Abstract
To evaluate the inhibitory effects of trimethoprim and sulfamethoxazole on cytochrome P450 (P450) isoforms, selective marker reactions for CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 were examined in human liver microsomes and recombinant CYP2C8 and CYP2C9. The in vivo drug interactions of trimethoprim and sulfamethoxazole were predicted in vitro using [I]/([I] +Ki) values. With concentrations ranging from 5 to 100 μM, trimethoprim exhibited a selective inhibitory effect on CYP2C8-mediated paclitaxel 6α-hydroxylation in human liver microsomes and recombinant CYP2C8, with apparent IC50(Ki) values of 54 μM (32 μM) and 75 μM, respectively. With concentrations ranging from 50 to 500 μM, sulfamethoxazole was a selective inhibitor of CYP2C9-mediated tolbutamide hydroxylation in human liver microsomes and recombinant CYP2C9, with apparent IC50 (Ki) values of 544 μM (271 μM) and 456 μM, respectively. With concentrations higher than 100 μM trimethoprim and 500 μM sulfamethoxazole, both drugs lost their selectivity for the P450 isoforms. Based on estimated total hepatic concentrations (or free plasma concentrations) of the drugs and the scaling model, one would expect in vivo in humans 80% (26%) and 13% (24%) inhibition of the metabolic clearance of CYP2C8 and CYP2C9 substrates by trimethoprim and sulfamethoxazole, respectively. In conclusion, trimethoprim and sulfamethoxazole can be used as selective inhibitors of CYP2C8 and CYP2C9 in in vitro studies. In humans, trimethoprim and sulfamethoxazole may inhibit the activities of CYP2C8 and CYP2C9, respectively.
Trimethoprim is frequently combined with sulfamethoxazole as cotrimoxazole, a broad-spectrum antibacterial agent, to treat a wide range of infections. Although trimethoprim is mainly excreted unchanged in urine, a significant amount (20%) of the dose is metabolized by the hepatic cytochrome P450 (P4501) isoforms (Gleckman et al., 1981). In individuals with severe liver damage, the elimination half-life of trimethoprim can be lengthened up to 2-fold (Rieder and Schwartz, 1975). Sulfamethoxazole is eliminated mainly by metabolism, and CYP2C9 plays an important role in itsN4-hydroxylation (Cribb et al., 1995).
Trimethoprim and sulfamethoxazole have increased the plasma concentrations or effects of drugs such as tolbutamide, phenytoin, warfarin, and glipizide, resulting in clinically significant drug-drug interactions (Hansen et al., 1979; O'Reilly, 1980; Wing and Miners, 1985; Johnson and Dobmeier, 1990). It has been suggested that inhibition of oxidative drug metabolism by trimethoprim and sulfamethoxazole is the likely mechanism of these drug-drug interactions (Wing and Miners, 1985). In previous in vitro studies, sulfamethoxazole has been shown to inhibit tolbutamide hydroxylation (a CYP2C9 marker reaction) with an apparentKi value of about 250 μM (Back et al., 1988; Komatsu et al., 2000a). However, it seems that there are no published in vitro studies investigating the effects of trimethoprim and sulfamethoxazole on different P450 isoforms. We have studied the inhibitory effect of trimethoprim and sulfamethoxazole on major P450 isoform activities in human liver microsomes and recombinant P450s using selective marker reactions.
Experimental Procedures
Materials.
Dextromethorphan and dextrorphan were obtained from Orion Pharma (Espoo, Finland). Sulfamethoxazole, trimethoprim, phenacetin, paracetamol, coumarin, 7-hydroxycoumarin, tolbutamide, chlorzoxazone, paclitaxel, testosterone, and NADPH were purchased from Sigma-Aldrich (St. Louis, MO). Hydroxytolbutamide, 6-hydroxychlorzoxazone, S-mephenytoin, 4′-hydroxymephenytoin, 6β-hydroxytestosterone, and 6α-hydroxypaclitaxel were purchased from Ultrafine Chemicals (Manchester, UK). Midazolam and 1′-hydroxymidazolam were kindly provided by F. Hoffmann-La Roche (Basel, Switzerland). Pooled human liver microsomes (prepared from five male, and five female human liver microsomal samples) containing representative activities of CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 were obtained from Gentest Corp. (Woburn, MA). Microsomes from baculovirus-infected cells engineered to express the cDNA encoding human CYP2C8 and CYP2C9 were also purchased from Gentest Corp. Other chemicals and reagents were obtained from Merck (Darmstadt, Germany).
