Abstract
CYP2C9 is involved in the metabolism of the oral anticoagulants warfarin, phenprocoumon, and acenocoumarol. It is also responsible for the 5′-hydroxylation of the nonsteroidal anti-inflammatory drug lornoxicam. Therefore, lornoxicam and the oral anticoagulants are potential inhibitors of their metabolism. Their inhibitory potency was investigated in microsomes from six human livers. An approach to predict pharmacokinetic interactions of lornoxicam from in vitro inhibition data was developed. Where possible, the forecasts were verified by comparison with data from clinical interaction studies. The following increases in steady-state plasma concentrations or areas under the plasma concentration-time curve of the oral anticoagulants by concomitant lornoxicam medication were predicted (values in parentheses are for healthy volunteers): (S)-warfarin, 1.58-fold (1.32-fold for racemate); racemic-acenocoumarol, 1.28-fold (1.09-fold); (R)-acenocoumarol, 1.10-fold (1.0-fold); racemic-phenprocoumon, 1.11-fold (1.18-fold); and (S)-phenprocoumon, 1.13-fold (1.24-fold). Lornoxicam 5′-hydroxylation was competitively inhibited in vitro by both phenprocoumon (Ki = 1.2 ± 0.4 μM) and acenocoumarol (Ki = 5.5 ± 3.5 μM). The present results indicate that relatively close predictions of the interactions of lornoxicam with oral anticoagulants from in vitro data are possible under the assumption that hepatic lornoxicam concentrations are similar to its total plasma concentrations. The degree of pharmacokinetic interactions exhibited by oral anticoagulants and lornoxicam is dependent on the respective contribution of CYP2C9 to their total clearance.
The prediction of in vivo drug interactions from in vitro metabolism experiments is important in the drug development process (Ball et al., 1995; Fuhr et al., 1996). Recently, drug regulators have encouraged the use of in vitro methods to rationally approach clinical interaction studies.
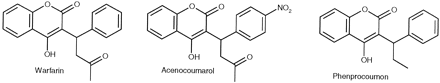
Marketed oral anticoagulants consist of the three 4-hydroxy coumarin derivatives: warfarin, phenprocoumon, and acenocoumarol (see Fig.1 for formulas). Although warfarin predominates medical use in the anticoagulant drug sectors of North America and Great Britain, phenprocoumon and acenocoumarol are widely prescribed in continental Europe. All three drugs are administered as racemates. Coumarin derivatives are drugs prone to cause potentially life-threatening drug/drug interactions because they exhibit a narrow therapeutic range and cytochrome P-450 (CYP)1-dependent, capacity-limited hepatic clearance (Harder and Thürmann, 1996). A flurry of pharmacokinetic interactions with oral anticoagulants have been described (Harder and Thürmann, 1996), and most of these appear to be related to the inhibition of CYP2C9, which is the major enzyme involved in the clearance of these drugs (Toon et al., 1985; Hermans and Thijssen, 1993; Jones et al., 1996; Miners and Birkett, 1998). Interestingly, there also are examples in which oral anticoagulants affected the pharmacokinetics and pharmacodynamics of concomitantly administered drugs such as tolbutamide and phenytoin (Harder and Thürmann, 1996), both of which are predominantly cleared by CYP2C9 (Miners and Birkett, 1998).
Structures of the oral anticoagulants.
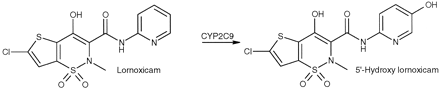
Lornoxicam is a relatively lipophilic representative of the oxicam group of nonsteroidal anti-inflammatory drugs (NSAIDs; Tsai et al., 1993). It is classified as a low extraction drug with predominantly hepatic clearance (Balfour et al., 1996). Its major metabolite is the pharmacologically inactive 5′-hydroxy lornoxicam (Balfour et al., 1996). 5′-Hydroxy lornoxicam is formed by CYP2C9 (Fig.2) in the liver (Bonnabry et al., 1996). From in vitro experiments with human liver microsomes, it is evident that lornoxicam 5′-hydroxylation accounts for up to 95% of total intrinsic lornoxicam clearance (Bonnabry et al., 1996).
