Abstract
Linezolid (Zyvox), the first of a new class of antibiotics, the oxazolidinones, is approved for treatment of Gram-positive bacterial infections, including resistant strains. The disposition of linezolid in human volunteers was determined, after a 500-mg (100-μCi) oral dose of [14C]linezolid. Radioactive linezolid was administered as a single dose, or at steady-state on day 4 of a 10-day, 500-mg b.i.d. regimen of unlabeled linezolid (n = 4/sex/regimen). Mean recovery of radioactivity in excreta was 93.8 ± 1.1% (range 91.2–95.2%, n = 15), of which 83.9 ± 3.3% (range 76.7–88.4%) was in urine and 9.9 ± 3.4% (range 5.3–16.9%) was in feces. There was no major difference in rate or route of excretion of radioactivity by dose regimen. Linezolid was excreted primarily intact, and as two inactive, morpholine ring-oxidized metabolites, PNU-142586 and PNU-142300. Other minor metabolites were characterized by high-performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry and 19F NMR spectroscopy. After the single radioactive dose, linezolid was the major circulating drug-related material accounting for about 78% (male) and 93% (female) of the radioactivity area under the curve (AUC). PNU-142586 (Tmax of 3–5 h) accounted for about 26% (male) and 9% (female) of the radioactivity AUC. PNU-142300 (Tmax of 2–3 h) accounted for about 7% (male) and 4% (female) of the radioactivity AUC. Overall, mean linezolid and PNU-142586 exposures at steady-state were similar across sex. In conclusion, linezolid circulates in plasma mainly as parent drug. Linezolid and two major, inactive metabolites account for the major portion of linezolid disposition, with urinary excretion representing the major elimination route. Formation of PNU-142586 was the rate-limiting step in the clearance of linezolid.
Linezolid ((S)-N-[[3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-acetamide, Zyvox, PNU-100766; Fig. 1) is the first of a new class of antibiotics, the oxazolidinones. Linezolid is approved in the United States and other countries worldwide for the treatment of Gram-positive bacterial infections, including those caused by resistant organisms. The oxazolidinones are synthetic compounds that selectively inhibit the initiation phase of bacterial protein synthesis (Swaney, 1996; Demyan et al., 1997; Lin et al., 1997; Shinabarger et al., 1997).
Linezolid metabolic pathways in mouse, rat, dog, and human.
Linezolid is dosed intravenously or orally at 400 or 600 mg b.i.d. Because bioavailability is approximately 100%, no dosage adjustment is needed when changing from intravenous to oral therapy. After an oral 600-mg dose, steady-state peak plasma concentrations of 21.2 ± 5.78 μg/ml are obtained at a Tmax of 1.03 ± 0.62 h. The plasma elimination half-life is 5.40 ± 2.06 h. Clearance, which occurs by both renal and nonrenal (65%) mechanisms is 80 ± 29 ml/min. Linezolid is neutral in the physiological pH range and undergoes renal tubular reabsorption. Plasma protein binding is low at 31%, and the volume of distribution approximates total body water (40–50 liters) (Pawsey et al., 1996,Stalker et al., 1997a,b; Pharmacia Corporation, 2000). In a single-dose study, female human subjects had about 20% lower body weight-normalized clearance than males (Sisson et al., 1999).
The objective of this study was to determine the pharmacokinetics, metabolism, and excretion of linezolid following single-dose and steady-state administration of [14C]linezolid to healthy human subjects.
Materials and Methods
Study Design.
The study enrolled 16 healthy volunteers (four subjects per sex for each regimen) aged 20 to 61 years. All subjects provided written informed consent prior to enrollment. Radiation exposure estimates for human tissues were predicted using maximum internal radiation dose (MIRD) software (Loevinger et al., 1991). The 100-μCi dose chosen for this study is similar to that given in most other human14C trials conducted in the United States (Dain et al., 1994).
