Abstract
Oxidative metabolism of the insect repellentN,N-diethyl-m-toluamide (DEET) by pooled human liver microsomes (HLM), rat liver microsomes (RLM), and mouse liver microsomes (MLM) was investigated. DEET is metabolized by cytochromes P450 (P450s) leading to the production of a ring methyl oxidation product,N,N-diethyl-m-hydroxymethylbenzamide (BALC), and an N-deethylated product,N-ethyl-m-toluamide (ET). Both the affinities and intrinsic clearance of HLM for ring hydroxylation are greater than those for N-deethylation. Pooled HLM show significantly lower affinities (Km) than RLM for metabolism of DEET to either of the primary metabolites (BALC and ET). Among 15 cDNA-expressed P450 enzymes examined, CYP1A2, 2B6, 2D6*1 (Val374), and 2E1 metabolized DEET to the BALC metabolite, whereas CYP3A4, 3A5, 2A6, and 2C19 produced the ET metabolite. CYP2B6 is the principal cytochrome P450 involved in the metabolism of DEET to its major BALC metabolite, whereas CYP2C19 had the greatest activity for the formation of the ET metabolite. Use of phenotyped HLMs demonstrated that individuals with high levels of CYP2B6, 3A4, 2C19, and 2A6 have the greatest potential to metabolize DEET. Mice treated with DEET demonstrated induced levels of the CYP2B family, increased hydroxylation, and a 2.4-fold increase in the metabolism of chlorpyrifos to chlorpyrifos-oxon, a potent anticholinesterase. Preincubation of human CYP2B6 with chlorpyrifos completely inhibited the metabolism of DEET. Preincubation of human or rodent microsomes with chlorpyrifos, permethrin, and pyridostigmine bromide alone or in combination can lead to either stimulation or inhibition of DEET metabolism.
N,N-Diethyl-m-toluamide, commonly known as DEET1, is the principle active ingredient in most personal insect repellents worldwide and is highly effective against a broad spectrum of insect pests, including potential disease vectors such as mosquitoes, biting flies, and ticks (including ticks that may carry Lyme disease). DEET was first developed and patented in 1946 by the U.S. Army for use by military personnel and later registered for general public use in 1957 (Schoenig et al., 1999). Every year, approximately one-third of the U.S. population (75,000,000) uses DEET-containing insect repellent products with DEET concentrations ranging from 10 to 100% in a variety of liquids, lotions, gels, sprays, sticks, and impregnated materials and more than 30 million packages of DEET-containing products are sold annually (Veltri et al., 1994). Approximately 230 products containing DEET are currently registered with the Environmental Protection Agency by about 70 different companies.
DEET is generally considered a benign chemical; however, isolated reports involving heavy and excessive exposure indicate a variety of toxic side effects, including toxic encephalopathy, seizure, acute manic psychosis, cardiovascular toxicity, and dermatitis (Robbins and Cherniack, 1986; Veltri et al., 1994). Because DEET was widely used during the Gulf War, it is one of several chemicals believed to have potential for possible detrimental interactions with other chemicals (Chaney et al., 1997, 1999; Haley and Kurt, 1997; McCain et al., 1997). For example, DEET has been shown to synergize seizures produced by pyridostigmine bromide and vice versa (Chaney et al., 1997, 1999). Subsequent investigations in rats have shown that DEET in combinations with pyridostigmine bromide or permethrin can lead to significant neurobehavioral deficits associated with significant inhibition of brainstem acetylcholinesterase activities (Abou-Donia et al., 2001). Mechanisms of these chemical interactions are currently unknown but may include the ability of one chemical to inhibit and/or induce the metabolism of another chemical. For example, the phosphorothioate organophosphate pesticide chlorpyrifos is capable of inhibiting metabolism of other chemicals due to its ability to irreversibly inhibit metabolizing enzymes such as cytochromes P450 (P450s) (Butler and Murray, 1997). Thus, an understanding of how DEET is metabolized in humans, the isoforms responsible for such metabolism, and the possible interactions of DEET in metabolism of other chemicals will aid in the evaluation of the possible role that DEET may play in deployment-related illnesses.