Predominant metabolic route of lornoxicam mediated by CYP2C9.
The investigation of the potential mutual inhibition of the metabolism of the coumarins and NSAIDs such as lornoxicam is of interest for two reasons. First, NSAIDs inhibit platelet aggregation and therefore may interfere with hemostasis and coagulation (Balfour et al., 1996), which in turn might lead to a potentiation of the coumarin effect (Harder and Thürmann, 1996). Second, both NSAIDs and the anticoagulants are substrates of CYP2C9, and thus there is the possibility of a pharmacokinetic interaction by the mutual inhibition of their metabolism (Miners and Birkett, 1998).
The purpose of this investigation was to test the hypothesis that it is possible to predict pharmacokinetic interactions between lornoxicam and oral anticoagulants from in vitro data. To this end, in vitro experiments were conducted in human liver microsomes to determine enzyme kinetic parameters. With aid of these parameters, we attempted to predict pharmacokinetic interactions between the drugs. Where possible, these forecasts were verified by comparison with data from clinical interaction studies. The approach was validated by investigating whether the presence or absence of pharmacokinetic interactions between lornoxicam and warfarin and between tenoxicam and acenocoumarol, respectively, could have been correctly predicted from in vitro literature data.
Materials and Methods
Drugs.
Lornoxicam, 5′-hydroxy lornoxicam, and tenoxicam were synthesized by Nycomed Austria (R&D, Department of Chemistry; Linz, Austria). Their identity and purity were checked by the Department of Quality Control (Nycomed Austria). Phenprocoumon and acenocoumarol were kindly provided by Hoffmann-La Roche (Basel, Switzerland) and CIBA-Geigy (Basel, Switzerland), respectively. [14C]Tolbutamide was purchased from Amersham (Vienna, Austria). BSA, KCl, MgCl2 · 6H2O,dl-isocitric acid, and HPLC-grade solvents were obtained from E. Merck (Darmstadt, Germany). Tolbutamide, NADP, and isocitric dehydrogenase (NADP), highly purified, were purchased from Sigma (Deisenhofen, Germany).
Human Liver Microsomes.
Human microsomes were prepared from the livers of six donors. The livers were provided by the International Institute for the Advancement of Medicine (Exton, PA). Liver tissue was healthy but medically unsuitable for transplantation. Homogenate (25%; w/v) of livers was prepared in Tris (50 mM)/KCl (154 mM) buffer, pH 7.4, in the usual manner (Lake, 1987). Washed liver microsomes were then prepared through ultracentrifugation (Lake, 1987) and stored as pellets without buffer at −80°C before use. These microsomes were characterized as to protein content, spectral CYP content, and tolbutamide methyl hydroxylase (CYP2C9) activity. The protein content was estimated according to the method of Lowry et al. (1951) with BSA as standard. The CYP content was determined through use of the CO-difference spectroscopic method with a UV-1601 UV-VIS spectrophotometer (Shimadzu, Vienna, Austria) as described by Omura and Sato (1964). Tolbutamide methyl hydroxylase activity was determined as described later in the presence of 100 μM tolbutamide and 0.2 μCi of [ring-14C(U)]tolbutamide. At the end of the incubation period, 200 μl of 1 M HCl was added, and the samples were extracted with 5 ml of diethyl ether (analytical grade). Samples were centrifuged, and the organic phases were evaporated in a centrifugal evaporator (RC 10.22; Jouan, Saint-Herblain, France). Samples were reconstituted in 200 μl of acetonitrile/water/phosphoric acid (85%) [40:60:0.04 (v/v)]. Then, 20 μl was subjected to isocratic radio-HPLC on a system consisting of a gradient pump (L6200 A; Merck-Hitachi, Darmstadt, Germany), autosampler (9090; Varian, Darmstadt, Germany), column oven (BFO 04; W.O. Industrial Electronics, Vienna, Austria), a UV detector SPD-6A (Shimadzu) set at 228 nm, and a radioactivity monitor (Radiomatic 515 TR Flo-one Beta; Canberra Packard, Vienna, Austria) containing a YSi Solarscint solid phase cell (volume, 420 μl; Canberra Packard). Data were acquired with the Flo-one 2.0 software (Canberra Packard). The mobile phase was acetonitrile/water/phosphoric acid (85%) [40:60:0.04 (v/v)] on an ODS Hypersil column (RP-18, 5 μm, 250 × 4.6 mm; Bischoff, Leonberg, Germany) at a flow rate of 2.0 ml/min and a column temperature of 30°C.
