Abstract
Phenacetin O-deethylation (POD) exhibits biphasic kinetics in human liver microsomes. Although cytochrome P-450 (CYP) 1A2 is responsible for the high-affinity component of POD, the enzyme(s) that catalyzes the low-affinity reaction is still unknown. We examined the roles of human CYPs in POD by using human liver microsomes and recombinant CYPs from baculovirus-infected insect cells. Of the recombinant CYPs studied, CYP1A2 showed the highest POD activity. CYP1A1, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 also showed POD activity at 500 μM phenacetin. KM values of recombinant CYP1A2 and CYP2E1 (28 ± 2 μM and 785 ± 125 μM, respectively) were similar to those of the high- and low-affinity components of POD in pooled human liver microsomes (15 ± 5 and 894 ± 189 μM, respectively). Fluvoxamine (10 μM) and anti-CYP1A2 antibodies potently inhibited POD activity at 500 μM phenacetin in pooled human liver microsomes to 22.8 and 34.2% of controls, respectively. CYP2E1 inhibitors diethyldithiocarbamate and aniline also reduced POD activity. The combination of fluvoxamine (10 μM) and aniline (1 mM) further inhibited the residual POD activity not inhibited by fluvoxamine alone. Microsomal POD activity in 12 human livers in the absence of fluvoxamine was correlated with immunoquantified CYP1A2 levels (r = 0.961,p < .001) and, in the presence of 10 μM fluvoxamine, was correlated with immunoquantified CYP2E1 levels (r = 0.589, p < .01) or chlorzoxazone 6-hydroxylase activity (r = 0.823,p < .001). These results suggest that CYP2E1 is responsible for the low-affinity component of POD in human liver microsomes.
Cytochrome P-450 (CYP)1 consists of a superfamily of heme-containing monooxygenases (Nelson et al., 1996) that play important roles in the biotransformation of numerous endogenous compounds and xenobiotics, including steroids, fatty acids, drugs, and carcinogens. CYP isoforms exhibit distinct but frequently overlapping substrate specificities (Rendic and Di Carlo, 1997). Fifteen or more different CYP isoforms involved in xenobiotic compound metabolism have been characterized in humans (Parkinson, 1996). The identification of human CYP isoforms responsible for the metabolism of therapeutic agents may predict or explain clinical or toxicological observations, such as drug-drug interactions. Therefore, we use an in vitro approach to characterize the substrate specificity of several CYP isoforms expressed in human liver microsomes.
CYP1A2 is one of two enzymes in the CYP1A subfamily (Nelson et al., 1996). In the human liver, CYP1A2 constitutes approximately 13% of total CYP protein (Shimada et al., 1994) and catalyzes the metabolism of a large variety of drugs and carcinogens (Rendic and Di Carlo, 1997). There is wide inter- and intraindividual variation in CYP1A2 activity, and it is known that cigarette smoking causes marked induction of the enzyme (Sesardic et al., 1988; Nakajima et al., 1994).
Phenacetin undergoes oxidative O-deethylation to yield acetaminophen by CYP1A2 and has therefore been used to assess the catalytic activity of CYP1A2 in vivo and in vitro or to investigate its regulation (Butler et al., 1989; Xiaodong et al., 1994; Bartoli et al., 1996). However, several studies have reported that the kinetics of phenacetin O-deethylation (POD) in human liver microsomes is biphasic (Boobis et al., 1981; Kahn et al., 1985; Gillam and Reilly, 1988; Tassaneeyakul et al., 1993), which could indicate the involvement of more than one isoform of CYP. The high-affinity component of POD is well established as a marker reaction for CYP1A2 function in human liver microsomes. However, it is unclear which enzyme(s) catalyzes the low-affinity reaction. Thus, we examined the roles of several human CYPs, as well as CYP1A2, in POD by using human liver microsomes and microsomes from baculovirus-infected insect cells expressing individual human CYPs.
Materials and Methods
Chemicals.
