Abstract
The pharmacokinetics and biotransformation of the antiretroviral agent nevirapine (NVP) after autoinduction were characterized in eight healthy male volunteers. Subjects received 200-mg NVP tablets once daily for 2 weeks, followed by 200 mg twice daily for 2 weeks. Then they received a single oral dose (solution) of 50 mg containing 100 μCi of [14C]NVP. Biological fluids were analyzed for total radioactivity, parent compound (HPLC/UV), and metabolites (electrospray liquid chromatography/mass spectroscopy and liquid chromatography/tandem mass spectroscopy). Mean recovery of radioactivity was 91.4%, with 81.3% excreted in urine and 10.1% recovered in the feces over a period of 10 days. Circulating radioactivity was evenly distributed between whole blood and plasma. At maximum plasma concentration, parent compound accounted for ∼75% of the circulating radioactivity. Mean plasma elimination half-lives for total radioactivity and NVP were 21.3 and 20.0 h, respectively. Several metabolites were identified in urine including 2-hydroxynevirapine glucuronide (18.6%), 3-hydroxynevirapine glucuronide (25.7%), 12-hydroxynevirapine glucuronide (23.7%), 8-hydroxynevirapine glucuronide (1.3%), 3-hydroxynevirapine (1.2%), 12-hydroxynevirapine (0.6%), and 4-carboxynevirapine (2.4%). Greater than 80% of the radioactivity in urine was made up of glucuronidated conjugates of hydroxylated metabolites of NVP. Thus, cytochrome P-450 metabolism, glucuronide conjugation, and urinary excretion of glucuronidated metabolites represent the primary route of NVP biotransformation and elimination in humans. Only a small fraction of the dose (2.7%) was excreted in urine as parent compound.
Nevirapine (NVP)1 is a non-nucleoside, reverse transcriptase inhibitor approved for use in the treatment of the HIV infection in humans. NVP (Fig. 1) is a dipyridodiazepinone that binds directly to reverse transcriptase and blocks the RNA- and DNA-dependent DNA polymerase activities by causing a disruption of the catalytic site of the enzyme (Merluzzi et al., 1990; Richman et al., 1991). When used in combination with other antiretroviral drugs (e.g., nucleosides and protease inhibitors), NVP has demonstrated significant antiviral activity at the established therapeutic dose of 200 mg twice daily (Cheeseman et al., 1995; Havlir et al., 1995).
Structure of NVP.
∗, position of 14C-radiolabel.
In clinical studies, NVP is readily (>90%) absorbed after oral administration in healthy volunteers and in patients with the HIV infection (Lamson et al., 1995a). After a single 200-mg dose, plasma NVP concentrations reach a maximum of 2 μg/ml by 4 h postdose and decline log linearly thereafter, resulting in a terminal phase half-life of ∼45 h. In vitro studies and early Phase I studies have demonstrated that NVP is an autoinducer of cytochrome P-450 (CYP) metabolism. Therapeutic dosing has been shown to result in an ∼2-fold increase in NVP apparent clearance and reduction in NVP half-life from 45 to 30 h after 2 weeks of dosing with 200 mg per day compared with a single dose (Lamson et al., 1995b). The purpose of the present study was to evaluate the pharmacokinetics and biotransformation of NVP under conditions of autoinduction as part of the clinical development of the compound. The study was performed in eight healthy male volunteers who were administered 200 mg of NVP once daily for 14 days, followed by 200 mg twice daily (400 mg/day) for an additional 14 days, followed by a single oral dose (solution) of 50 mg containing 100 μCi of [14C]NVP.
Materials and Methods
Reference Compounds.
NVP (5,11-dihydro-11-cyclopropyl-4-methyl-6H-dipyrido [3,2-b:2′,3′-e][1,4]diazepin-6-one) was synthesized at Boehringer Ingelheim Pharmaceuticals, Inc. (Ridgefield, CT), along with the following compounds: [14C]NVP (Fig. 1), 12-hydroxynevirapine, 2-hydroxynevirapine, 3-hydroxynevirapine, 5-hydroxynevirapine, 7-hydroxynevirapine, 8-hydroxynevirapine, A-ring and C-ring N-oxide derivatives of NVP, 5-methylnevirapine, and descyclopropylnevirapine. [14C]NVP was further purified by preparative HPLC, isotopically diluted by addition of unlabeled NVP and recrystallized from aqueous hydrochloric acid solution by neutralization with sodium hydroxide to afford [14C]NVP (specific activity, 90.9 μCi/mg, 3363 MBq/g) as the hemihydrate. Chemical identity was established by comparison of its melting point, infrared spectrum, and chromatographic behavior with those of authentic NVP. Radiochemical purity was established at 99.9% by HPLC and 98.6% by thin-layer chromatography.