Microsomal Incubations.
All incubations were carried out in triplicate in a thermostated water bath at 37°C. One incubate contained 0.5 mg of microsomal protein (i.e., 1 mg of microsomal protein/ml). Cofactors were added as NADPH regenerating system, consisting of 1 mM NADP, 5 mM MgCl2 · 6H2O, 5.5 mMdl-isocitric acid, and 0.5 U of isocitric dehydrogenase, highly purified, per incubate. All compounds were dissolved in Tris · HCl buffer (100 mM, pH 7.5). NADPH regenerating system was omitted from control incubations. Lornoxicam stock solutions (0.5 mM) were made up in Tris · HCl buffer (100 mM, pH 7.5) containing 1 μl of 4 M NaOH/ml. Stock solutions of phenprocoumon and acenocoumarol (both 0.5 mM) were prepared in Tris · HCl buffer (100 mM, pH 7.5) containing 3 and 5 μl of 4 M NaOH/ml, respectively. In all experiments, samples were preincubated with cofactors, substrate, and inhibitor for 5 min at 37°C, and then ice-cold microsomes were added. The incubation time was 2 h. Incubations were performed at substrate concentrations of 5, 10, 20, 50, and 100 μM and inhibitor concentrations of 0, 10, 20, 50, and 100 μM. Incubation conditions (microsomal protein content, incubation time) had been optimized with regard to linearity and metabolic turnover.
Sample Preparation and HPLC Analysis.
Immediately after the incubation period, samples were put on ice, and the reaction was stopped by addition of 500 μl of ice-cold methanol. Then, 10 μl of internal standard stock solution (0.5 mM) was added. Samples were stored at −20°C overnight to complete the precipitation of proteins. The next morning, samples were centrifuged for 30 min at 14,000 rpm (centrifuges 5415 and 5417; Eppendorf, Hamburg, Germany). Next, 100 μl of the supernatant was used for HPLC analysis. HPLC assays were run on a system consisting of a gradient pump (L6200 A; Merck-Hitachi), autosampler (9090; Varian), column oven (BFO 04; W.O. Industrial Electronics), and a UV detector (SPD-6A; Shimadzu) equipped with the HPLC Manager version 2 software (Merck-Hitachi). Isocratic HPLC assays were developed on an ODS Hypersil column (RP-18, 5 μm, 250 × 4.6 mm; Bischoff) operated at a flow rate of 1.0 ml/min and at 30°C for the determination of lornoxicam and 5′-hydroxy lornoxicam in presence of phenprocoumon or acenocoumarol (system 1) and for the determination of acenocumarol in presence of lornoxicam and for the determination of phenprocoumon in presence of lornoxicam (both system 2). The run time was 10 min in all cases. For system 1, the mobile phase consisted of 0.1 M NaH2PO4, pH 6.0/acetonitrile [70:30 (v/v)], UV detection was at 371 nm, and tenoxicam was used as internal standard. System 2 was run with a mobile phase of 0.5% (v/v) acetic acid/acetonitrile [45:55 (v/v)] at 312 nm (UV detection), with phenprocoumon serving as internal standard for acenocoumarol determinations, and vice versa. The limit of detection for 5′-hydroxy lornoxicam with system 1 was 0.5 ng/injection (system 1 was used for monitoring the appearance of 5′-hydroxy lornoxicam). With system 2, the disappearance of acenocoumarol and phenprocoumon, respectively, was monitored, and the lowest concentration measured was far in excess of the lowest concentration run in the calibration samples (0.5 μM). An enantiospecific HPLC assay was used for the investigation of the stereoselective metabolism of acenocoumarol. This assay was carried out on an HP 1050 series instrument equipped with gradient pump, autosampler, column oven, and degasser (all Hewlett-Packard, Vienna, Austria) linked to a UV-975 detector (Jasco, Großumstadt, Germany). The data acquisition system was the HP Chem Station (Hewlett Packard). Separation of acenocoumarol enantiomers (de Vries and Schmitz-Kummer, 1993) was accomplished at 30°C on a chiral AGP column (100 × 4. 0 mm; Chrom Tech, Hägersten, Sweden) using a linear gradient system (A: 0.01 M NaH2PO4, pH 7.0, containing 1 mM dimethyl octylamine; B: 2-propanol containing 1 mM dimethyl octylamine): 0 to 10.0 min, 0 to 20% B; 10.1 to 15.0 min, 20% B; 15.1 to 16.0 min, 20 to 0% B; and 16.1 to 26 min, 0% B (equilibration). The flow rate was 0.9 ml/min. Peaks were quantified according to their peak area. Calibration was performed in the range from 0 to 100 μM concentration of the respective analyte (6–9 points).