Fluvoxamine maleate was a gift from Solvay Meiji (Tokyo, Japan). Sulfaphenazole and S-mephenytoin were purchased from Daiichi Pure Chemicals (Tokyo, Japan). Troleandomycin (TAO) was purchased from Sigma (St. Louis, MO). NADP+, glucose 6-phosphate, and glucose 6-phosphate dehydrogenase were purchased from Oriental Yeast (Tokyo, Japan). HPLC-grade acetonitrile and methanol, and analytical grade phenacetin, paracetamol (acetaminophen), aniline hydrochloride, caffeine, quinidine sodium,N,N-diethyldithiocarbamate (DDC) trihydrate, and other reagents were purchased from Wako Pure Chemical Industries (Osaka, Japan). Anti-rat CYP1A1, CYP1A2, CYP2C, CYP2D6, and CYP3A rabbit sera and monoclonal antibodies against human CYP2A6 or CYP2E1 were obtained from Daiichi Pure Chemicals.
Enzyme Preparations.
Pooled human liver microsomes (lot 2) consisted of a mixture of microsomes prepared from six individual donors, and individual microsomes from 12 human liver specimens (HG3, HG6, HG23, HG30, HG42, HG43, HG56, HG66, HG70, HG89, HG93, and HG112) were obtained from Gentest (Woburn, MA). Immunochemically determined CYP contents and isoform specific activities of each CYP isoform in the microsomes were provided by the manufacturer as follows. Levels of CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1, and CYP3A4 were determined by immunoblot analysis. Specific activities of CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A were determined by the activities against phenacetin O-deethylase (200 μM), coumarin 7-hydroxylase (200 μM), S-mephenytoinN-demethylase (100 μM), diclofenac 4′-hydroxylase (100 μM), S-mephenytoin 4′-hydroxylase (100 μM), bufuralol 1′-hydroxylase (25 μM), chlorzoxazone 6-hydroxylase (100 μM), and testosterone 6β-hydroxylase (200 μM), respectively.
Microsomes prepared from baculovirus-infected insect cells expressing CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP2D6, CYP2E1, CYP3A4+b5 (CYP3A4 coexpressed with cytochrome b5), CYP3A4 (CYP3A4 without b5), CYP3A5, and CYP4A11 were obtained from Gentest. All recombinant CYPs were coexpressed with NADPH CYP oxidoreductase.
POD Assay.
Microsomal POD activity was determined by measuring the rate of acetaminophen formation at 37°C as described previously (Kobayashi et al., 1998). Briefly, a typical incubation mixture consisted of 0.1 mg/ml human liver microsomal protein or a 20 pmol/ml CYP concentration from baculovirus-infected insect cells, 0.1 M potassium phosphate buffer (pH 7.4), 0.1 mM EDTA, an NADPH-generating system (0.5 mM NADP+, 2.0 mM glucose 6-phosphate, 1 IU/ml glucose 6-phosphate dehydrogenase, 4 mM MgCl2), and phenacetin in a final volume of 250 μl. Phenacetin dissolved in methanol was added to test tubes and evaporated with a vacuum evaporator at 40°C. The incubation mixture, except for microsomes and the NADPH-generating system, were redissolved by sonication. The mixture including microsomes and the NADPH-generating system was incubated at 37°C for 30 min. After the reaction was stopped by adding 100 μl of cold acetonitrile, 50 μl of caffeine (5 μg/ml in methanol) was added as an internal standard. The mixture was centrifuged at 10,000g for 5 min, and the supernatant was evaporated by a vacuum evaporator at 40°C for 15 min. Fifty microliters of the remaining sample was analyzed by HPLC.