Subjects, Dosing, and Sample Collection.
Eleven healthy male volunteers (20–34 years of age, weighing 62.6–84.4 kg) who met the eligibility criteria were enrolled in the study. Eight subjects completed all phases of the study including administration of [14C]NVP. Subjects were to be nonsmokers and received the same NVP treatment: 200 mg once daily for 2 weeks, followed by 200 mg every 12 h (400 mg/day) for 2 additional weeks. Volunteers then fasted from 10:00 PM the night before until 4 h after administration of [14C]NVP, prepared as an oral solution (50 mg in 200 ml in 1.25% citric acid solution, 100 μCi/200 ml), at 8:00 AM. Except for acetaminophen and ibuprofen for headache, no medications were administered during the 28-day pretreatment period with 200 and 400 mg of NVP per day. No medications were administered to the volunteers after administration of the radiolabeled dose. Additionally, no coffee, tea, cola, or alcohol ingestion was permitted during the course of the study.
Serial blood samples (10 ml) were obtained at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 8, 12, 24, 48, 72, 96, 120, 144, 168, 192, 216, and 240 h postdose. Both whole blood and plasma were analyzed for radioactivity. Urine samples were collected before dosing and at 0 to 12 and 12 to 24 h after dosing and daily thereafter for 10 days. Fecal samples were collected for 10 days. All samples were stored at approximately −20°C until analyzed.
Analysis of Radioactivity.
Whole blood and fecal samples were combusted before counting using a Packard 306 Sample Oxidizer. Aliquots of 200 μl from a 2-ml sample of whole blood and 500 μl from a 25% (w/v) fecal homogenate were placed in sample oxidizer combustion cones, weighed, air dried, and combusted in a sample oxidizer. Blood and fecal samples prepared in this manner, along with plasma (200 μl) and urine (500 μl) samples mixed with 10 ml of Insta-Gel XF, were counted for total radioactivity using a Packard 2500 TR liquid scintillation analyzer (Packard Instrument Co., Downers Grove, IL).
Quantitation of NVP in Plasma.
Plasma samples were prepared utilizing solid phase extraction (SPE) and analyzed for NVP concentrations using a validated HPLC assay at Boehringer Ingelheim Pharmaceuticals, Inc. The assay covered the range of 0.025 to 10 μg/ml, had a limit of quantitation of 0.025 μg/ml, and had coefficients of variation for accuracy and precision to within <15%.
Radiochromatographic Analysis of NVP and Metabolites in Plasma, Urine, and Feces.
Plasma and urinary metabolites
Urine samples taken over 0 to 96 h from each subject were centrifuged (2500 rpm) at 4°C for 15 min using an IEC Centra MP4R centrifuge (International Equipment Co., Needham Heights, MA). The volume of urine corresponding to 200,000 dpm of radioactivity was determined. For analysis of plasma samples, 1-ml aliquots were obtained from each pooled serial time point (2.4 ml). For either matrix (urine or plasma), a SPE technique was used for the analysis. The extraction process utilized a Visiprep 24 Port-DL Solid Phase Extraction Manifold (Supelco, Bellefonte, PA) equipped with a Supelco SPE Vacuum Pump Trap kit attached to a Welch GEM 8890 vacuum pump (Welch Vacuum Pump Technology, Inc., East Hanover, NJ) to provide continuous vacuum. Each sample was loaded into an activated Sep-Pak Vac (6 ml, 1 g) C18 cartridge (Waters Corp., Milford, CT) and eluted slowly through the cartridge in dropwise fashion. The vacuum in the manifold never dropped below −15 mm Hg. Next, the columns were washed with 6 ml of Omnisolv HPLC water (EM Science, Gibbstown, NJ). The entire aqueous portion was removed, and the cartridge was allowed to dry under vacuum for 1 h. Finally, the cartridge was eluted with 3 ml of methanol and dried under nitrogen at 40°C in a Zymark Turbo Vap LV Evaporator (Zymark Corp., Hopkinton, MA). The dried urine and plasma extracts were taken up in 200 μl of KH2PO4 (0.05 M) with triethylamine 0.1% (v/v). Every step of the procedure was evaluated with respect to extraction efficiency of total radioactivity.