In a randomized, open-label, parallel group study, the single-dose group received a single 500-mg oral dose of [14C]linezolid solution (100 μCi), administered on the morning of day 1. In the steady-state group, two 250-mg linezolid tablets were given every 12 h on days 1 to 3. On day 4 a single 500-mg oral dose of [14C]linezolid solution (100 μCi) was given as the AM dose then two 250-mg linezolid tablets as the PM dose, followed on days 5 to 10 by two 250-mg linezolid tablets every 12 h. Adverse events were all nonserious and mild to moderate in intensity, with no clinically significant laboratory or ECG abnormalities.
Formulations and Dose Administration.
Linezolid 250-mg tablets were from lot 27,721 (single-dose) or lot 27,872 (steady-state dose). Linezolid was labeled with14C on the carbonyl carbon of the acetamide moiety. The radiolabel was formulated as a sterile solution and packaged in tared 500-ml amber bottles containing 250 ml of [14C]linezolid (2 mg/ml, 0.4 μCi/ml). The measured percentage of radiopurity was 98.38 ± 0.08% CV.1 The measured mean concentration of linezolid in the formulation was 2.0075 ± 0.26% CV mg/ml and 2.054 ± 0.64% CV mg/ml for single-dose and steady-state dose groups, respectively. Corresponding measured specific activities were 0.1967 and 0.1952 μCi/mg.
Subjects were required to fast from 10:00 PM on the day prior to the radioactive dose until 4 h after administration of the [14C]linezolid solution. Subjects had free access to water during the fasting period, except that no fluids or water were allowed during the 2 h preceding and 1 h following dosing. Each subject consumed the entire contents of the bottle and a bottle rinse.
Radiochemical Excretion and Pharmacokinetic Analysis.
Urine, feces, blood, and plasma were collected over the 7 days following the radioactive doses. Subject demographics and dosimetry are summarized in Table 1. Actual radiation exposure, calculated for each subject using the MIRD method (Loevinger et al., 1991), was well below the limits permitted by the Food and Drug Administration (CFR 21, Part 361.1, 1998).
Summary of subject demographics and dosimetry
Biofluid collection.
Blood samples (5 or 10 ml) were collected into K3EDTA vacutainers at time 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 10, 12, 14, 16, 24, 36, 48, 72, 96, 120, 144, and 168 h after the radioactive dose. In the steady-state group, a predose (day 1) blood sample was collected, and Cminsamples were drawn prior to each subsequent nonradioactive dose. Hematocrit measurements were done at 0, 12, 24, and 168 h following the radiolabel dose. Plasma was collected by centrifugation (4°C) for radioanalysis and then frozen (−20°C).
After the radiolabel dose, urine was collected as voided, and pooled in tared containers over the following intervals: 0 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 36, 36 to 48, 48 to 60, 60 to 72, 72 to 84, 84 to 96, 96 to 120, 120 to 144, and 144 to 168 h. Subjects were asked to void at the end of each collection interval. Urine was stored at 4°C until aliquots were obtained and was then frozen at −20°C.
Individual bowel movements were collected and frozen immediately (−20°C). Samples were later thawed and pooled over 24-h intervals for radioanalysis and metabolite profiling.
Radioactivity analysis.
All assays were done gravimetrically by direct capture of sample weights by DEBRA, version 4.1c (LabLogic, Sheffield, UK). Radioactivity analysis was performed using Packard Tri-Carb liquid scintillation spectrometers (model 1900 or 2300TR; Packard Instrument Co., Meriden, CT). Fecal homogenates and blood were combusted with a Packard Tri-Carb sample oxidizer (model 387) and analyzed by liquid scintillation counting (LSC) in Carbosorb E/Permafluor E+ (Packard Instrument Co.). Radioactivity in plasma and urine was determined by LSC in Ultima Gold.
Radioactivity data analysis.