HPLC analysis was performed with an L-6000 pump (Hitachi, Tokyo, Japan), an L-4000 UV detector (Hitachi), an AS-2000 autosampler (Hitachi), a D-2500 integrator (Hitachi), and a CAPCELL PAK C18 UG120 column (4.6 mm × 250 mm, 5 μm; Shiseido, Tokyo, Japan). The mobile phase consisted of 50 mM potassium dihydrogen phosphate and acetonitrile at a ratio of 85:15, v/v (%), delivered at a flow rate of 0.7 ml/min. The eluate was monitored at a wavelength of 245 nm. The column temperature was maintained at 30°C. Acetaminophen was quantified by comparison with standard curves by using the peak-height ratio method.
Kinetic Analyses.
Kinetic studies were performed with pooled human liver microsomes and recombinant CYPs from baculovirus-infected insect cells (CYP1A1, CYP1A2, CYP2C19, CYP2D6, CYP2E1, and CYP3A4+b5). POD activities were determined with phenacetin concentrations ranging from 1 μM to 1 mM. All reactions were performed within a linear range with respect to protein concentration and incubation time. Briefly, 0.1 mg/ml human liver microsomes, 8 pmol/ml recombinant CYP1A1 or CYP1A2, 20 pmol/ml recombinant CYP2C19, CYP2D6, or CYP2E1, and 40 pmol/ml recombinant CYP3A4+b5 were incubated for 30 min. Michaelis-Menten kinetic parameters for POD in human liver microsomes were estimated by fitting the data to the following equation:
Inhibition Study.
CYP isoform-specific inhibitors or substrates (i.e., compounds able to act as competitive inhibitors) were used to study POD activity with 500 μM phenacetin in pooled human liver microsomes. Isoform-specific inhibitors and alternative substrates used were 0.1 to 100 μM fluvoxamine (CYP1A; Pastrakuljic et al., 1997), 1 to 100 μM sulfaphenazole (CYP2C9; Newton et al., 1995), 1 to 100 μMS-mephenytoin (CYP2C19; Küpfer and Preisig, 1984), 1 to 100 μM quinidine (CYP2D6; Guengerich et al., 1986), 1 to 1000 μM DDC (CYP2E1; Newton et al., 1995), 1 to 1000 μM aniline (CYP2E1;Morgan et al., 1982), and 1 to 100 μM TAO (CYP3A; Newton et al., 1995). Incubations were carried out as mentioned above except for TAO and DDC, which were preincubated in the presence of the NADPH-generating system at 37°C for 15 min, and the reaction was initiated by the addition of substrate dissolved in water.
The immunoinhibition of POD activity was examined by preincubating human liver microsomal samples (25 μg of microsomal protein) with various amounts of antibodies for 1 h on ice. Phenacetin (500 μM) and other components of the incubation medium were then added, and the reaction was carried out as described above. The amounts of anti-rat CYP1A1, CYP1A2, CYP2C, CYP2D6, or CYP3A rabbit sera and monoclonal antibodies against human CYP2A6 or CYP2E1 used were up to 200 and 100 μl/mg microsomal protein, respectively. These amounts were verified to inhibit the specific activities of corresponding CYP isoforms in human liver microsomes (Daiichi Pure Chemicals, unpublished data).
Correlation Study.
Correlations between the POD activities at a 500 μM substrate concentration in the absence or presence of 10 μM fluvoxamine and the catalytic activity of phenacetin O-deethylase (CYP1A2), coumarin 7-hydroxylase (CYP2A6), S-mephenytoinN-demethylase (CYP2B6), diclofenac 4′-hydroxylase (CYP2C9),S-mephenytoin 4′-hydroxylase (CYP2C19), bufuralol 1′-hydroxylase (CYP2D6), chlorzoxazone 6-hydroxylase (CYP2E1), and testosterone 6β-hydroxylase (CYP3A) were studied by using microsomes from 12 human livers. Similarly, POD activity at a 500 μM substrate concentration in the absence or presence of 10 μM fluvoxamine in microsomes from 12 human livers was compared with immunoquantified levels of CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1, and CYP3A4. For correlation analysis, the isoform specific activities and immunochemically determined CYP levels for each microsomal CYP isoform were provided by the manufacturer.
Statistical Analysis.