Fecal metabolites.
Fecal samples taken over 24 to 48 h and 48 to 72 h from the two subjects with the highest recovery of radioactivity (subject nos. 3 and 11) were used to represent the study population. Each sample was manually mixed to ensure homogeneity, after which a 30-ml aliquot was decanted to an appropriately labeled, 50-ml disposable plastic centrifuge tube and centrifuged in a Beckman Model TJ-6 Centrifuge for 15 min at a minimum of 4000 rpm. The fecal sample supernatants were transferred to another set of 50-ml disposable plastic centrifuge tubes and weighed on a Sartorius AC210S analytical balance (Sartorius Corp., Gottingen, Germany). Single 100-μl aliquots were counted for the determination of the total radioactivity in each fecal supernatant using the Beckman LS 5000 TA. After centrifugation, 90% or more of the radioactivity was determined to be in the fecal supernatants. An appropriate volume of supernatant was then extracted by the SPE procedure described above, with every step of the procedure, as with plasma and urine, evaluated with respect to extraction efficiency of total radioactivity. Finally, the dried fecal extracts were taken up in 200 μl of KH2PO4 (0.05 M) with triethylamine 0.1% (v/v).
HPLC.
For the chromatographic analysis, a Hewlett Packard 1090 Win Liquid Chromatography System was used along with an HPLC3D ChemStation (DOS Series) system. The latter was equipped with an auto-injector, UV diode-array detector, column oven, PV5 solvent delivery system and HP 1090 Win System Software Rev. A.0205. An ODS Hypersil C18(4.0 × 20 mm) guard column and a Waters Nova-Pak C18 (3.9 × 300 mm, 4-μm particle size) analytical column were used for the chromatographic separation. The mobile phase for solvent A was comprised of potassium phosphate monobasic buffer (0.05 M, pH 4.6) with triethylamine 1% (v:v), and the mobile phase for Solvent B was acetonitrile. The gradient conditions were 0 to 35% of solvent B over 60 min with data acquisition for 60 min. Other pertinent information included: injection volume, 100 μl; flow rate, 1 ml/min; detection, A: UV diode-array detector wavelength (λ), 240 nm; Radiomatic FLO-ONE/β Radio-Chromatography detector (Series A-500); scintillation cocktail, ULTIMA-FLO M; liquid scintillation pump flow rate, 3 ml/min; and TR-LSC Flow cell volume, 0.5 ml/min.
Radiochromatographic analysis.
Radiolabeled NVP mobile phase standards with concentrations ranging from 0.05 to 50 ng/μl were made from a methanolic 1 μg/μl standard stock solution. The specific activity of these standards was 19.32 μCi/mg or 5.33 mCi/mmol. From these standards, calibration curves in mobile phase, urine, and plasma were constructed with concentrations ranging from 10 to 10,000 ng/ml. External standardization was used for the quantitation of parent compound and/or metabolites (dpm/ml and/or μg/ml). The volume of pooled urine associated with 200,000 dpm and the fecal sample volumes associated with 100,000 dpm were extracted such that half the radioactivity found in the methanolic extract was injected on the column to yield the matrix metabolite patterns. For the analysis of plasma samples, 1-ml aliquots were obtained from each pooled serial time point (2.4 ml) and also extracted such that half the radioactivity extracted was injected on the column. For the incubation mixtures, the methanolic extracts were dissolved in 200 μl of 50 mM KH2PO4 with 0.1% triethylamine (v/v), and 20 μl of each sample was injected onto the HPLC system. All of the metabolite pattern radiochromatograms were similarly integrated, and peak percentages were determined from the total peak area sum.
Enzymatic Hydrolysis.