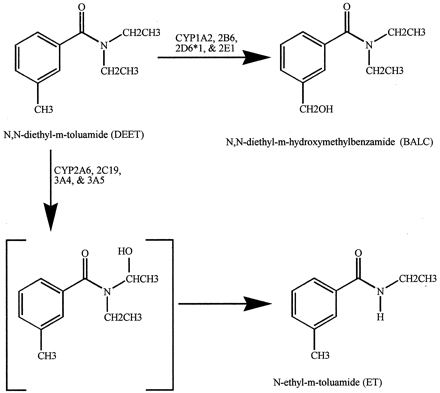
The available literature on the pharmacokinetics and metabolism of DEET has been reviewed (Qui et al., 1998). In rat, oxidative metabolism seems to account for most, if not all, of the metabolites derived from DEET. The major metabolites areN,N-diethyl-m-hydroxymethylbenzamide (BALC) andN-ethyl-m-toluamide (ET) (Fig.1), indicating that either theN-ethyl or the ring methyl groups can be oxidized (Taylor, 1986; Yeung and Taylor, 1988; Taylor and Spooner, 1990; Schoenig et al., 1996; Constantino and Iley, 1999). These two major and several minor metabolites were characterized in liver microsomes from phenobarbital-pretreated rats (Taylor, 1986; Yeung and Taylor, 1988;Constantino and Iley, 1999). Studies of the metabolism of DEET by human enzymes are lacking. Studies of absorption and metabolism in humans have been conducted only at the level of determining urinary metabolites and suggest that 5 to 8% of topically applied DEET is rapidly absorbed and excreted. As many as six metabolites were recovered from the human urine samples (Selim et al., 1995).
Primary in vitro metabolites of DEET.
The main objectives of the present study were to identify and quantify the oxidative metabolism of DEET by human liver microsomes and to compare the metabolism of DEET in human liver microsomes with that in rat and mouse liver microsomes. Further objectives were to identify the human P450 isoforms responsible for DEET metabolism, and to investigate potential interactions of DEET with other xenobiotics.
Materials and Methods
Chemicals.
DEET, chlorpyrifos, chlorpyrifos-oxon, 3,5,6-trichloro-2-pyridinol, and permethrin (50:50 cis-trans) were purchased from ChemService (West Chester, PA). ET and BALC were synthesized as described previously (Taylor, 1986). Pyridostigmine bromide was purchased from Roche Molecular Biochemicals (Indianapolis, IN). HPLC grade acetonitrile and tetrahydrofuran were purchased from Fisher Scientific (Pittsburgh, PA) and Mallinckrodt Baker, Inc. (Paris, KY), respectively. All other chemicals were purchased, if not specified, from Sigma Chemical (St. Louis, MO).
Rodent Liver Microsome Preparation.
Rat liver microsomes (RLM) and mouse liver microsomes (MLM) were prepared from adult male Long Evans rats and adult male CD-1 mice (Charles River Laboratories, Raleigh, NC), respectively, according to the method of Cook and Hodgson (1983). Briefly, immediately after sacrificing the animals, the fresh livers were excised, weighed, minced, and washed with ice-cold homogenized buffer (50 mM potassium phosphate buffer, pH 7.5; 0.1 mM EDTA; 1.15% potassium chloride). Samples were homogenized with a Polytron homogenizer in ice-cold homogenization buffer and centrifuged at 10,000g for 15 min. The supernatant was filtered through glass wool, centrifuged at 100,000g for 1 h, and the microsomal pellet was resuspended in storage buffer (50 mM potassium phosphate, pH 7.5; 0.1 mM EDTA; 0.25 M sucrose). All processes were performed at 0–4°C. The microsomal preparation was aliquoted and stored at −80°C until used.
Total cytochrome P450 content was determined by the CO-difference spectrum method of Omura and Sato (1964). Protein concentration was determined using the Bio-Rad protein assay with bovine serum albumin as standard (Bradford, 1976).
Human Liver Microsomes and Human Cytochrome P450 Isoforms.
Pooled human liver microsomes (HLM) (pooled from 10 donors) and human P450 isoforms expressed in baculovirus-infected insect cells (Sf9) (BTI-TN-5B1-4), CYP1A1, 1A2, 2A6, 1B1, 2B6, 2C8, 2C9*1 (Arg114), 2C18, 2C19, 2D6*1 (Val374), 2E1, 3A4, 3A5, 3A7, and 4A11 were purchased from GENTEST (Woburn, MA). Gender specific HLM (pooled from five male donors or five female donors, respectively) were purchased from GENTEST. Gender specific HLM (pooled from 10 male donors or 10 female donors, respectively) were also purchased from Xenotech (Kansas City, KS). Selected individual human liver microsomes (HG042, HG043, and HG095) were purchased from GENTEST.