Statistical significance was determined with the Student’st test for unpaired samples, with p < .05 considered statistically significant. Data represent the mean of duplicate or triplicate measurements for every experiment. Correlation coefficients (r) were determined by Pearson’s product-moment method.
Results
Kinetics of POD in Pooled Human Liver Microsomes.
The Eadie-Hofstee plot for POD in pooled human liver microsomes in the present study was biphasic (data not shown), as reported previously (Boobis et al., 1981; Kahn et al., 1985; Gillam and Reilly, 1988; Tassaneeyakul et al., 1993). ApparentKM values for the high- and low-affinity components were 15 ± 5 and 894 ± 189 μM, respectively. The Vmax values for the high- and low-affinity components were 537 ± 156 and 1660 ± 479 pmol/min/mg of protein, respectively.
Activity and Kinetics of POD in Recombinant CYPs.
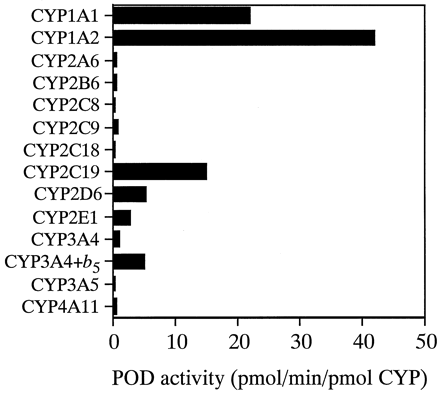
POD activity at 500 μM substrate in microsomes from baculovirus-infected insect cells expressing individual human CYPs was determined (Fig. 1). Of the recombinant CYPs studied, CYP1A2 showed the highest activities (42.1 pmol/min/pmol of CYP). CYP1A1, CYP2C19, CYP2D6, and CYP2E1 showed POD activities of 22.0, 15.1, 5.4, and 2.9 pmol/min/pmol of CYP, respectively. CYP3A4+b5 showed higher POD activity than did CYP3A4 (5.2 versus 1.2 pmol/min/pmol of CYP). Negligible POD activity (<10 pmol of product) was detected in control microsomes and microsomes expressing CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C18, CYP3A5, and CYP4A11.
POD activity in microsomes from baculovirus-infected insect cells expressing individual human CYPs.
Substrate (500 μM phenacetin) was incubated at 37°C for 30 min with microsomes (20 pmol of CYP/ml) from baculovirus-infected insect cells expressing CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A4+b5, CYP3A5, and CYP4A11. Each column represents the mean of duplicate experiments.
Kinetic analyses were performed with microsomes from baculovirus-infected insect cells expressing CYP1A1, CYP1A2, CYP2C19, CYP2D6, CYP2E1, and CYP3A4+b5. POD activity of recombinant CYP1A1, CYP1A2, and CYP2E1 exhibited typical Michaelis-Menten kinetics, and kinetic parameters were estimated for CYP1A1, CYP1A2, and CYP2E1 as described above. As shown in Table1, CYP1A2 showed the highestVmax values (47 ± 0.6 pmol/min/pmol of CYP), whereas CYP1A1 and CYP2E1 exhibited approximately 2- and 11-fold lower Vmax values, respectively. The apparent KM value of CYP2E1 (785 ± 125 μM) was approximately 30-fold higher than those of CYP1A1 and CYP1A2 (27 ± 3 and 28 ± 2 μM, respectively), indicating its lower affinity for phenacetin. KMvalues of recombinant CYP2C19, CYP2D6, and CYP3A4+b5 could not be estimated because plots of V versus S were linear (data not shown).
Michaelis-Menten kinetic parameters of POD in microsomes from baculovirus-infected insect cells expressing CYP1A1, CYP1A2, or CYP2E1
Inhibition Study.