Glucuronidase incubations were performed for the isolated glucuronide metabolites of NVP. For the control incubation, no enzyme or inhibitor was added to the flask. The glucuronidase incubation had 25 mg (41,667 U) of β-glucuronidase type IX-A (G-7396 Lot # 33H6846; Sigma Chemical Co., St. Louis, MO) or 100 μl (20,000 U) of β-glucuronidase type X-A (G-7896 Lot # 63H6838; Sigma) added to the flask. Both forms of β-glucuronidase were isolated from Escherichia coli and lacked sulfatase activity. Type X-A is a highly purified form of type IX-A. For the inhibited incubation, 25 mg ofd-saccharic acid 1,4-lactone (S-0375 Lot # 128F3887; Sigma), a known inhibitor of β-glucuronidase, was added to the flask before enzyme. The samples were then vortexed, covered with Parafilm (American Can Co., Greenwich, CT), and incubated overnight at 37°C in a shaking water bath. After the incubation period, the samples were extracted by the SPE procedure.
Metabolite Identification.
HPLC
The preliminary determination of metabolite structures was achieved using UV spectral analysis and chromatographic overlays of the NVP analog standards with each urine radiochromatogram. The UV spectral analysis entailed the exact spectral matching of the metabolite to the NVP analog standard. The chromatographic overlays compared the metabolite retention times to those of the authentic standards.
Liquid chromatography/mass spectroscopy (LC/MS) and LC/tandem mass spectroscopy (LC/MS/MS).
Radiolabeled (1 mg) and nonlabeled NVP (4 mg) were mixed and dissolved in methanol to yield a 1 μg/μl standard stock solution. From this solution, mobile phase standards were made with concentrations ranging from 0.05 to 50 ng/μl. The specific activity of these standards was 18.2 μCi/mg or 4.8 μCi/mmol. The standards were used for the generation of parent compound radiochromatograms and served as HPLC performance standards.
The mass spectrometric analyses were conducted on a Finnegan MAT TSQ700 (Finnegan Corp., San Jose, CA) equipped with an electrospray interface attached to a Waters 600 MS Multisolvent Delivery System (Waters Corp.). A Metachem Inertsil ODS-2, 5 μm, 1.0 × 150-mm column set at 40°C was used for the metabolite separations. A linear gradient from 0 to 30% acetonitrile over 30 min was used with an 80 μl/min flow rate. Positive ion mode mass spectra were obtained.
A mixture of NVP analog standards (200 ng/μl per standard) was diluted with 25 mM ammonium acetate (pH 4.0) such that 33.3 ng per standard was injected (1 μl) onto the HPLC system. The 0- to 96-h extracted sample from the pooled urine collection (n = 7 and 261,027 dpm) was brought up in 100 μl of 25 mM ammonium acetate (pH 4.0), vigorously mixed, and 2 μl was injected onto the HPLC system. Final determination of metabolite structures was achieved by mass spectral analysis, UV spectral analysis, and chromatographic overlays of the NVP analog standards with each radiochromatogram. In addition, the structural determination of the conjugated metabolites was completed by several β-glucuronidase incubations. The structural confirmation of the male rat urinary 4-carboxynevirapine major metabolite was achieved with additional NMR and mass spectroscopy identification (Riska et al., 1996). This information yielded additional structural confirmation, which led to the identification of 4-carboxynevirapine as a human minor metabolite.
Pharmacokinetic Analysis.
The pharmacokinetics of NVP were characterized by fitting a one-compartmental model with first order absorption and elimination to the NVP plasma concentration-time data using WinNonlin, Version 2.1. An inverse concentration weighting function (1/C) was used for the nonlinear regression analysis. Goodness of fit was evaluated by examination of the predicted values and residual plots. The following pharmacokinetic parameters were estimated for NVP: first order absorption (ka) and elimination (k) rate constants, half-life (T1/2 32 = 32 ln 32 2/k), apparent clearance (CL/F), volume of distribution (V/F), and average steady-state NVP plasma concentration (Css).
Results
Mass Balance Excretion of Radiocarbon.
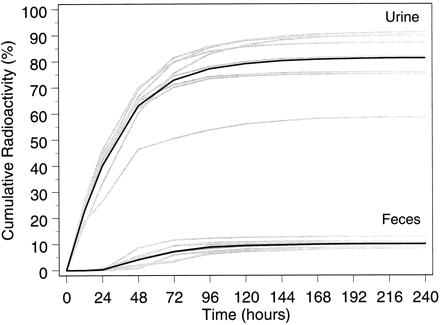
After multiple therapeutic doses of NVP (200 mg once daily for 2 weeks, followed by 200 mg twice daily for 2 weeks), followed by a single 50-mg oral dose of [14C]NVP (100 μCi) to eight subjects, radioactivity was predominantly excreted in urine (Fig.2). Approximately 91.4 ± 10.5% (mean ± S.D.) of the radiolabeled dose was recovered, with urine (81.3 ± 11.1%) representing the primary route of excretion compared with feces (10.1 ± 1.5%). Total recovery for subject 9 (69.2%) was outside the S.D. for the study group as a whole, perhaps due to failure by this subject to collect complete samples. Otherwise, the total recovery of radioactivity in the remaining seven subjects was 94.5 ± 5.9%.