Determination of Enzyme Kinetic Parameters.
Apparent enzyme kinetic parameters were calculated using GraFit software (Leatherbarrow, 1992). Ki,Km, and Vmaxvalues were derived through iterative nonlinear regression after Michaelis-Menten representation of the experimental data (rate of metabolite formation as a function of substrate concentration). The adequate inhibition model was chosen according to the following criteria: 1) lowest χν2(reduced χ2), 2) lowest standard errors of computed parameters, and 3) how well the Kmand Vmax estimates with inhibitor agreed with those estimated in the absence of inhibitor.
Prediction of In Vivo Interaction from In Vitro Data.
Interactions were predicted as follows. An interaction in vivo was considered to be likely (Ito et al., 1998) if the following is true:
Results
Metabolism of Lornoxicam in Human Liver Microsomes.
Lornoxicam exhibited a Vmax value of 35.4 ± 21.9 pmol · min−1 · mg protein−1 with a range of 9.7 to 74.5 pmol · min−1 · mg protein−1 (141.5 ± 117.6 · min−1 · nmol of CYP−1; range, 27.2–368.8 pmol · min−1 · nmol of CYP−1) and a Kmvalue of 12.0 ± 4.6 μM (range, 7.6–20.1 μM) with regard to its 5′-hydroxylation in liver microsomes from six donors. This resulted in a Clint of 3.57 ± 2.24 μl · min−1 · mg protein−1 with a range of 0.52 to 7.54 μl · min−1 · mg protein−1 (14.01 ± 12.15 μl · min−1 · nmol of CYP−1; range, 1.46–37.33 μl · min−1 · nmol of CYP−1). The Vmaxvalue of lornoxicam 5′-hydroxylation and Clintcorrelated well with tolbutamide methylhydroxylase (CYP2C9) activity (r2 = 0.94 and 0.89, respectively).
To validate the procedure used for predictions, the warfarin/lornoxicam and warfarin/tenoxicam interactions were investigated using published data.
Warfarin/Lornoxicam.
In vitro biotransformation experiments in human liver microsomes suggest that lornoxicam has an affinity to CYP2C9 similar to that of (S)-warfarin (Km = 4–10 μM;He et al., 1995; Bonnabry et al., 1996). Hitherto, theKi value for the inhibition of (S)-warfarin metabolism by lornoxicam has not been reported. It is expected that the Ki value of lornoxicam for the competitive inhibition of (S)-warfarin equals the Km value because CYP2C9 is solely responsible for the 5′-hydroxylation of lornoxicam (Bonnabry et al., 1996), and more than 85% of the total clearance of (S)-warfarin is mediated by CYP2C9 (Miners and Birkett, 1998). Table 1 shows the predicted influence of lornoxicam coadministration on the pharmacokinetics of (S)-warfarin and a comparison with the clinical data, which indicated a significant interaction between warfarin and lornoxicam (Ravic et al., 1990). The pharmacokinetic interaction was borne out by an enhanced warfarin effect in terms of prolonged prothrombin time and elevated international normalized ratio (by ≈20% each).