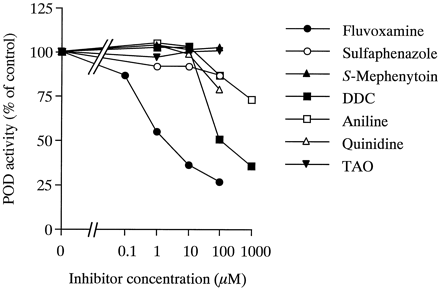
CYP isoform-specific xenobiotic compounds were screened for inhibitory effects on POD activity in pooled human liver microsomes (Fig.2). Fluvoxamine inhibited POD activity in a concentration-dependent manner, with an IC50value of 1.8 μM. DDC inhibited POD activity by >50% at concentrations above 100 μM. POD activity was inhibited to 87 and 73% of control activity by 100 μM and 1 mM aniline, respectively. The extent of inhibition by quinidine or sulfaphenazole on POD activity was slight (<20%), even at concentrations of 100 μM. No effects ofS-mephenytoin (CYP2C19 substrate) and TAO (CYP3A inhibitor) were observed with inhibitor concentrations up to 100 μM.
Effects of specific inhibitors for CYP isoforms on POD activity in pooled human liver microsomes.
The concentration of phenacetin used was 500 μM, and the range of inhibitor concentrations was 0.1 to 100 or 1000 μM. Each data point represents the mean of duplicate experiments.
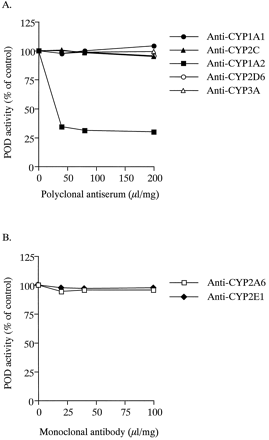
Similar results were obtained in antibody inhibition studies. As shown in Fig. 3, anti-CYP1A2 serum inhibited POD activity to 34.2% of control activity at 40 μl/mg microsomal protein. No effects were observed with antibodies against CYP1A1, CYP2C, CYP2D6, CYP3A, CYP2A6, or CYP2E1.
Immunoinhibition of POD activity by antibodies against CYP isoforms in pooled human liver microsomes.
Human liver microsomes (25 μg of microsomal protein) were preincubated with various amounts of antibodies for 1 h on ice before incubation with 500 μM phenacetin. Each data point represents the mean of duplicate experiments.
Effects of Fluvoxamine and Aniline on POD Activity.
The effects of 10 μM fluvoxamine and 1 mM aniline, alone and in combination, on POD activity in pooled human liver microsomes were examined. The concentrations of fluvoxamine and aniline were selected based on the following observations. Fluvoxamine (10 μM) inhibited CYP1A2-catalyzed POD activity to 19% of control activity but had little effect on CYP2E1-catalyzed activity. Conversely, 1 mM aniline completely inhibited CYP2E1-catalyzed POD activity but had little effect on CYP1A2-catalyzed activity (M. Nakajima, K. Kobayashi, K. Oshima, N. Shimada, S. Tokudome, K. Chiba and T. Yokoi, submitted). As shown in Fig. 4, 10 μM fluvoxamine potently inhibited POD activity to 22.8 ± 1.0% of control (p < .005). Aniline (1 mM) also significantly inhibited POD activity to 76.3 ± 9.2% of control (p < .05). The combination of fluvoxamine and aniline inhibited POD activity to 5.3 ± 0.02% of control (p < .005). The inhibition by combined fluvoxamine and aniline was significantly more potent as compared with fluvoxamine (p < .005) or aniline (p < .01) alone.
Effects of fluvoxamine and aniline on POD activity in pooled human liver microsomes.
The concentration of phenacetin used was 500 μM. Each column represents the mean ± S.D. of three different experiments. Residual activities with 10 μM fluvoxamine and 1 mM aniline, alone and in combination, are expressed as percentages of control activity. Control activity was 1156 ± 86 pmol/min/mg. *p < .05; ***p < .005 compared to control;†††p < .005 compared to 10 μM fluvoxamine; §§p < .01 compared to 1 mM aniline.