Inhibition Studies.
The effects of trimethoprim and sulfamethoxazole on eight different P450 isoform-specific marker reactions were studied. PhenacetinO-deethylation was used to probe for CYP1A2, coumarin 7-hydroxylation for CYP2A6, paclitaxel 6α-hydroxylation for CYP2C8, tolbutamide hydroxylation for CYP2C9, S-mephenytoin 4′-hydroxylation for CYP2C19, dextromethorphanO-demethylation for CYP2D6, chlorzoxazone 6-hydroxylation for CYP2E1, and midazolam 1′-hydroxylation and testosterone 6β-hydroxylation for CYP3A4. All incubations were performed in duplicate, and the mean values were used. Briefly, each incubation was performed with 20 μg human liver microsomes or recombinant P450 isoforms in a final incubation volume of 0.2 ml, after diluting from their original concentrations (20 mg/ml, 3 mg/ml, 2.1 mg/ml for human liver microsomes, recombinant CYP2C8, and CYP2C9, respectively). The incubation medium contained 0.1 M sodium phosphate buffer (pH 7.4) and 5 mM MgCl2. To determine whether the inhibition of P450 isoforms by trimethoprim and sulfamethoxazole could be mechanism-based, trimethoprim (dissolved in 2 μl of methanol, final concentration 5–500 μM) and sulfamethoxazole (dissolved in 2 μl of methanol, final concentration 50–1000 μM) were preincubated with the incubation medium at 37°C for 15 min, either in the presence or absence of 1.0 mM NADPH. An equal volume (2 μl) of methanol was added to the noninhibitor controls. After the preincubation, probe substrates were added either with or without 1.0 mM NADPH for measurement of the corresponding marker activities.
Testosterone (dissolved in 2 μl of methanol, final concentration 25 μM) and paclitaxel (dissolved in 2 μl of methanol, final concentration 1–5 μM) were incubated with the incubation medium for 6 and 20 min, respectively. Acetonitrile (100 μl) was used to terminate the reactions. For the other reactions, the incubation conditions including solvents, incubation times, quenching methods, and the effects of specific inhibitors have been reported elsewhere (Wen et al., 2001). The time of incubation and concentration of microsomes (100 μg/ml) used in each assay were determined to be in the linear range for the rate of metabolite formation. After incubation at 37°C for a specific period of time, the reaction was quenched by adding an appropriate chemical to precipitate the proteins. The incubation mixtures were then centrifuged for 5 min at 10,000g. An aliquot of the supernatant fraction was subjected to analysis using high-performance liquid chromatography (HPLC).
Incubations with the recombinant CYP2C8 and CYP2C9 isoforms were performed using the same conditions as the incubations with human liver microsomes, except that the incubation mixture contained 100 μg/ml of CYP2C8 and CYP2C9 supersomes and was incubated for 20 min (CYP2C8) and 30 min (CYP2C9), respectively.
HPLC Analysis.
Assays for the respective products of P450 marker reactions were carried out using HPLC (Stewart and Carter, 1986; Harris et al., 1994;Wang et al., 2000; Wen et al., 2001). The HPLC system consisted of a Pharmacia LKB 2150 pump (LKB, Uppsala, Sweden), a Hewlett Packard 1050 autosampler (Hewlett Packard, Mississauga, ON), a Hewlett-Packard 3396 integrator (Hewlett Packard), a SPD-10AV Shimadzu UV detector (Shimadzu, Kyoto, Japan; for analysis of CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2E1, and CYP3A4 activities), a RF-551 Shimadzu fluorescence detector (Shimadzu; for analysis of CYP2A6 and CYP2D6 activities) or model 5100A Coulochem electrochemical detector (ESA Inc., Bedford, MA; for analysis of the inhibitory effect of sulfamethoxazole on CYP2E1 activity). The intraday and interday coefficients of variation for all assays were less than 7% at relevant concentrations (n = 6).