Predicted lornoxicam/(S)-warfarin interactions
There are no clinical data available with regard to the influence of warfarin coadministration on lornoxicam pharmacokinetics. As forecast from in vitro experiments, (S)-warfarin coadministration does not exhibit a clinically significant influence on the pharmacokinetics of lornoxicam (Table 1).
Warfarin/Tenoxicam.
Tenoxicam, the 6-deschloro derivative of lornoxicam, is another substrate of CYP2C9 (Zhao et al., 1992). In vitro data on the metabolism of tenoxicam (Zhao et al., 1992) and warfarin (He et al., 1995) were taken from the literature. Because the in vitro inhibition of the metabolism of (S)-warfarin by tenoxicam and vice versa have not been described, the equivalence ofKm and Kivalues of the drugs toward CYP2C9 was assumed. CYP2C9 is the major CYP isozyme involved in the metabolism of both drugs (Zhao et al., 1992; He et al., 1995). Warfarin-induced anticoagulation was not affected by concomitant medication with tenoxicam in healthy volunteers (Eichler et al., 1992). Likewise, racemic (rac)-warfarin concentrations were not altered by tenoxicam. The influence of warfarin on the pharmacokinetics of tenoxicam was tested only after a single dose of rac-warfarin, which led to a slight, but not significant, increase in tenoxicam plasma levels (Eichler et al., 1992). The interaction forecast regarding the effect of tenoxicam on warfarin agrees with the clinical observation (Table 2).
Predicted tenoxicam/(S)-warfarin interactions
The influence of (S)-warfarin on the pharmacokinetics of tenoxicam is predicted to be negligible (Table 2).
Acenocoumarol/Lornoxicam.
The in vitro metabolism of rac-acenocoumarol was assessed by its disappearance from the incubation mixture because reference compounds for the acenocumarol enantiomers and metabolites were not available. It was, however, confirmed that the metabolism and inhibition of acenocoumarol metabolism were enantioselective. The assignment of enantiomer identity was performed using the enantiospecific HPLC assay described by de Vries and Schmitz-Kummer (1993; see Materials and Methods). The metabolic turnover of both of the acenocoumarol enantiomers in the absence of lornoxicam was in agreement with the literature (Hermans and Thijssen, 1993): At 5 μM rac-acenocoumarol, the (S)-enantiomer was metabolized about three times as fast as the (R)-enantiomer. The IC50 of lornoxicam for the inhibition of the metabolism of 2.5 μM (S)-acenocoumarol was 18 ± 1.9 μM, whereas the metabolism of (R)-acenocoumarol remained practically unaffected by lornoxicam (results not shown). Further data were obtained with a nonenantiospecific assay, and the results must be interpreted with the awareness that the kinetic parameters derived are hybrid constants rather than specific for a single metabolic reaction.
Table 3 shows the enzyme kinetic parameters and the Ki value of lornoxicam for competitive inhibition in livers from six human donors.
Kinetic parameters for the metabolism of rac-acenocoumarol and its inhibition by lornoxicam
Because individual results varied widely, parameters obtained with microsomes from donor 0102921 were used for predictions of pharmacokinetic interactions (Table 4) as a worst case scenario.
Predicted interactions between lornoxicam and acenocoumarol
Inhibition of lornoxicam 5′-hydroxylation by rac-acenocoumarol was competitive in six livers with a Ki value of 5.5 ± 3.5 μM (range, 3.2–12.3 μM). Table 4 displays the predicted increase in lornoxicam steady-state concentrations and AUC under concomitant treatment with rac-acenocoumarol.
Phenprocoumon/Lornoxicam.
The effect of lornoxicam on the metabolism of rac-phenprocoumon was not investigated in vitro because the microsomal metabolism of rac-phenprocoumon is characterized by very low turnover rates, which renders the drug metabolically stable under in vitro conditions, if the metabolism is assessed on the basis of disappearance of parent compound (results not shown). Because synthetic standards of the phenprocoumon metabolites were not available, in the present study, we had to rely on published data for the prediction of a clinical interaction. Hitherto, the in vitro metabolism of phenprocoumon in human liver microsomes has not been described. However, the inhibition constantKi values of (R)- and (S)-phenprocoumon toward CYP2C9 are 0.5 and 0.6 μM, respectively (Jones et al., 1996). Under the assumption that phenprocoumon is a competitive inhibitor and a substrate of CYP2C9, theKm value of phenprocoumon should match theKi value (Miners et al., 1995; Miners and Birkett, 1998). The same applies to lornoxicam, whose rate-limiting metabolic pathway was shown to be competitively inhibited by phenprocoumon (see later). Therefore, a prediction of the interaction of phenprocoumon and lornoxicam under clinical conditions was attempted using the experimental in vitro data obtained from the investigation of the inhibition of the 5′-hydroxylation of lornoxicam by phenprocoumon (Table 5).