In Vitro DEET Metabolism.
Enzyme kinetic assays for microsomes were performed by incubation in 1.5-ml microcentrifuge tubes of serial concentrations of DEET (final concentrations 31.24–3000 μM) with microsomes in 20 mM Tris-HCl buffer, pH 8.3 at 37°C, containing 5 mM MgCl2 (final volume 1.0 ml) for 5 min. Preliminary studies demonstrated that a pH of 8.3 was optimal for the production of DEET metabolites as had previously been reported for metabolites of DEET by rat liver microsomes (Yeung and Taylor, 1988). The microsomal protein concentrations used in assays were 1.5 mg/ml for HLM, RLM, and MLM. After preincubation at 37°C for 5 min, reactions were initiated by the addition of ice-cold microsomes to prewarmed buffer/substrate/cofactors. The final concentration of the NADPH-generating system was 0.25 mM NADP, 2.5 mM glucose 6-phosphate, and 2 U/ml glucose-6-phosphate dehydrogenase. The controls were performed in the absence of the NADPH-generating system. Reactions were terminated by the addition of an equal volume of acetonitrile and vortexing. After 10-min centrifugation at 15,000 rpm in a microcentrifuge, the supernatants were analyzed for BALC and ET concentrations by HPLC as described below.
The initial metabolic activity assays for human P450 isoforms were performed by incubation of DEET (final concentrations 1000 or 3000 μM) with P450 isoforms (final P450 contents 100–200 pmol/ml) for 20 min in P450-specific buffers recommended by the supplier (GENTEST). The controls were performed in the absence of an NADPH-generating system. For CYP1A1, 1A2, 1B1, 2D6*1 (Val374), 3A4, 3A5, and 3A7 a 100 mM potassium phosphate buffer with 3.3 mM MgCl2, pH 7.4, was used. For CYP2B6, 2C8, 2C19, and 2E1 a 50 mM potassium phosphate buffer with 3.3 mM MgCl2, pH 7.4, was used. For CYP2C9*1 (Arg144), 2C18, and 4A11 a 100 mM Tris-HCl buffer with 3.3 mM MgCl2, pH 7.5, was used. For CYP2A6 a 50 mM Tris-HCl buffer with 3.3 mM MgCl2, pH 7.5, was used.
Enzyme kinetic assays for human CYP1A2, 2B6, and 2D6*1 (Val374) were performed by incubations of serial concentrations of DEET (final concentrations 31.25–1000 μM) (final P450 content 50 pmol) for 10 min in P450-specific buffers recommended by the supplier (GENTEST).
Assays of five pooled male and female HLM purchased from GENTEST and 10 pooled male and female HLM purchased from Xenotech were performed by incubations of DEET (final concentration 1000 μM) with microsomes (final protein concentration 1.5 mg/ml) for 10 min. Similar conditions were used for assays of individual HLM.
Induction.
Adult male CD-1 mice, 28 to 30 g, were obtained from Charles River Laboratories and acclimated for 4 days. Low (2 mg/kg/day), medium (20 mg/kg/day), and high (200 mg/kg/day) doses of DEET in 100 μl of corn oil were given intraperitoneally daily for 3 days. The dose range for DEET was selected to not exceed a dose known to produce a physiological effect (Chaney et al., 1999). Doses approximating LD10 values for phenobarbital (80 mg/kg/day) in 100 μl of water or 3-methylcholanthrene (20 mg/kg/day) in 100 μl of corn oil were also administered intraperitoneally, to separate groups of mice, daily for 3 days. Controls were given corn oil only or water. Microsomes were prepared from livers of fed mice on the 4th day as described above.
The following substrates were used as indicators of the activities for the following isozymes: ethoxyresorufin O-deethylation (EROD) and methoxyresorufin O-demethylation (MROD) for CYP1A1/2 (Burke and Mayer, 1974; Nerurkar et al., 1993), pentoxyresorufin O-dealkylation (PROD) for CYP2B10 (Lubet et al., 1985), and benzyloxyresorufin O-dealkylation (BROD) for CYP2B (Nerurkar et al., 1993). Assays were conducted as described byPohl and Fouts (1980). Briefly, assays were initiated by the addition of an NADPH-regenerating system and incubated for 5 min at 37°C. Product formation for EROD, MROD, PROD, and BROD activities were determined spectrofluorometrically (F-2000 fluorescence spectrophotometer; Hitachi, Tokyo, Japan) by comparison with a standard curve generated with resorufin.