Dose weights (mg of linezolid), specific activity (μCi/mg), body weight (kg), subject number, sample weight (g), aliquot weight (g), uncorrected LSC results (dpm), and LSC background (dpm) recorded in predose matrix were processed by DEBRA, version 4.1c. Excretion data for each matrix (urine, feces) and for the sum of all matrices were expressed as recovered percentage of administered radioactive dose per collection. The percentage of dose excreted during each collection interval calculated by DEBRA was transformed to cumulative percentage of dose excreted. The percentage of dose remaining to be excreted [amount remaining to be excreted (ARE)] was calculated using Microsoft Excel, version 5.0c (Microsoft, Redmond, WA). Harmonic mean ARE half-lives were calculated for urine data using linear regression of the log-linear terminal phase. Blood and plasma radioanalysis data were expressed as μg-Eq of linezolid/g of sample matrix. Hematocrit data from each subject were used to calculate the blood/plasma partition coefficient Kp (Sun et al., 1987), written in Microsoft Excel as follows:Kp = (Cb − (Cp · (1 − HCT)))/HCT/Cp, where HCT is the hematocrit, expressed as a fraction, Cb is concentration in blood, and Cp is the concentration in plasma.
Metabolite Profiling and Quantitation.
Excreta from each subject were profiled separately. Urine samples containing more than 1% of dose, combined feces samples containing greater than 1% of dose, and pooled plasma samples were quantitatively profiled using reversed phase HPLC with radiochemical detection.
Metabolites were characterized by comparing their HPLC-UV retention times and mass spectra (HPLC-APCI-MS and -MS-MS) to those of authentic synthetic standards, when available. Unknown metabolites and metabolites lacking a synthetic standard were characterized by HPLC-APCI-MS and HPLC-APCI-MS-MS. Major drug-related peaks in selected urine samples were also quantified by 19F NMR spectroscopy, and results were compared with quantitative radiometric HPLC data. A Student's t test was used to compare metabolite abundance by dose group and by sex. All calculations were performed using Microsoft Excel, version 5.0c.
Sample extraction and concentration for metabolite profiling.
Excreta collections were pooled proportional to total sample weight. Recoveries were calculated for all extraction steps. Acidification was required to extract acidic metabolites, and retention times of acidic metabolites were sensitive to mobile phase pH. Due to the instability of PNU-142586 in acidic solution, acidified samples were kept at 4°C during sample preparation, and in the autoinjector tray. Decomposition of PNU-142586 during sample preparation was shown to be insignificant under these extraction and analysis conditions, in comparison to samples analyzed without acidification or extraction. Recovery from the HPLC column was quantitative, based on LSC of HPLC eluate with column in-line, versus column off-line. Comparison of radiometric HPLC peak integration data with data obtained by fraction collection with LSC showed that quenching did not occur during the HPLC gradient.
Urine (1 ml, 0–24-h samples) was acidified with 1 M ammonium acetate (pH 4.5) and 100 μl of acetonitrile. After centrifugation, 150-μl aliquots were analyzed by HPLC. For more dilute urine samples, a vacuum solid phase extraction (SPE) manifold was conditioned sequentially with methanol, acetonitrile, and 50 mM acetic acid. Urine (2 ml) was acidified to pH 3 to 4 with acetic acid and immediately slowly loaded onto C2 cartridges (3 cc/500 mg; Varian, Harbor City, CA) then prewashed with 10 mM acetic acid. Radioactivity was slowly eluted with two aliquots of elution solvent and evaporated to near dryness at room temperature. The elution solvent was 90% organic (97:3 acetonitrile:isopropanol) and 10% 100 mM ammonium acetate, pH 4.8. Extracts were dissolved in mobile phase, centrifuged, and aliquots were analyzed by HPLC.
Pooled feces homogenate (5–7 g) was centrifuged at 3200gfor 15 min and the supernatant was collected. Water (5 ml) was added to the pellet, followed by vortexing for 10 min, and centrifugation. The supernatants were combined and the process was repeated. SPE cartridges were loaded with 1 to 4 ml of acidified (pH < 4) feces extract. The SPE and HPLC analysis procedures were the same as for the urine samples.