Predicted lornoxicam/phenprocoumon interactions
Table 5 indicates that based on a competitive inhibition of CYP2C9, a 1.11-fold increase in AUC and steady-state concentrations of rac-phenprocoumon would be expected. A trend toward increased AUC and decreased clearance of (R)-phenprocoumon and a significant decrease in (S)-phenprocoumon clearance by 15% and an increase in (S)-phenprocoumon AUC by 24% were observed in healthy volunteers (Masche et al., 1999b).
Although the (S)-enantiomer is the more potent optical isomer, the pharmacokinetic effect of reduced clearance, and thereby increased exposure, was not borne out in a parallel pharmacodynamic effect. Conversely, the anticoagulant activity of phenprocoumon was reduced after lornoxicam coadministration (Masche et al., 1999b).
In vitro experiments suggest that rac-phenprocoumon is a potent inhibitor of lornoxicam 5′-hydroxylation (and of CYP2C9) with aKi value of 1.2 ± 0.4 μM in six human livers. However, Table 5 shows that this strong in vitro inhibition is forecast to have only minor significance under therapeutic conditions when rac-phenprocoumon and lornoxicam are coadministered.
Discussion
The prediction of pharmacokinetic drug/drug interactions from in vitro data has attracted increased interest of the scientific community during the past couple of years, in part in response to the fatal terfenadine/ketoconazole interaction. Retrospectively, this interaction was found to be predictable from in vitro data (von Moltke et al., 1994). Nevertheless, there remain a number of hitherto unresolved issues with the prediction of pharmacokinetic interactions (Bertz and Granneman, 1997; Ito et al., 1998; Lin and Lu, 1998).
Apart from problems with regard to the experimental estimation ofKi values, which are related to the nonspecific binding of drugs to lipoproteins in the microsomal preparations, the most crucial point in the forecasting procedure is the setting of [I] (Bertz and Granneman, 1997; Ito et al., 1998; Lin and Lu, 1998) [i.e., the effective in vivo concentration of the inhibiting drug at the site of metabolism (intracellular hepatocyte concentration)]. The nonspecific binding to microsomes has been shown to be low for acidic drugs such as warfarin (Obach, 1997); hence,Km and Kivalues of the acidic lornoxicam and the oral anticoagulants evaluated in this study are likely to be in the correct order of magnitude. The effective in vivo concentration of the inhibiting drug [I] is not directly amenable to measurement in humans; it is often regarded as being equal to total therapeutic drug concentrations in plasma (He et al., 1995; Ring et al., 1995; Kunze et al., 1996; von Moltke et al., 1996; Wrighton and Ring, 1999), although this contradicts a basic dogma of pharmacology according to which unbound drug only should be able to cross membranes and to reach intracellular targets (Lin and Lu, 1998). The argument for using total concentrations was mainly to include a conservative safety factor in the prediction of interactions (Wrighton and Ring, 1999). Using this method, some interactions have been correctly predicted with some variation in terms of quantitative aspects (Ring et al., 1995; Ito et al., 1998; Lin and Lu, 1998). On the other hand, the use of free plasma concentrations as an approximation of the cytosolic concentration in the hepatocyte could often underestimate the actual concentration. Some authors have adjusted these concentrations by experimental liver/water or liver/plasma partition ratios (von Moltke et al., 1996). It is conceivable that intrahepatic concentrations might by far exceed free plasma concentrations, as active hepatic uptake might be involved as a clearance mechanism (Ito et al., 1998).