Chlorpyrifos Metabolism.
Chlorpyrifos metabolism activity assays were performed by incubation of 100 μM chlorpyrifos with induced MLM for 5 min. The microsomal protein concentrations used in the assays were 1.0 mg/ml in 100 mM Tris-HCl buffer, pH 7.4 at 37°C, containing 5 mM MgCl2 and 3 mM EDTA. Reactions were stopped by the addition of acetonitrile followed by centrifugation at 15,000 rpm. The supernatant was analyzed for chlorpyrifos-oxon and 3,5,6-trichloro-2-pyridinol concentrations by HPLC as described by Tang et al. (2001).
Inhibition.
The inhibition of human CYP2B6 by chlorpyrifos and chlorpyrifos-oxon were studied by incubating these two compounds (final concentration 100 μM), CYP2B6 (50 pmol), NADPH-generating system, and 50 mM potassium phosphate buffer with 3.3 mM MgCl2, pH 7.4, for 5 min at 37°C before adding DEET (final concentration 1000 μM). The reaction was terminated after an additional 20 min by the addition of an equal volume of acetonitrile. After brief centrifugation the supernatant was analyzed for BALC concentration by HPLC.
To understand possible interactions produced by potential induction of metabolizing enzymes by DEET; effects on DEET metabolism were examined in HLM, MLM, and RLM after preincubations with chlorpyrifos, permethrin, and pyridostigmine bromide either alone or in combination (final concentration 100 μM). In addition, the effects of these compounds were also examined in some mice that had been induced with phenobarbital (80 mg/kg/day), 3-methylcholanthrene (20 mg/kg/day), or the high dose of DEET (200 mg/kg/day). Microsomes (final protein concentrations 1.5 mg/ml), NADPH-generating system, and 20 mM Tris-HCl buffer with 5 mM MgCl2, pH 8.3 at 37°C, were incubated for 5 min at 37°C before adding DEET (final concentration 1000 μM). The reaction was terminated after 10 min by the addition of an equal volume of acetonitrile. After centrifugation at 15,000 rpm, the supernatant was analyzed for BALC and ET concentrations by HPLC.
All assays were conducted in triplicate. The protein concentrations and incubation times used in the assays were found to be in the linear range in preliminary experiments. No metabolites were detected when incubations were carried out in the absence of an NADPH-generating system.
Analysis of Metabolites by HPLC.
Metabolites were analyzed using a Shimadzu HPLC system (Kyoto, Japan). The Shimadzu HPLC system used in this study consisted of two pumps (LC-10AT), a Shimadzu autoinjector (SIL-10AD VP), and a Waters 486 tunable absorbance detector (Waters, Milford, MA). For DEET metabolites, the mobile phase for pump A was 3.5% tetrahydrofuran, 96.5% water; for pump B 100% acetonitrile. A gradient system was used as described by Yeung and Taylor (1988) in the following manner: 0 to 3 min (10% B), 3 to 30 min (10–60% B), 30 to 32 min (60–10% B), and 32 to 35 min (10% B). The flow rate was 1 ml/min. For chlorpyrifos metabolites, the mobile phase for pump A was 10% acetonitrile, 89% H2O and 1% phosphoric acid; for pump B 99% acetonitrile and 1% phosphoric acid. A gradient system was initiated at 20% pump B and increased to 100% pump B in 20 min. The flow rate was 1 ml/min. Metabolites for DEET and chlorpyrifos were separated by a C18 column (Luna 5 μm, 150 × 3 mm; Phenomenex, Rancho Palos Verdes, CA) and detected at 230 nm. Under these conditions the retention times for BALC, ET, and DEET were 2.5, 5, and 11 min, respectively. The limit of detection for the two DEET metabolites was approximately 10 μM. Concentrations of metabolites were obtained by extrapolation of peak height from a standard curve.Km andVmax were obtained from Lineweaver-Burk plots (1/velocity versus 1/substrate concentration).