To concentrate sufficient radioactivity to observe minor peaks, equal weights of plasma samples obtained 0.25 to 6 h after dose administration were combined and processed. Plasma was acidified with 1 M acetic acid (< pH 4), centrifuged, and extracted by SPE, as described for urine, using an elution solvent composed of 91:6:3 methanol:acetonitrile:pH 7, 100 mM ammonium acetate.
HPLC radiochemical method.
The HPLC system consisted of a PerkinElmer series 410 pump (PerkinElmer, Norwalk, CT), a PerkinElmer ISS-200 autoinjector, a Waters 486 UV detector (255 nm) (Millipore Corp., Milford, MA), and a Packard Radiomatic Flow-One Beta Radio-Chromatography series A-525 detector (software version 3.55). HPLC separations were performed using a Waters Symmetry C8 analytical column (250 × 4.6 mm) and a Waters Sentry guard column. The HPLC mobile phase was a blend of acetonitrile and 100 mM acetic acid adjusted to pH 4.8 with ammonium hydroxide. The gradient sequentially defined as percentage of acetonitrile was initially 10% and held for 4 min, a 12-min nonlinear (PE#2) ramp to 25%, and held for 8 min, a 3-min linear ramp to 60%, and held for 5 min. Prior to each injection, the column was cleaned with 80% acetonitrile for 5 min and reequilibrated for 12 min. The mobile phase flow rate was 1 ml/min. No additional major HPLC peaks were uncovered using a formate-based alternative mobile phase gradient, or by 19F NMR spectroscopy.
The radiochemical detector used an update time of 6 s and an 0.5-ml time-resolved LSC cell. The liquid scintillant to HPLC flow rate ratio was 3:1. Peak integrations were in units of percentage of integrated peaks and were converted to percentage of dose using total percentage of recovery data.
Stability of PNU-142586 in acidic solution.
PNU-142620 (lactone) and PNU-142586 (ring-opened hydroxy-acid) exist in a pH dependent equilibrium, with acidic conditions favoring the lactone (Fig. 1). Concomitant irreversible decomposition of PNU-142586 to PNU-142618 also occurs in acid media. PNU-142586 decomposed almost completely to PNU-142618 in the analytical standards during storage at 4°C in 10 mM buffer, pH 4.8, over about a 2- to 4-week period. PNU-142620 decomposed to both PNU-142586 and PNU-142618 under the same conditions.
HPLC/MS of urine samples.
HPLC-APCI-MS and -MS-MS analyses were performed to verify the identity of metabolites and to assign structures to unknown peaks. The HPLC system was similar to the radiometric HPLC system, except a Hewlett-Packard series 1050 pump and autoinjector were used. The retention times using this system were slightly different from the radiometric HPLC system.
Mass spectrometric analysis was performed on a Finnigan MAT TSQ 7000 triple quadrupole mass spectrometer (Finnigan MAT, San Jose, CA) directly coupled to the HPLC system. The ionization mode was APCI. Data were collected on an Alpha Station 255 running Digital Unix 4.0b, Finnigan ICL version 8.3.2 and Finnigan ICIS version 8.3.0. The nebulizer was set to 450°C and the heated capillary was set to 240°C.
19F NMR spectroscopy.
Urine samples were filtered through 0.45-μm Gelman nylon acrodiscs. To 500 μl of urine filtrate was added 50 μl of 99.9% deuterated water plus 50 μl of 2 or 200 μM p-fluorobenzoic acid (p-fluorobenzoic acid-internal standard) in deuterated water. Linezolid and its metabolites were quantified by comparing the integrated intensities of their 19F resonances to that of p-fluorobenzoic acid. Concentration data (nmol/0.5 ml) were converted to percentage of dose.