Cumulative recovery of radioactivity in urine and feces.
Shaded curves, the cumulative recovery for each patient; darkened curves, the numerical average for the study group.
Plasma and Blood Radioactivity and Plasma NVP Concentration Profiles.
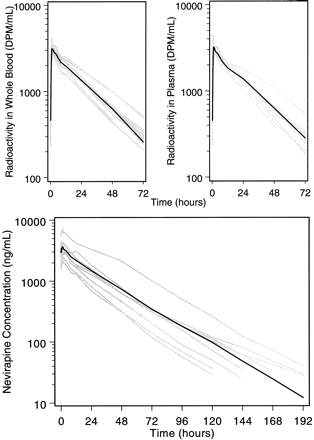
Plasma concentrations of NVP were quantitated using a validated HPLC assay with UV detection; the limit of quantitation was 25 ng/ml. Mean concentration versus time plots of radioactivity in plasma and whole blood were parallel as shown in Fig. 3. Circulating radioactivity was evenly distributed between whole blood and plasma. The slow decline of radioactivity in whole blood and of radioactivity and NVP in plasma (Fig. 3) over time indicates a prolonged disposition phase relative to the rate of absorption.
Concentrations of radioactivity in whole blood and plasma (top) and of NVP in plasma (bottom).
Shaded curves, the cumulative recovery for each patient; darkened curves, the numerical average for the study group.
Pharmacokinetics.
The pharmacokinetics of NVP were characterized from the plasma concentration data taken after administration of the multiple dose regimen and single 50-mg oral dose of [14C]NVP. The plasma concentration-time data were fitted by a one compartment model with first order absorption and elimination. The calculated pharmacokinetic parameters are shown in Table1. The mean elimination half-life of NVP in plasma (20.0 ± 3.4 h) closely approximated the mean elimination half-lives of radioactivity in plasma (21.3 ± 3.6 h) and whole blood (21.8 ± 2.5 h). The mean apparent systemic clearance and volume of distribution of NVP were 3.29 ± 0.89 liter/h and 92.0 ± 19.3 liters, respectively. In this group of subjects, the average steady-state NVP plasma concentration associated with the NVP 200 mg twice daily dosing regimen was 5.5 ± 1.9 μg/ml.
NVP pharmacokinetic parameters after autoinduction
Identification of Metabolites.
Urine
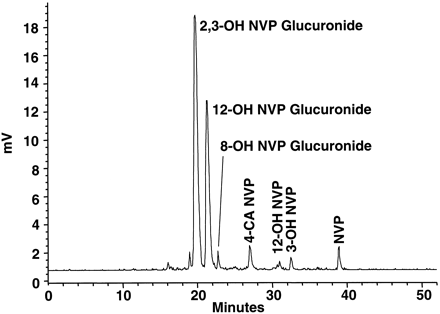
UV spectral analysis of the urine chromatograms, contrasted with chromatographic overlays of the NVP analog standards, were used for identification and quantitation of parent compound and major metabolites. A typical radiochromatogram (0–96 h) is shown in Fig.4. Two major metabolites of NVP, later identified as glucuronides of 2- and 3-hydroxynevirapine, coeluted on the radiochromatogram. Collectively, they accounted for 54.5 ± 5.1% of the total radioactivity in urine. Other glucuronides in urine included 12-hydroxynevirapine (29.1 ± 6.5%) and 8-hydroxynevirapine (1.6 ± 0.2%). Nonglucuronide metabolites, including 3-hydroxynevirapine (1.5 ± 0.3%), 12-hydroxynevirapine (0.7 ± 0.3%), NVP parent compound (3.3 ± 3.5%), and 4-carboxynevirapine (2.9%), accounted for only a small fraction of the total radioactivity in urine.