Statistics.
Significant differences between data sets were determined by one-way analysis of variance and multiple comparisons were performed with the Tukey-Kramer honestly significant different test by using a SAS program (SAS, 1989).
Results
Pooled HLM as well as RLM and MLM showed a much lowerKm (higher affinity) and higher intrinsic clearance for ring hydroxylation (BALC formation) than forN-deethylation (ET formation) from DEET (∼10-fold differences) (Table 1). HLM also exhibited higher Km values (lower affinities) than either RLM or MLM. HLM exhibited a lower intrinsic clearance rate [CLint(Vmax/Km)] for both metabolites than RLM. However, the CLintfor MLM was similar to that of HLM. When mice were treated with the high dose (200 mg/kg/day) there was a significant increase in theVmax and intrinsic clearance of BALC and ET, indicating that DEET induced its own metabolism.
Kinetic parameters for the ring methyl oxidation and N-deethylation of DEET by pooled HLM, RLM, MLM, and DEET treated mouse liver microsomes
Among 15 different human P450 isoforms screened, only CYP1A2, 2B6, 2D6*1 (Val374), and 2E1 displayed detectable BALC metabolite production (Table 2). The activity of CYP2E1 was significantly less than the activities of the other P450s producing the BALC metabolite. Production of the BALC metabolite was generally much higher than that of the ET metabolite. Isoforms producing detectable amounts of the ET metabolite included CYP3A4, 3A5, 2A6, and 2C19. These isoforms produced no detectable amounts of the BALC metabolite. CYP2C19 showed significantly higher activity than CYP3A4, 3A5, and 2A6. Isoforms CYP1A1, 1B1, 3A7, 2C8, 2C9*1 (Arg144), 2C18, and 4A11 were inactive in the production of either metabolite.
Ring methyl oxidation and N-deethylation activities toward DEET in human cytochrome P450 isoforms expressed in baculovirus-infected insect cells
Kinetic studies of CYP1A2, 2B6, and 2D6*1 (Val374) with respect to the production of the ring methyl oxidation product BALC are shown in Table3. No significant differences inKm,Vmax, and CLint(Vmax/Km) in production of the BALC metabolite were observed between CYP1A2 and 2B6. Activity of the CYP2D6 isoform was too low for accurate kinetic determinations. Comparisons of male and female differences in metabolism of DEET were performed using pooled liver microsomes from two different suppliers. Microsomes from GENTEST included five pooled males and females, whereas those from Xenotech included 10 individuals from each gender. In both cases, activity of females in the production of BALC and ET metabolites was greater than that of males; however, due to departure from randomness in the way these pooled samples were prepared the data are insufficient to demonstrate a definitive gender difference (data not shown).
Kinetic parameters for ring methyl oxidation of DEET by human cytochrome P450 isoforms expressed in baculovirus infected insect cells
To further determine the importance of CYP2B6 and 1A2 in ring methyl oxidation of DEET and, in addition, the importance of CYP3A4, 2C19, and 2A6 in N-deethylation of DEET, liver microsomes from three different individuals possessing varying levels of these isoforms were investigated with respect to their ability to metabolize DEET (Table4). The individual with high levels of both CYP2B6 and 1A2 (HG042) had significantly greater ability to produce the BALC metabolite than the other two individuals. In contrast, individuals with high levels of CYP1A2 (HG043) or CYP2D6 (HG095) had significantly lower ability to metabolize DEET to the BALC metabolite, indicating the importance of CYP2B6 in formation of this metabolite. The individual with high levels of CYP3A4 and 2A6 but low level of CYP2C19 (HG042) had the highest activity for production of the ET metabolite. The individual (HG043) with the highest level of CYP2C19 but low levels of CYP3A4 and 2A6 had significantly greater ability to produce the ET metabolite than the individual (HG095) with very low levels of these isoforms.
Ring methyl oxidation and N-deethylation activities toward DEET in individual human liver microsomes
Experiments were conducted to examine the potential of DEET to induce enzymes involved in metabolism. These experiments included the prototypical P450 inducers phenobarbital and 3-methylcholanthrene. Doses of DEET were low, medium, and high (2, 20, and 200 mg/kg/day). As expected, phenobarbital and 3-methylcholanthrene induced P450 content. Phenobarbital induced BROD (5.2-fold) and PROD (30-fold) activities, whereas 3-methylcholanthrene induced EROD (23-fold) and MROD (10.5-fold) activities. No significant levels of induction were observed for the two lower doses of DEET. In contrast, the high dose of DEET produced significant increases in BROD (3.5-fold) and in PROD activities (4.0-fold).