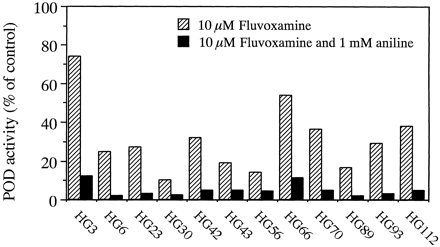
To clarify the contributions of CYP1A2 and CYP2E1 to POD at 500 μM phenacetin in individual microsomes of human livers, the effects of 10 μM fluvoxamine alone and in combination with 1 mM aniline on POD activity in microsomes from 12 human livers were determined. Although 10 μM fluvoxamine inhibited POD activity in all human liver microsomes, large interindividual differences were observed in the inhibitory effects of fluvoxamine in the 12 human liver microsome preparations (Fig. 5). Residual POD activity ranged from 10.3 (HG30) to 73.9% (HG3) of control activities. The combination of fluvoxamine and aniline potently inhibited POD activity to less than 12% of control activities in all 12 human liver microsome samples.
Effects of fluvoxamine alone and in combination with aniline on POD activity in 12 human liver microsomes.
The concentration of phenacetin used was 500 μM. The residual activities with 10 μM fluvoxamine and in combination with 1 mM aniline were expressed as percentages of control activity. Each column represents the mean of duplicate experiments.
Correlation Study.
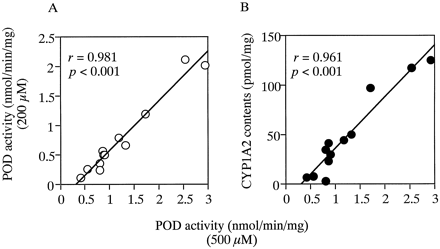
As shown in Fig. 6, POD activity in the 12 human liver microsome preparations at 500 μM phenacetin in the absence of fluvoxamine was significantly correlated with POD activity at 200 μM phenacetin (r = 0.981, p < .001) and also correlated with immunoquantified CYP1A2 levels (r = 0.961, p < .001). No other significant correlations were observed between POD activity in the absence of fluvoxamine and catalytic activities of coumarin 7-hydroxylase (r = 0.145), S-mephenytoinN-demethylase (r = 0.092), diclofenac 4′-hydroxylase (r = 0.563), S-mephenytoin 4′-hydroxylase (r = 0.333), bufuralol 1′-hydroxylase (r = 0.078), chlorzoxazone 6-hydroxylase (r = 0.110), or testosterone 6β-hydroxylase (r = 0.000). POD activity in the absence of fluvoxamine was also not correlated with the immunoquantified levels of CYP2A6 (r = 0.092), CYP2B6 (r = 0.285), CYP2D6 (r = 0.342), CYP2E1 (r = 0.000), or CYP3A4 (r = 0.210).
Correlation between POD activity at 500 μM phenacetin and that at 200 μM phenacetin or immunoquantified levels of CYP1A2 in 12 human liver microsomes.
POD activity in 12 human liver microsomes at 500 μM phenacetin was compared with POD activity at 200 μM phenacetin (A) or immunoquantified levels of CYP1A2 (B). Each data point represents the mean of duplicate experiments.
In the presence of 10 μM fluvoxamine, POD activity at a 500 μM substrate concentration was not significantly correlated with POD activity at 200 μM phenacetin (r = 0.295) and immunoquantified CYP1A2 levels (r = 0.282). However, POD activity at a 500 μM substrate concentration in the presence of fluvoxamine was significantly correlated with chlorzoxazone 6-hydroxylase activity (r = 0.823, p < .001) and also with immunoquantified CYP2E1 levels (r = 0.589, p < .01) as shown in Fig.7. POD activity in the presence of fluvoxamine was not significantly correlated with catalytic activities of coumarin 7-hydroxylase (r = 0.285),S-mephenytoin N-demethylase (r = 0.044), diclofenac 4′-hydroxylase (r = 0.255),S-mephenytoin 4′-hydroxylase (r = 0.110), bufuralol 1′-hydroxylase (r = 0.567), or testosterone 6β-hydroxylase (r = 0.045) and with immunoquantified levels of CYP2A6 (r = 0.141), CYP2B6 (r= 0.095), CYP2D6 (r = 0.348), or CYP3A4 (r = 0.114).