Samples were run on a Bruker DRX-500 spectrometer, using a fluorine observe frequency of 470.532 MHz and a proton decoupling frequency of 500.130 MHz. The 19F NMR spectra consisted of single lines at unique chemical shifts for the fluorinated components in the urine sample. The major resonance peaks were identified by authentic standard addition to urine.
Quantitative analysis of linezolid and metabolites in human plasma.
Plasma samples were assayed for linezolid and metabolites PNU-142586 and PNU-142300 by HPLC-MS-MS on a Finnigan TSQ-700 mass spectrometer. Plasma samples (0.020 ml) were diluted with water, and proteins were precipitated with acetonitrile containing the internal standard PNU-108812. After centrifugation, an aliquot of supernatant was removed, dried under nitrogen, reconstituted in water:acetonitrile (95:5, v/v), and injected. Separation was done using an isocratic mobile phase of 0.2% trifluoroacetic acid/methanol (50:50, v/v) at a flow rate of 0.3 ml/min. The analytical column was a Zorbax SB-CN (2.1 × 150 mm, 5 μm; MacMod Analytical, Inc., Chadds Ford, PA).
Quantitative mass spectrometry was performed using an APCI source operated in the positive ion mode. Detection was done by selected reaction monitoring of the product ion at m/z 296 (molecular ion at m/z 338) for linezolid,m/z 324 (molecular ion atm/z 370) for PNU-142586,m/z 328 (molecular ion atm/z 370) for PNU-142300, andm/z 276 (molecular ion atm/z 320) for the internal standard. Retention times of linezolid, its metabolites, and the internal standard were approximately 2.0 to 3.0 min.
Quantitation was done using peak area ratios from calibration standard curves, using a weighted (1/concentration) linear, least-squares regression. The linear calibration range for linezolid was 0.0054 to 53.6 μg/ml, for PNU-142586 was 0.006 to 30.0 μg/ml, and for PNU-142300 was 0.0049 to 4.9 μg/ml.
Standard curve correlation coefficients for all components were ≥0.999. The mean ± CV recovery for the linezolid calibration standards was 103 ± 5%, 101 ± 7% for the PNU-142300 calibration standards, and 104 ± 8% for the PNU-142586 calibration standards. Intraday accuracy and precision were monitored by analysis of at least three quality control standards. The intra-assay mean recovery ± CV% for the high- and low-quality control analysis were 110 ± 8% (22.4 μg/ml) and 114 ± 6% (0.0224 μg/ml) for linezolid. For PNU-142586 mean recoveries were 100 ± 14% (2.97 μg/ml) and 95 ± 13% (0.0297 μg/ml). For PNU-142300 means recoveries were 91 ± 22% (1.01 μg/ml) and 93 ± 15% (0.0101 μg/ml).
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic parameters were calculated using formulas described by Gibaldi and Perrier (1982). Calculations were done using a validated internal, SAS-based clinical pharmacokinetics analysis package (CPAP, version 1.0; Trilogy Consulting Corporation, 1996). Cmax andTmax for intact linezolid, metabolites in plasma, and radioactivity in plasma and whole blood were determined from individual subject concentration-time curves. The apparent terminal elimination half-life (t1/2) was calculated as (ln 2/λz). Area under the curve values for intact linezolid, and metabolites in plasma, and radioactivity in plasma and whole blood [AUC0-t(last)] were determined using trapezoidals from time 0 to the last quantifiable drug concentration [Ct(last)]. For single-dose data, area under the plasma linezolid, metabolite, and radioactivity concentration time-curves through infinite time (AUC0-∞) were calculated by addingCt(last)/λzto AUC0-t(last). Area under the curve was also calculated from 0 to 12 h by trapezoidal rule (AUC0–12) from day 4 data and was used to calculate steady-state clearance for the parent compound. Total apparent oral systemic clearance (CL or CL/F) of linezolid was calculated as actual dose/AUC, and assumes a bioavailability of 100% (Pharmacia Corporation, 2000). Renal clearance (CLr/F) was estimated from total recovery of linezolid in urine/AUC. The apparent volume of distribution (Vz/F) was calculated as CL/λz. Following the administration of the labeled dose on day 4, AUC0-∞ was calculated for total radioactivity in plasma and whole blood. Pharmacokinetic parameters determined for each sex were compared using ttest analyses. All statistical tests were performed using the SAS system (version 6, SAS Institute, Cary, NC). A p value of less than 0.05 was considered statistically significant.