Studies were also conducted to examine the possible effect of DEET, phenobarbital, and 3-methylcholanthrene to induce metabolism of chlorpyrifos in mice. No significant differences were observed in the dearylation of chlorpyrifos with any treatment. However, significant increases in chlorpyrifos desulfuration activity were observed with phenobarbital (5.5-fold) and the high dose of DEET (2.8-fold).
The possibility that chlorpyrifos or chlorpyrifos-oxon may inhibit DEET metabolism by human CYP2B6 was investigated by incubating 100 μM concentration of each substrate for 5 min before addition of DEET as a substrate. Chlorpyrifos preincubation resulted in 100% inhibition of the production of the BALC metabolite, whereas for chlorpyrifos-oxon, 58% inhibition was observed.
The effects of chlorpyrifos, permethrin, and pyridostigmine bromide alone or in combination on DEET metabolism were investigated using human, rat, and mouse liver microsomes (Table5). Preincubation of pooled HLM with permethrin, pyridostigmine bromide, and permethrin + pyridostigmine bromide significantly increased the production of BALC. Preincubation of pooled HLM with chlorpyrifos alone or in combination with any other compound significantly decreased the production of BALC. Preincubation of pooled HLM with chlorpyrifos, permethrin, pyridostigmine bromide alone or in combination showed no significant differences on the production of ET. Generally, preincubation of pooled HLM, RLM, MLM, DEET-treated MLM, phenobarbital-treated MLM, and 3-methylcholanthrene-treated MLM with chlorpyrifos alone or in combination of any other compound significantly inhibited the production of BALC and ET.
Effects of chlorpyrifos (CPS), permethrin (PMT), and pyridostigmine bromide (PYB) alone and in combination on DEET metabolism by pooled HLM, RLM, MLM, DEET-treated MLM, phenobarbital (PB)-treated MLM, and 3-methylcholanthrene (3-MC)-treated MLM
Discussion
Despite the extensive use of DEET as an insect repellent worldwide, no in vitro studies have been carried out using human enzymes and none of the rat studies have been at the level of individual isoforms. The mean metabolic intrinsic clearance rates, as estimated byVmax/Km, indicate that RLM metabolize DEET more efficiently than pooled HLM or MLM and that the BALC metabolite is produced more readily than the ET metabolite.
A previous study using rat liver microsomes demonstrated a significant gender difference in the metabolism of DEET with males having nearly 3-fold greater metabolizing ability than females (Yeung and Taylor, 1988). Although some pooled female HLM showed significantly higher activities in BALC and ET production from DEET than pooled male HLM the results could not be regarded as definitive as noted above. Further research with large randomly selected pooled male and female HLM or microsomes from individuals is required to better understand possible gender differences in humans.
It is of interest that although eight different isoforms are capable of metabolizing DEET, each isoform produced only one metabolite. Results from individual incubations of DEET with various P450 isoforms suggested that CYP1A2 and 2B6 were highly active in production of the BALC metabolite, whereas CYP3A4, 2C19, and 2A6 were important in the formation of the ET metabolite. Inasmuch as different individuals have varying levels of each of these isoforms, we selected microsomes from individuals possessing widely varying activities of these isoforms to represent contrasting levels of predicted metabolic activity. As expected, the individual with high levels of CYP2B6 and 1A2 had the greatest ability to produce the BALC metabolite. In contrast, individuals possessing high levels of either CYP3A4 or 2C19 had the greatest levels of the ET metabolite production, whereas the individual with the lowest levels of these isoforms had the lowest ET metabolite production. Based on these results it seems reasonable to suggest that individuals, regardless of gender, with varying activities of these P450 isoforms, will be more or less efficient in the metabolism of DEET.
To determine which P450 isoforms were inducible by DEET, substrate-specific assays were conducted using microsomes from DEET-treated mice. DEET treatment did not induce CYP1A1/1A2 as determined from EROD and MROD activity measurement. In contrast, PROD and BROD activities, measures of CYP2B isoform activity, significantly increased in the animals treated with the highest dose of DEET. These induction studies corroborated our results, which indicated that CYP2B6, similar to phenobarbital-induced CYP2B isoforms in rodents (Levi et al., 1988; Fabrizi et al., 1999), is one of the most important P450 isoforms in ring methyl oxidation of DEET and, furthermore, that DEET induces its own metabolism.