Data Analysis.
The IC50 values (concentration of inhibitor to cause 50% inhibition of original enzyme activity) were determined graphically. The apparent inhibitory constant (Ki) values were calculated by nonlinear regression analysis using Systat for Windows 6.0.1 (SPSS Inc., Chicago, IL). Different models of enzyme inhibition (i.e., competitive, noncompetitive, uncompetitive, and mixed-type inhibition) were fitted to the kinetic data (Segel, 1975). An assessment of goodness of fit of the models was made using the size of the residual sum of squares and the random distribution of the residuals, the standard error, and the 95% confidence interval of the parameter estimates.
Results
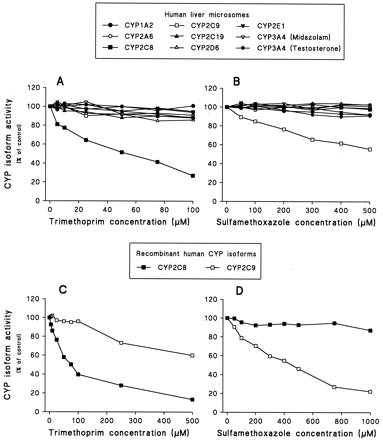
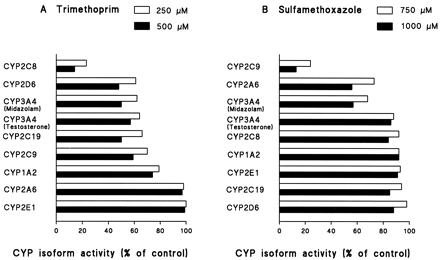
With concentrations ranging from 5 to 100 μM, trimethoprim exhibited a selective inhibitory effect on CYP2C8-mediated paclitaxel 6α-hydroxylation with an apparent IC50(Ki) value of 54 μM (32 μM) in human liver microsomes and an IC50 of 75 μM in recombinant CYP2C8 (Fig. 1, Table1). The pattern of inhibition was competitive (Table 1). However, trimethoprim lost its isoform selectivity at concentrations higher than 100 μM (Fig.2). As much as 20 to 50% of CYP1A2-, CYP2C9-, CYP2C19-, CYP2D6- and CYP3A4- mediated activities were inhibited at concentrations of 250 and 500 μM (Fig. 2).
Inhibitory effect of trimethoprim and sulfamethoxazole on P450-catalyzed reactions in human liver microsomes (A, B) and in recombinant CYP2C8 and CYP2C9 (C, D).
Trimethoprim and sulfamethoxazole were incubated using conditions described under Experimental Procedures. The enzyme reactions evaluated were CYP1A2-catalyzed phenacetinO-deethylation, CYP2A6-catalyzed coumarin 7-hydroxylation, CYP2C8-catalyzed paclitaxel 6α-hydroxylation, CYP2C9-catalyzed tolbutamide hydroxylation, CYP2C19-catalyzedS-mephenytoin 4′-hydroxylation, CYP2D6-catalyzed dextromethorphan O-demethylation, CYP2E1-catalyzed chlorzoxazone 6-hydroxylation, CYP3A4-catalyzed midazolam 1′-hydroxylation, and testosterone 6β-hydroxylation. Phenacetin, coumarin, paclitaxel, tolbutamide, S-mephenytoin, dextromethorphan, chlorzoxazone, midazolam, and testosterone were used at concentrations around their correspondingKm values (i.e., 50, 1, 5, 50, 40, 1.5, 25, 2, and 25 μM, respectively). Each data point represents an average of duplicates.
Inhibition of CYP2C8 and CYP2C9 isoforms by trimethoprim and sulfamethoxazole in human liver microsomes and recombinant P450 isoforms and the predicted in vivo inhibition of the metabolism of coadministered CYP2C8 and CYP2C9 substrates by trimethoprim and sulfamethoxazole, respectively, from in vitro data
Inhibitory effects of high concentrations of trimethoprim (A) and sulfamethoxazole (B) on CYP1A2-catalyzed phenacetin O-deethylation, CYP2A6-catalyzed coumarin 7-hydroxylation, CYP2C8-catalyzed paclitaxel 6α-hydroxylation, CYP2C9-catalyzed tolbutamide hydroxylation, CYP2C19-catalyzed S-mephenytoin 4′-hydroxylation, CYP2D6-catalyzed dextromethorphan O-demethylation, CYP2E1-catalyzed chlorzoxazone 6-hydroxylation, CYP3A4-catalyzed midazolam 1′-hydroxylation, and testosterone 6β-hydroxylation in human liver microsomes.