In this work, the classification of low clearance drugs into restrictively and nonrestrictively eliminated drugs (Wilkinson, 1987;Verbeeck and Wallace, 1994) has been used to provide a rationale for the setting of [I]. Total plasma concentrations of nonrestrictively eliminated drugs such as lornoxicam and acenocoumarol are believed to be available for hepatic clearance, with this borne out by a hepatic extraction ratio higher than their unbound fraction (Wilkinson, 1987; Verbeeck and Wallace, 1994). Therefore, for lornoxicam and acenocoumarol, hepatic concentrations were assumed to equal total plasma concentrations. In contrast, restrictively eliminated drugs such as phenprocoumon, warfarin, and tenoxicam appear to follow the classic pattern of clearance being limited to their free fraction. Thus, for the restrictively eliminated drugs, unbound plasma concentrations were set as cytosolic hepatic concentrations. This procedure proved to be sufficiently accurate to correctly predict the interaction of warfarin and lornoxicam (Table 1) and the absence of interaction between warfarin and tenoxicam (Table 2) from published in vitro data. The increase in steady-state plasma concentrations of rac-warfarin under lornoxicam comedication is somewhat less than predicted. The apparent overprediction could be due to the fact that only rac-warfarin was measured in the clinical study (Ravic et al., 1990). This could have partially obscured the differences in plasma concentrations, because (R)-warfarin concentrations are unlikely to be affected by lornoxicam. (R)-Warfarin is not metabolized by CYP2C9 (Kunze et al., 1996).
The effect of the presence of lornoxicam on the metabolism of rac-acenocoumarol and the effect of the presence of rac-acenocoumarol or rac-phenprocoumon on the major metabolic reaction of lornoxicam were assessed in vitro. The pharmacological features of lornoxicam, such as its interference with platelet function (Balfour et al., 1996), could cause an increase in bleedings if coadministered with oral anticoagulants, particularly if its therapeutic concentrations were increased by oral anticoagulants: warfarin, phenprocoumon, acenocoumarol, and lornoxicam are all metabolized by CYP2C9, and this enzyme contributes a major proportion to their clearance (Hermans and Thijssen, 1993; Bonnabry et al., 1996; Miners and Birkett, 1998).
From the in vitro data presented here, however, interactions are forecast only for rac-acenocoumarol (Table 4). In contrast to the other two anticoagulants, acenocoumarol is a nonrestrictively eliminated drug, and it is assumed to reach sufficiently high hepatic concentrations to significantly inhibit the metabolism of lornoxicam. Clinical pharmacokinetic data are not available for verification of this prediction. The absence of a relevant change in the anticoagulative activity of acenocoumarol during concomitant administration of lornoxicam (Masche et al., 1999a) makes it likely that the forecast increase in lornoxicam concentrations will have no pharmacodynamic consequences.
The values for Km,Vmax, and Clint for the metabolism of rac-acenocoumarol (Table 3) are in a similar range as those described previously (Hermans and Thijssen, 1993). Table 4suggests that a pharmacokinetic interaction by metabolic inhibition would be expected in some patients. The effect did not have significant bearings on a clinical study (Masche et al., 1999a). Lornoxicam nearly exclusively inhibits the metabolism of (S)-acenocoumarol, whereas (R)-acenocoumarol remains largely unaffected. In a clinical interaction study, lornoxicam increased the AUC of (S)-acenocoumarol by 24%, whereas there was no significant effect on the (R)-enantiomer (Masche et al., 1999a).
The pharmacokinetic effect of lornoxicam coadministration on (S)-acenocumarol has no clinical consequences (Masche et al., 1999a) because the effect of rac-acenocoumarol is largely conveyed by the (R)-enantiomer (Hermans and Thijssen, 1993; Harder and Thürmann).