Representative radiochromatogram of a pooled urine sample (0–96 h) from subject 3.
By increasing the gradient from 60 to >90 min, it was possible to separate the peaks associated with the 2- and 3-hydroxynevirapine glucuronide metabolites in urine, although the profiles were not completely resolved (Fig. 5). As shown in Fig. 6, incubation of the two coeluted metabolites, 2- and 3-hydroxynevirapine glucuronide, with β-glucuronidase yielded the hydrolyzed aglycon byproducts 2-hydroxynevirapine (42%) and 3-hydroxynevirapine (58%). Glucuronidase incubations with the other isolated glucuronide metabolites of NVP revealed no evidence of additional coeluted metabolites. The recovery of NVP and its metabolites in urine is presented in Table 2. Overall, >90% of the radioactivity in urine was made up of glucuronide conjugates of hydroxylated NVP metabolites.
Magnified view of HPLC radiochromatogram of a pooled urine sample (120-min gradient).
Representative UV chromatogram overlay of isolated 2- and 3-hydroxynevirapine glucuronide before and after incubation with β-glucuronidase.
Recovery of radioactivity in urine
Plasma.
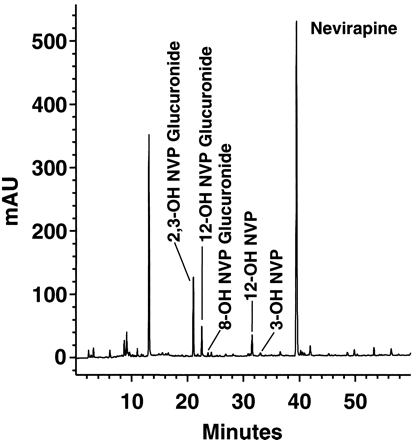
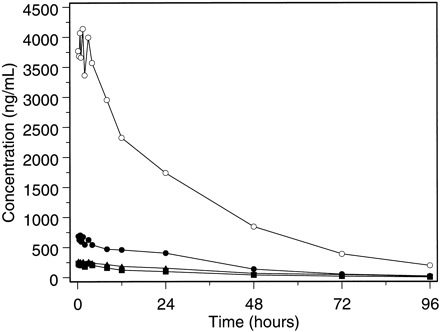
The concentration of NVP and major metabolites of NVP were quantitated in plasma by pooling samples from all eight subjects at each time point over 0 to 96 h. The pooled samples were quantitated and profiled using HPLC with UV diode array detection (λ = 240 nm). A typical chromatographic profile from the pooled sample taken at 1.5 h after radiolabeled dose (peak concentration) is shown in Fig.7. NVP accounted for most (75%) of the total analyte signal in the plasma chromatogram. Several major metabolites also were identified in plasma including the coeluted 2- and 3-hydroxynevirapine glucuronides (12%), 12-hydroxynevirapine glucuronide (5%), 12-hydroxynevirapine (4%), and 3-hydroxynevirapine (0.6%). Collectively, NVP and the metabolites identified in the plasma chromatogram accounted for >95% of the radioactivity in plasma. The concentration profiles of NVP and major metabolites in plasma as determined from the chromatograms are shown in Fig.8.
HPLC radiochromatogram of a pooled plasma sample taken at 1.5 h after administration of [14C]NVP.
Plasma concentration-time profiles for NVP (○), 2/3-hydroxynevirapine glucuronide (●), 12-hydroxynevirapine glucuronide (▴), and 12-hydroxynevirapine (▪).
The plasma NVP concentrations in this study were within the clinical range of concentrations associated with therapeutic dosing with NVP, although they were not representative of steady state because the treatment dose (200 mg) was followed by a 50-mg radiolabeled dose. However, the profiles illustrate the relative concentrations of major metabolites and NVP under conditions of autoinduction. The decline of NVP and metabolite concentrations were consistent with the decline of total radioactivity over the same time interval. The ratios of parent compound to individual metabolites remained relatively constant. At 96 h, the concentration of NVP declined to 197 ng/ml, whereas the concentration of each metabolite was at or below the limit of quantitation (25 ng/ml). The plasma chromatograms indicate that most of the analyte in plasma was associated with NVP parent compound and that the pharmacokinetic disposition of radioactivity was rate-limited by biotransformation of NVP to its hydroxylated metabolites rather than by excretion of the metabolites into feces and urine.
Feces.