Tang et al. (2001) reported that CY2B6 has the highest activity for desulfuration of chlorpyrifos, an activation process. In this study, mice treated intraperitoneally with the highest dose of DEET had significant induction of CYP2B. This induction was demonstrated to result in increased chlorpyrifos desulfuration (2.8-fold), resulting in the production of the activation product, chlorpyrifos oxon. The lower doses of DEET were not effective inducers of CYP2B6. Although these results may imply that DEET could increase organophosphate toxicity, the high levels necessary to produce the effects, combined with the mode of administration, might suggest that risks through epidermal exposures to humans may be minimal. On the other hand, preincubation of chlorpyrifos with CYP2B6 resulted in complete inhibition of DEET metabolism to the BALC metabolite. Thus, chlorpyrifos exposure could inhibit the subsequent metabolism of DEET in humans by inhibiting the isoforms involved in DEET metabolism.
Serious side effects caused by drug-drug interactions have been reported (Guengerich, 1997). Either inhibition or induction can modulate the activity of an enzyme; P450s may exhibit stimulation (positive cooperativity) in the presence of certain xenobiotic compounds (Guengerich, 1997; Szklarz and Halpert, 1998). Our data show stimulation of pooled HLM, wherein conversion of DEET to BALC increased significantly in the presence of permethrin, pyridostigmine bromide, and permethrin + pyridostigmine bromide. However, no stimulation was observed with RLM, MLM, and MLM induced with DEET, phenobarbital, or 3-methylcholanthrene in the presence of permethrin, pyridostigmine bromide, and permethrin + pyridostigmine bromide. Inhibition in a sense may be considered more serious than enzyme induction because inhibition happens quickly, not taking time to develop, as with induction (Guengerich, 1997). Our data show the inhibition of pooled HLM, RLM, MLM, and MLM induced with DEET, phenobarbital, or 3-methylcholanthrene, wherein conversion of DEET to its metabolites, BALC and ET, decreased significantly in the presence of chlorpyrifos alone or in combination of any other tested compound.
In summary, the present investigation has demonstrated that human liver microsomes appear to have generally lower activities for DEET metabolism than those from rodent livers. A screen of human P450 isoforms demonstrated that different sets of isoforms are responsible for the production of each metabolite (BALC and ET). Individuals with varying levels of activities of these human P450 isoforms, regardless of gender, are more or less active in their metabolism of DEET. DEET induction studies, conducted in mice, demonstrate that exposure could result in the induction of CYP2Bs, resulting in potential interactions with other chemicals such as chlorpyrifos. MLM from DEET-treated mice metabolized chlorpyrifos more readily to chlorpyrifos-oxon, a potent anticholinesterase, than control MLM. Preincubation of chlorpyrifos alone with CYP2B6 completely inhibited the metabolism of DEET, whereas preincubation of microsomes with chlorpyrifos, permethrin, and pyridostigmine bromide alone or in combinations may lead to either stimulation or inhibition of DEET metabolism.
Footnotes
-
This research was supported by U.S. Army Cooperative Agreement DAMD 17-00-2-0008. Preliminary studies were presented at the Conference on Illnesses among Gulf War Veterans: A Decade of Scientific Research, Alexandria, Virginia, 2001; and part of the studies will be presented at the 41st Society of Toxicology annual meeting in Nashville, TN, 2002.
- Abbreviations used are::
- DEET
- N,N-diethyl-m-toluamide
- P450
- cytochrome P450
- BALC
- N,N-diethyl-m-hydroxymethylbenzamide
- ET
- N-ethyl-m-toluamide
- HPLC
- high-performance liquid chromatography
- RLM
- rat liver microsomes
- MLM
- mouse liver microsomes
- HLM
- human liver microsomes
- EROD
- O-deethylation
- MROD
- methoxyresorufinO-demethylation (MROD)
- PROD
- pentoxyresorufinO-dealkylation (PROD)
- BROD
- benzyloxyresorufinO-dealkylation
- Received September 13, 2001.
- Accepted December 4, 2001.
- U.S. Government