Each data point represents an average of duplicates.
Sulfamethoxazole selectively and competitively inhibited tolbutamide hydroxylase activity with concentrations ranging from 50 to 500 μM in human liver microsomes and recombinant CYP2C9, with apparent IC50 (Ki) values of 544 μM (271 μM) and 456 μM, respectively (Fig. 1; Table1). Very little (<20%) or no inhibition of other P450 isoforms was found in this concentration range. However, at concentrations higher than 500 μM, sulfamethoxazole showed a modest (30–40%) inhibitory effect on CYP2A6-mediated coumarin 7-hydroxylation and CYP3A4-mediated midazolam 1′-hydroxylation (Fig. 2).
Preincubation of trimethoprim and sulfamethoxazole with NADPH for 15 min prior to the addition of the specific substrates did not increase the degree of inhibition (data not shown).
Discussion
The results of the present study indicate that trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 (Ki = 32 μM) and CYP2C9 (Ki = 271 μM), respectively, in human liver microsomes at concentrations ranging from 5 to 100 μM trimethoprim and 50 to 500 μM sulfamethoxazole. With concentrations higher than 100 μM trimethoprim and 500 μM sulfamethoxazole, both drugs lost their selectivity toward the P450 isoforms and became inhibitors of several P450 isoforms. The results are in agreement with previous in vitro studies showing that sulfamethoxazole competitively inhibited tolbutamide hydroxylase activity, with an apparentKi value of 246 μM (Back et al., 1988) or 283 μM (Komatsu et al., 2000a) in human liver microsomes.
Theoretically, drug-drug interactions based on inhibition of hepatic drug metabolism (i) can be predicted by theKi value and the concentration of the inhibitor [I] around the metabolic enzyme in the liver using the following scaling model: i = [I]/([I] +Ki), assuming that the substrate concentration is much lower than itsKm value (von Moltke et al., 1998). After oral administration of 200 mg trimethoprim and 800 mg sulfamethoxazole twice daily, the mean peak plasma concentrations of trimethoprim and sulfamethoxazole were approximately 20 and 250 μM, respectively (Moore et al., 1996; Dollery, 1999). Although the exact liver/plasma partition ratios of trimethoprim and sulfamethoxazole in humans are unknown, animal experiments in monkeys have shown that the liver/plasma partition ratios are about 6.5 for trimethoprim and 0.15 for sulfamethoxazole (Craig and Kunin, 1973), which agree well with the relatively small volume of distribution of sulfamethoxazole (about 0.2 l/kg) in humans (Dollery, 1999). Accordingly, it can be estimated that the total liver concentrations of trimethoprim and sulfamethoxazole are around 130 and 40 μM, respectively. Consequently, based on these total hepatic concentrations, one would expect approximately 80 and 13% inhibition of the metabolic clearance of CYP2C8 and CYP2C9 substrates by trimethoprim and sulfamethoxazole, respectively (Table1). Alternatively, assuming that 55% of trimethoprim and 34% of sulfamethoxazole are unbound in plasma (Dollery, 1999) and that equal unbound concentrations are found in plasma and at the enzyme site, approximately 26 and 24% inhibition of CYP2C8 and CYP2C9 by trimethoprim and sulfamethoxazole would be expected, respectively.