UV spectral analysis of the fecal chromatograms taken from two subjects (nos. 3 and 11) at 24 to 48 and 48 to 72 h after radiolabeled dose were analyzed with chromatographic overlays of NVP analog standards. There was no evidence of glucuronide conjugates of NVP in feces, although this does not take into account the possibility of hydrolysis by the intestinal microflora. Most of the fecal radioactivity was represented by NVP parent compound (1.8–4.3%), 2-hydroxynevirapine (7.7–11.7%), 3-hydroxynevirapine (19.4–23.9%), 12-hydroxynevirapine (1.6–3.7%), carboxynevirapine (4.7–7.7%), and 8-hydroxynevirapine (1.2–2.3%).
Estimation of level of metabolites.
On the basis of the percentage of radioactivity excreted in urine (81.3%), the percentage of the dose represented by NVP and its various metabolites is presented in Table 2. Overall, ∼76.2% of the radiolabeled dose was recovered in the urine in the form of glucuronidated hydroxyl metabolites of NVP (69.3%). Only a small fraction of the dose (2.7%) was recovered in the urine as parent compound.
Discussion
In this study conducted in eight healthy male volunteers dosed to steady state with NVP 200 mg twice daily followed by a single 50-mg dose of [14C]NVP, ∼91.4 ± 10.5% of the radiolabeled dose was recovered in urine and feces within 10 days of dosing. Renal excretion was the primary mode of NVP elimination, accounting for 81.3 ± 11.1% of the radiolabeled dose compared with feces (10.1 ± 1.5%). Excretion of NVP parent compound in urine represented ∼2.7% of the dose. Although no radioactivity was detected in plasma by 96 h postdose, NVP plasma concentrations quantitated by HPLC with UV detection at 144 to 192 h postdose indicated that residual NVP was still being eliminated from the systemic circulation, despite radioactivity concentrations falling below detectable levels, and partially explains the incomplete recovery (<10% of the dose) of radiocarbon.
Recovery of 81% of the radiocarbon in urine indicates that nevirapine was well absorbed orally. This finding was consistent with earlier estimates of >90% bioavailability in humans (Lamson et al., 1995a). NVP pharmacokinetics were characterized by a relatively prolonged pharmacokinetic disposition phase, even under conditions of autoinduction. The magnitude of autoinduction is further depicted by the change in NVP exposure after 4 weeks of dosing with NVP 200 to 400 mg/day when compared with lower doses or a single dose. NVP clearance in the present study was ∼2-fold higher (single dose CL/F,1.5 ± 0.4 liter/h versus multiple dose CL/F, 3.3 ± 0.9 liter/h), and NVP half-life was proportionally lower (single dose T1/2, 44.0 ± 12.9 h versus multiple dose T1/2, 20.0 ± 3.4 h) when compared with a single 200-mg exposure (Lamson et al., 1995b;Murphy and Montaner, 1996). Collectively, these data suggest that the higher NVP clearance observed in the present study was due to a change in NVP metabolism rather than to altered bioavailability.
At the time of maximum concentration of NVP, parent compound accounted for 75% of the radioactivity in plasma. Plasma concentrations of NVP and radioactivity declined log-linearly at approximately the same rate thereafter. Therefore, the results demonstrate that NVP was the largest single component of the circulating radiocarbon in plasma. The plasma profiles and half-lives of radiocarbon (21.3 h) and NVP (20.0 h) suggest that biotransformation of NVP parent represents the rate-determining step in the elimination of radiocarbon from the systemic circulation. The presence of multiple radioactive peaks in the UV chromatograms of plasma and urine, together with the mass spectrometric analysis, indicates that NVP biotransformation involves extensive hydroxylation and glucuronidation of hydroxylated metabolites. The metabolites were then largely excreted into the urine, where 2-hydroxynevirapine glucuronide (22.9%), 3-hyroxynevirapine glucuronide (31.6%), and 12-hydroxynevirapine glucuronide (29.0%) appeared in the highest quantities relative to total urine radioactivity. Collectively, the major metabolites accounted for 68% of the total radiolabeled dose.
