglucuronic acid to form 2169W94glucuronide. This glucuronide conjugate accounts for 30% of the total amount excreted in the bile. In the lysate of long-term cultured hepatocytes, only ∼3% of the total amount incubated was detected as the 2169W94 glucuronide conjugate. These results indicate that the long-term cultured hepatocytes were not capable of theO-demethylation reaction. Consequently, negligible glucuronide conjugate was formed and excreted in bile. However, after incubation of the monolayers with 2169W94, theO-demethylated metabolite of 264W94, 58.5% of2169W94 was converted to glucuronide conjugates and significant biliary excretion was observed in the cultured hepatocytes (Fig. 9B). Evidently, phase I metabolic activities such asO-demethylation deteriorate significantly, whereas the phase II metabolic activities such as glucuronide conjugation are maintained, at least in part, in the long-term sandwich-cultured hepatocytes. This observation was consistent with previous studies indicating that activities of phase II enzymes are better preserved in cultured hepatocytes than those of phase I enzymes, although long-term cultured hepatocytes lose both phase I and phase II enzyme activity (Niemann et al., 1991; Rogiers and Vercruysse, 1993; LeCluyse et al., 1996). The present studies indicate that sandwich-cultured hepatocytes can be used to predict in vivo biliary excretion of a substrate in its parent form. The application of this in vitro model system to study and to predict in vivo biliary excretion of metabolites requires consideration of the status of metabolic activities in the monolayers.
The uncertainty in predicting the biliary excretion of drug metabolites should not limit the utility of this in vitro model as a screening tool for predicting the biliary excretion of drug candidates in vivo. This in vitro model may provide adequate information regarding the biliary clearance of a drug candidate in its parent from. The deterioration of phase I metabolic activity with maintenance of biliary transport may represent an advantage of this in vitro model system to differentiate the biliary excretion of parent drug versus metabolites.
Previous studies indicate that the extent to which individual P-450 enzymes are expressed in cultured hepatocytes depends greatly on the medium and matrix conditions (Utesch et al., 1991; Kocarek et al., 1993; Donato et al., 1994; LeCluyse et al., 1996). To predict the biliary excretion of metabolites, culture conditions will need to be optimized to maintain both hepatic transport as well as phase I and phase II enzyme activities. In addition, recently it was reported that extensive bile canalicular networks form in sandwich-cultured human hepatocytes (Kono et al., 1997). Whether biliary excretion in cultured human hepatocytes correlates with biliary excretion in vivo in humans is the subject of ongoing investigations.
In summary, results of the present study suggest that biliary excretion measured by the biliary excretion index and biliary clearance in sandwich-cultured rat hepatocytes correlates with in vivo biliary excretion in rats. Biliary clearance represents a useful indicator of in vivo biliary excretion. Application of this in vitro model to predict in vivo biliary excretion for drug metabolites is possible if the relevant metabolic activities in the in vitro model are maintained.
Footnotes
-
Send reprint requests to: Dr. Kim L.R. Brouwer, Pharm.D., Ph.D., Division of Drug Delivery and Disposition, School of Pharmacy, CB# 7360, Beard Hall, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7360. E-mail: kbrouwer{at}unc.edu
-
This work was supported in part by National Institutes of Health Grant GM41935. X.L. was supported in part by a fellowship sponsored by Glaxo Wellcome, Inc.
- Abbreviations used are::
- DMEM
- Dulbecco’s modified Eagle’s medium
- Received July 9, 1998.
- Accepted February 24, 1999.
- The American Society for Pharmacology and Experimental Therapeutics