Results
Excretion and Recovery of Radioactivity.
Subject demographics and dosimetry are shown in Table 1. Data on the cumulative excretion of radioactivity along with urine ARE half-lives are summarized in Table 2 and illustrated in Fig. 2. The mean rate and route of radioactivity excretion across single-dose and steady-state regimens were similar. The overall mean total recovery of radioactivity in urine and feces was 93.0 ± 0.9% of dose (n = 7) and 94.5 ± 0.8% of dose (n = 8) for each dose regimen, respectively. These high recoveries show that the reversible hydrolytic loss of the acetamide radiolabel to PNU-105368 (and exhaled14CO2) is at most, less than 5 to 7% of dose (see the metabolism scheme in Fig. 1).
Summary of cumulative recovery and urine ARE half-life of radioactivity in human subjects after oral administration of [14C]linezolid
Plot of cumulative excretion of radioactivity in urine and feces.
Single-dose group.
Excretion in urine was the dominant route of elimination, accounting on average for 83 to 84% of dose. Fecal excretion accounted for 10 to 11% of dose in three of four groups. Excretion in feces was lower in female subjects in the single-dose group (12.2 ± 3.8 versus 6.5 ± 1.4%, for males and females, respectively), and correspondingly higher in urine (mean 81.4 ± 3.8 versus 85.8 ± 2.2%, for males and females, respectively). This small shift to urinary excretion in single-dose females is in accord with relatively less metabolism of linezolid to PNU-142586 in this dose group. This difference was not observed at steady-state.
About 80% of the radioactive dose was recovered in the first 24 h, and radioactivity recovery was essentially complete within 48 h (Fig. 2). Accordingly, harmonic mean terminal ARE half-lives of radioactivity in urine were 4.8 ± 0.8 and 6.2 ± 1.0 h in single-dose males and females, respectively. This difference (p = 0.054) is in accord with 20% lower clearance in female subjects (Sisson et al., 1999). The difference in excretion rate was not present in the steady-state group, where more variability was introduced by one male subject with a longer half-life (202, 8.3 h). Mean ARE half-lives at steady-state were 4.7 ± 1.4 and 5.0 ± 0.6 h.
Quantitative Radiometric HPLC Profiles of Urine, Feces, and Plasma.
The metabolic pathways of linezolid are summarized in Fig. 1. Urine and feces samples from each subject were separately profiled by radiometric HPLC. Metabolite abundance data are in Table3. Retention times,19F NMR chemical shifts, and observed MH+ for major and most minor drug-related peaks are summarized in Table 4.
Summary of relative abundance of radioactive metabolites accounting for more than 1% of dose in urine and feces
Correlation summary of radiometric HPLC and HPLC MS peaks characterized in human excreta
Representative radiochromatograms of urine, feces, and plasma from single-dose subjects are compared in Fig.3. The three major excreted compounds were linezolid and the carboxylic acid metabolites PNU-142586 and PNU-142300. The mean parent drug and metabolite abundance in urine was similar across three of the groups; however, the three females in the single-dose group excreted relatively less PNU-142586 [16.4 ± 7.3% (females) versus 36.0 ± 4.0% (males)] and concomitantly more unchanged linezolid [57.7 ± 11.7% (females) versus 30.9 ± 6.4% (males)]. In accord with the ARE data, male subject 202 had lower PNU-142586 excretion in urine (22.8% of dose versus 48.2, 44.3, and 45.1% of dose) and higher linezolid excretion (49.6% of dose versus 20.2, 22.5, and 26.0% of dose) than the other three subjects in his group. Individuals with lower total clearance had a longer urine ARE half-life and excreted less PNU-142586 and more linezolid, therefore this metabolic pathway appears to be rate limiting in the clearance of linezolid.