Correlation between POD activity at 500 μM phenacetin in the presence of fluvoxamine and chlorzoxazone 6-hydroxylase activity and immunoquantified levels of CYP2E1 in 12 human liver microsomes.
POD activity in 12 human liver microsomes at 500 μM phenacetin in the presence of 10 μM fluvoxamine was compared to chlorzoxazone 6-hydroxylase activity (A) or immunoquantified levels of CYP2E1 (B). Each data point represents the mean of duplicate experiments.
Discussion
POD is widely used as one of the prototypical reactions catalyzed by CYP1A2 in human liver microsomes. However, several studies have reported that the kinetics of POD in human liver microsomes are biphasic (Boobis et al., 1981; Kahn et al., 1985; Gillam and Reilly, 1988; Tassaneeyakul et al., 1993), suggesting the involvement of more than one CYP isoform. Therefore, we examined the roles of several human CYPs in POD by using 13 recombinant CYP isoforms: CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5, and CYP4A11. Figure 1 shows that CYP1A2 exhibited the highest specific activity, followed by CYP1A1 and CYP2C19. CYP2D6, CYP2E1, and CYP3A4 coexpressed with b5 also interacted with phenacetin, although with low activities. These results suggested that CYP1A2, CYP1A1, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 could catalyze POD in human liver microsomes.
The biphasic liver microsomal POD kinetics observed in the present study are consistent with results of previous human in vitro kinetic studies (Boobis et al., 1981; Kahn et al., 1985; Gillam and Reilly, 1988; Tassaneeyakul et al., 1993). The apparentKM values for the high- and low-affinity components of POD activity in pooled human liver microsomes obtained from this study were of a similar order to those from several previous reports (Boobis et al., 1981; Kahn et al., 1985; Gillam and Reilly, 1988; Tassaneeyakul et al., 1993). To determine the contribution of individual CYP isoforms to the high- and low-affinity components of POD in human liver microsomes, the apparent KMvalues of recombinant CYP isoforms for POD were compared with those of the high- and low-affinity components of POD in human liver microsomes. The KM value of the high-affinity component in pooled human liver microsomes was close to that of recombinant CYP1A2 or CYP1A1 (Table 1). In addition, anti-CYP1A2 antibodies specifically inhibited POD activity at 500 μM phenacetin, but anti-CYP1A1 antibodies did not (Fig. 3). Therefore, CYP1A1 was considered not to contribute to POD in human liver microsomes. These observations are in agreement with the proposal (Sesardic et al., 1988) that CYP1A2 is responsible for the high-affinity component of POD in human liver microsomes and for the low expression or lack of expression of CYP1A1 in uninduced human liver (Murray et al., 1993).
The KM value of the low-affinity POD component in pooled human liver microsomes was similar to that of recombinant CYP2E1 (Table 1). To our knowledge, this is the first report that CYP2E1 is responsible for POD activity as the low-affinity enzyme in human liver microsomes. Therefore, additional studies, including selective inhibition and correlation analyses, were conducted to evaluate the role of CYP2E1 in POD. The contribution of CYP2E1 to POD was supported by a number of observations. First, CYP2E1 inhibitors, DDC and aniline, reduced POD activity at 500 μM phenacetin in pooled human liver microsomes (Fig. 2). Second, the combination of fluvoxamine and aniline further inhibited the residual POD activity not inhibited by fluvoxamine alone in both pooled and individual microsome preparations from human livers (Figs. 4 and 5). Third, POD activity in 12 human liver microsome preparations at 500 μM phenacetin in the presence of 10 μM fluvoxamine significantly correlated with immunoquantified CYP2E1 levels and chlorzoxazone 6-hydroxylase activity, which is catalyzed by CYP2E1 (Fig. 7). Taken together, these observations suggested that CYP2E1 was responsible for the low-affinity component of POD in human liver microsomes and was involved in the reaction at high substrate concentrations.