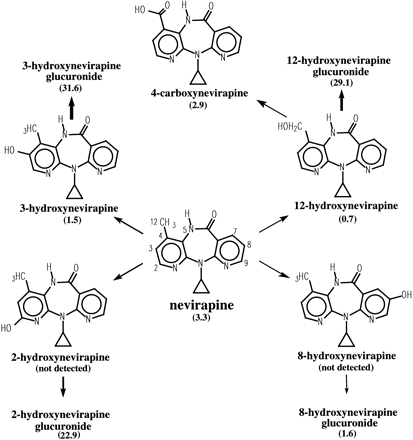
Two of the major NVP metabolites (2- and 3-hydroxynevirapine glucuronide) coeluted in the urine radiochromatogram and were separated after enzymatic hydrolysis with β-glucuronidase. Other less prominent metabolites in urine identified from the UV chromatograms, LC/MS, and LC/MS/MS analyses included 2-, 3-, 12-, and 8-hydroxynevirapine, and 4-carboxynevirapine that was formed by secondary oxidation of 12-hydroxynevirapine. Many of the same metabolites, albeit in markedly smaller quantities, were also found in the feces. The proposed metabolic biotransformation of NVP in humans under conditions of autoinduction is shown in Fig.9.
NVP biotransformation pathways in humans.
The percentage of radioactivity recovered in urine is indicated within the brackets.
Although significant differences in the rate of elimination of NVP have been noted between various animal species in vivo and in vitro and humans (i.e., dog > mouse > monkey > male rat > female rat > chimp > human), qualitatively the metabolism of NVP is similar (Riska et al., 1996). NVP autoinduction also has been observed across species. In animals and humans, NVP undergoes significant oxidative metabolism to 2-, 3-, and 8-hydroxynevirapine, 4-carboxynevirapine, and 12-hydroxynevirapine, followed by glucuronidation and renal excretion. The only exception was the beagle dog; after administration of [14C]NVP 20 mg/kg, ∼41 to 46% of the dose was recovered in the feces as parent compound.
In vitro experiments with human liver microsomes containing specific cDNA-expressed CYP isoforms suggest that the formation of 2- and 3-hydroxynevirapine are mediated exclusively by CYP3A and CYP2B6, respectively, and that formation of 12- and 8-hydroxynevirapine are mediated by multiple enzymes including CYP3A and CYP2D6 (D. Erickson, G. Mather, R. Levy, J.K., submitted). With 2-, 3-, and 12-hydroxynevirapine as major metabolites, the results of the present study suggest that CYP3A4 and/or CYP2B6 are pathways that are induced by NVP. The significance of NVP induction of CYP3A with respect to interactions with other drugs, particularly protease inhibitors, has been extensively investigated (Sahai et al., 1997; Murphy et al., 1999). However, the significance of the potential for NVP to induce the CYP2B6 pathway is not well known and is the subject of additional investigation.
In summary, after administration of therapeutic doses to induce hepatic enzymes, a single dose of [14C]NVP was well absorbed orally and nearly completely (91.4%) recovered in urine (81.3%) and feces (10.1%). Greater than 80% of the radioactivity in urine was made up of glucuronidated conjugates of hydroxylated metabolites of NVP. Thus, CYP metabolism, glucuronide conjugation, and urinary excretion of glucuronidated metabolites represent the primary routes of NVP biotransformation and elimination in humans.
Acknowledgments
We thank Suzannah Cort, Heather Macy, and Dr. Philip Leese (Innovex, Inc.) for assistance in conducting the study; Clark Perry for synthesizing [14C]NVP; and Deborah Korpalski, Lois Rowland, Wayne Yu, and David Joseph for their chromatography support. In addition, we thank Randy Press of Oread Laboratories, Inc. for the determination of matrix radioactivity and Greg Rahn of Oneida Research Services for assistance in the LC/MS/MS metabolite identification.
Footnotes
-
Send reprint requests to: Paul Riska, Ph.D., Department of Drug Metabolism and Pharmacokinetics, Boehringer Ingelheim Pharmaceuticals, Inc., 900 Ridgebury Rd., Ridgefield, CT 06877. E-mail:priska{at}rdg.boehringer-ingelheim.com
- Abbreviations used are::
- NVP
- nevirapine
- SPE
- solid phase extraction
- LC/MS
- liquid chromatography/mass spectroscopy
- LC/MS/MS
- LC/tandem mass spectroscopy
- CYP
- cytochrome P-450
- Received November 5, 1998.
- Accepted April 27, 1999.
- The American Society for Pharmacology and Experimental Therapeutics