CYP2C8 is primarily responsible for the metabolism of e.g., taxol, cerivastatin, rosiglitazone and troglitazone and also involved in the metabolism of zopiclone, carbamazepine, verapamil, and amiodarone (Ohyama et al., 2000; Ong et al., 2000). However, most previous clinical drug-drug interaction studies involving trimethoprim have focused on substrates of CYP2C9. For example, trimethoprim used alone inhibited the metabolic clearance of tolbutamide (14%) and phenytoin (30%) (Hansen et al., 1979; Wing and Miners, 1985). As can be seen from Figs. 1 and 2, CYP2C8 is much more susceptible to the inhibitory effect of trimethoprim than CYP2C9. Although tolbutamide and phenytoin are metabolized primarily by CYP2C9, they are metabolized to a minor extent also by CYP2C8 (Komatsu et al., 2000a,b). Therefore, inhibition of CYP2C8 by trimethoprim may explain the reported modest reduction in the metabolic clearance of tolbutamide and phenytoin. Trimethoprim at very high concentrations (i.e., 250 μM and 500 μM) inhibited 30 and 40% of CYP2C9 activity, respectively. Thus, because trimethoprim is distributed extensively into tissues such as liver, there is a possibility that trimethoprim may also slightly reduce the activity of hepatic CYP2C9.
Consistent with our prediction, sulfamethoxazole used alone has modestly inhibited the metabolic clearance of the CYP2C9 substrates tolbutamide (14%) and phenytoin (10%) (Hansen et al., 1979; Wing and Miners, 1985). When coadministered with trimethoprim, sulfamethoxazole increased the area under the plasma concentration-time curve ofS-warfarin, another CYP2C9 substrate, by 20% (O'Reilly, 1980). When combined with trimethoprim, sulfamethoxazole inhibited the metabolic clearance of tolbutamide more than when trimethoprim was used alone (25 versus 14%) (Wing and Miners, 1985).
With the negligible inhibitory effects on the other P450 isoforms tested, trimethoprim and sulfamethoxazole with normal therapeutic doses are unlikely to produce clinically relevant interactions by inhibiting these P450 isoforms. In line with these findings, trimethoprim/sulfamethoxazole had no significant effects on the pharmacokinetics of theophylline (a CYP1A2 substrate) and nifedipine (a CYP3A4 substrate) (Jonkman et al., 1985; Edwards et al., 1990).
No chemical has been previously identified as a selective inhibitor of CYP2C8 (Ong et al., 2000). Quercetin has been used as an inhibitor of CYP2C8, but it is also a potent inhibitor of CYP1A2 (Dierks et al., 2001). Also sulfaphenazole, a commonly used potent inhibitor of CYP2C9 (Ki = 0.3 μM), has some inhibitory effects toward the other CYP2C isoforms, [e.g., CYP2C8 (Ki = 63 μM) and CYP2C18 (Ki = 29 μM)] (Mancy et al., 1996).
Our results indicate that when used at concentrations lower than 100 μM (trimethoprim) and 500 μM (sulfamethoxazole), trimethoprim and sulfamethoxazole can be used as selective inhibitors of CYP2C8 and CYP2C9, respectively, in in vitro studies. In addition, even with a concentration reaching 1000 μM, sulfamethoxazole is a very selective inhibitor of CYP2C9 among the CYP2C isoforms. However, the inhibitor concentration must be selected carefully. As noted above, at the concentration of 500 μM, trimethoprim inhibited CYP1A2, 2C9, 2C19, 2D6, and 3A4 activities by about 25, 40, 50, 50, and 50%, respectively. Similarly, 1000 μM sulfamethoxazole reduced the activities of CYP2A6 and 3A4 by 45 and 40%, respectively.
In conclusion, our study demonstrated that trimethoprim (5–100 μM) and sulfamethoxazole (50–500 μM) are selective inhibitors of CYP2C8 and CYP2C9 activities, respectively. At clinically relevant concentrations, trimethoprim strongly inhibits CYP2C8 activity, and sulfamethoxazole moderately inhibits CYP2C9 activity. The inhibition of CYP2C8 activity by trimethoprim and CYP2C9 by sulfamethoxazole may be the mechanisms involved in the drug-drug interactions between trimethoprim/sulfamethoxazole, and tolbutamide, phenytoin, warfarin, and glipizide.
Acknowledgments
We thank Lisbet Partanen for skillful technical assistance.
Footnotes
-
This study was supported by grants from the Helsinki University Central Hospital Research Fund and the National Technology Agency of Finland (Tekes), Finland.
- Abbreviations used are::
- P450
- cytochrome P-450
- HPLC
- high-performance liquid chromatography
- Received December 5, 2001.
- Accepted February 19, 2002.
- The American Society for Pharmacology and Experimental Therapeutics