Experiments with pooled human liver microsomes revealed that anti-CYP1A2 antibody specifically inhibited POD activity at a substrate concentration of 500 μM, but anti-CYP2E1 antibody did not (Fig. 3). In addition, the inhibition of POD activity by 1 mM aniline was weaker than that by 10 μM fluvoxamine (Fig. 4). These results suggested that CYP1A2 predominantly catalyzed POD at substrate concentrations of 500 μM in pooled human liver microsomes and that CYP2E1 was involved in the reaction as a minor enzyme. However, inhibition experiments performed on microsomes prepared from 12 individual human livers indicated that large differences between individuals were observed in the inhibitory effects of 10 μM fluvoxamine (Fig. 5). The residual percentages of POD activity not inhibited by 10 μM fluvoxamine ranged from 10.3 (HG30) to 73.9% (HG3) of control activities. Interestingly, the residual percentages of POD activity not inhibited by 10 μM fluvoxamine negatively correlated with immunoquantified levels of CYP1A2 (r = −0.752, p < .01). These results suggested that there were large interindividual differences in the contribution of CYP1A2 to POD at 500 μM phenacetin in human liver microsomes, and that these differences were dependent on CYP1A2 levels in individual microsomes of human livers. In addition, the combination of 10 μM fluvoxamine and 1 mM aniline potently inhibited POD activity at 500 μM phenacetin in all human liver microsome preparations studied. Therefore, the contribution of CYP2E1 to POD at 500 μM phenacetin may be significant in human liver microsomes with low CYP1A2 contents.
The correlation study with the 12 human liver microsome preparations indicated that POD activity did not correlate with the specific activities and immunoquantified levels of CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, or CYP3A, regardless of the presence or absence of fluvoxamine. In addition, antibodies against CYP2A6, CYP2B6, CYP2C, CYP2D6, and CYP3A did not inhibit POD activity at 500 μM phenacetin in pooled human liver microsomes (Fig. 3). In experiments with recombinant CYPs, CYP2A6, CYP2B6, and CYP2C9 failed to catalyze POD (Fig. 1). Although recombinant CYP2C19, CYP2D6, and CYP3A4 exhibited the significant POD activities (Fig. 1), KMvalues could not be estimated because of linear plots of Vversus S. These results suggested that CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 play negligible roles in POD in human liver microsomes.
In conclusion, it appears that CYP1A2 and CYP2E1 are primarily involved in POD in human liver microsomes as high- and low-affinity enzymes, respectively. To our knowledge, this is the first study to present data that CYP2E1 is responsible for POD in human liver microsomes as the low-affinity enzyme. In human liver microsomes with low CYP1A2 contents, CYP2E1 may make a significant contribution to POD. At least in vivo, POD is considered to be mainly catalyzed by CYP1A2, which is a high-affinity enzyme of POD (Xiaodong et al., 1994; Bartoli et al., 1996). However, we should take into account the possibility that CYP2E1 may also contribute to POD activity at high substrate concentrations, when POD is used as a marker reaction for CYP1A2 function in human liver microsomes.
Footnotes
-
Send reprint requests to: Kaoru Kobayashi, Ph.D., Laboratory of Biochemical Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Chiba University, Yayoi-cho 1-33, Inage-ku, Chiba 263-8522, Japan. E-mail: kaoruk{at}p.chiba-u.ac.jp
- Abbreviations used are::
- CYP
- cytochrome P-450
- POD
- phenacetin O-deethylation
- TAO
- troleandomycin
- DDC
- N,N- diethyldithiocarbamate
- Received February 8, 1999.
- Accepted May 3, 1999.
- The American Society for Pharmacology and Experimental Therapeutics