Because experimental data could not be obtained due to the metabolic stability of phenprocoumon in vitro, the inhibitory potency of lornoxicam toward the metabolism of phenprocoumon (Table 5) was predicted with the assumption of competitive inhibition and identity ofKi and Km. This assumption is supported by the fact that lornoxicam has been shown to not inhibit CYP3A4, CYP2C19, and CYP2D6 (Bonnabry et al., 1996). The inhibitory potency of lornoxicam toward phenprocoumon is, therefore, likely to be founded on the inhibition of CYP2C9. Because both phenprocoumon and lornoxicam are metabolized by CYP2C9 and because no evidence was seen that lornoxicam might be a mechanism-based inhibitor, the Ki value of lornoxicam toward phenprocoumon metabolism should be in the range of itsKm value (Miners et al., 1995). Under these conditions, a 1.13-fold increase in AUC of the more potent (S)-phenprocoumon during concomitant treatment with lornoxicam is predicted (Table 5), which is a slight underestimation of the 1.24-fold increase seen in the clinic setting (Masche et al., 1999b). Interestingly, this pharmacokinetic effect was not paralleled by the expected pharmacodynamic response (Masche et al., 1999b). In contrast, significantly decreased anticoagulation was observed. This finding is the more surprising because lornoxicam inhibits clot formation (Balfour et al., 1996). On the other hand, there have been reports that NSAIDs such as indomethacin can have procoagulant effects in vitro (Nygaard et al., 1995). Whether this observation can explain the contradictory effect of lornoxicam on the anticoagulant response to phenprocoumon requires further investigation.
Phenprocoumon coadministration was predicted to not cause significant inhibition of lornoxicam metabolism in vivo (Table 5), although phenprocoumon was found to be a potent CYP2C9 inhibitor in vitro (Ki = 1.2 μM). The forecast that this high affinity toward CYP2C9 will not result in inhibition in vivo hinges on the assumption that the phenprocoumon concentration in the hepatocyte available for enzyme inhibition will be equal to the unbound fraction (0.053 μM) of its therapeutic plasma concentration. This assumption is based on the restrictive elimination of phenprocoumon: Only its free fraction should be available to enter the hepatocyte.
In addition to an effect on their metabolism by CYP2C9, lornoxicam and the 4-hydroxycoumarin anticoagulants could mutually affect transport processes possibly involved in their pharmacokinetics and disposition. However, neither of the drugs have hitherto been shown to be substrates or inhibitors of transporters such as P-glycoprotein or organic anion transport protein, which can be involved in the disposition of drugs.
The three oral anticoagulants differ in the extent to which CYP2C9 contributes to their overall clearance and, therefore, in their propensity toward interactions with CYP2C9 substrates and inhibitors. Although CYP2C9 is responsible for about 85% of the clearance of the more potent (S)-warfarin (Miners and Birkett, 1998), it accounts for only about 40% of the clearance of the more potent (R)-enantiomer of acenocoumarol (Hermans and Thijssen, 1993) and 50% of the clearance of rac-phenprocoumon (Toon et al., 1985), the enantiomers of which are more similar in terms of potency and pharmacokinetics. This explains why the competitive CYP2C9 inhibitor lornoxicam exhibits a greater inhibition of warfarin clearance compared with acenocoumarol and phenprocoumon.
In conclusion, a useful approach was developed to predict pharmacokinetic interactions of the new NSAID lornoxicam. The work presented here indicates that relatively close predictions of the interactions of lornoxicam with oral anticoagulants from in vitro data are possible under the assumption that hepatic lornoxicam concentrations are similar to its total plasma concentrations. The respective degree of pharmacokinetic interactions between these agents can be rationalized on the basis of the contribution of CYP2C9 to their total clearance. With growing understanding of the underlying principles of pharmacokinetic drug interactions, predictions from in vitro data will become more reliable. However, there remains much uncertainty regarding the effective concentrations of drugs at the site of interaction, and better insights into this aspect are needed to improve precision in the forecasting of drug/drug interactions.
Footnotes
-
Send reprint requests to: Dr. Christopher Kohl, Department of Drug Metabolism, Pfizer Central Research, Ramsgate Road, Sandwich, Kent CT13 9NJ, England. E-mail:christopher_kohl{at}sandwich.pfizer.com
- Abbreviations used are::
- CYP
- cytochrome P-450
- AUC
- area under the plasma concentration-time curve
- NSAID
- nonsteroidal anti-inflammatory drug
- rac
- racemic
- Received June 28, 1999.
- Accepted October 12, 1999.
- The American Society for Pharmacology and Experimental Therapeutics