Comparison of representative single-dose HPLC-radiochemical profiles for urine (top), feces (middle), and plasma (bottom).
The three major peaks in the feces were the carboxylic acid metabolites PNU-142586, PNU-142300, and PNU-173558. Linezolid accounted for less than 0.2% of the dose in feces for all subjects, in accord with 100% bioavailability.
Most minor metabolites were by-products of PNU-142586 or PNU-142300, which arose in the separate “lactone” and “lactam” pathways (Fig. 1). The most prominent minor metabolites were PNU-173558, PNU-142618, and PNU-143131, accounting for means of 3.3, 1.0, and 1.0% of dose, respectively. Approximately 14 other minor radioactive peaks together accounted for a total of 2.7% of dose. There was a small amount of reversible deacetylation to form nonradioactive PNU-105368. Peaks M16 and M17 were known process impurities. Some of the other very minor peaks in the radiochemical HPLC profile were not associated with detectable diagnostic ions in mass spectra and were not identified. Some may be by-products of lost 14C-acetate from the acetamide moiety, and therefore not drug related. The individual percentage of dose estimates for most of these trace level peaks were well below 0.5% of dose each.
The 0.5- to 6.0-h plasma samples were combined from each subject and profiled by radiometric HPLC (Fig. 3). Pooling was done to improve signal-to-noise ratios and thus see minor peaks, although this probably biased the relative abundance of peaks in favor of parent drug. Nonetheless, linezolid was the major drug-related material in the plasma, followed by small amounts of PNU-142586, PNU-142300, PNU-142618, PNU-173558, and PNU-141960.
Metabolite Quantitation by 19F NMR Spectroscopy.
The abundance of linezolid and metabolites in urine samples was quantified by 19F NMR spectroscopy. These results were compared with those obtained by HPLC with radiochemical detection. In general, the resonances falling between −120 and −124 ppm were tertiary amines and those between −132 and −134 ppm were secondary amines derived from metabolism of the morpholine ring.
As was observed in the radiometric HPLC profiles, linezolid, PNU-142586, PNU 142300, and PNU-173558 were the only metabolites representing more than 1% of the dose. 19F NMR-calculated mass balance for these four compounds was 88 ± 7% (range 82–105%, n = 8) of the results obtained by radiochemical detection. NMR was also used to determine the abundance of coeluting, nonradioactive, or acid-labile metabolites. PNU-143131 and PNU-143011 coeluted by HPLC but were resolved by NMR, showing the ratio of PNU-143131:PNU-143011 to be approximately 3:1. PNU-105368 and PNU-100440 were not radioactive due to the loss of the14C-acetyl group. PNU-105368 accounted for less than 0.1% of the dose, and PNU-100440 accounted for approximately 0.4 to 0.8% of the dose. Noticeably absent in the19F NMR urine samples was PNU-142618, which amounted to 0.8 and 0.5% of dose for subjects 1 and 2, respectively, by HPLC radiochemical detection. This is evidence that PNU-142618 may be a chemical degradation artifact arising from sample processing under acidic conditions and is not necessarily an in vivo metabolite.
Pharmacokinetic Parameters of Radioactivity in Plasma and Blood.
The pharmacokinetic parameters for radioactivity in plasma and blood are shown in Tables 5 and6. Plasma concentrations were higher than blood concentrations. Plasma and blood AUC andCmax values were significantly lower in males relative to females in both single-dose and steady-state groups. These differences are at least in part due to the 1.33- and 1.17-fold higher doses given to female subjects per kilogram of body weight. There were no significant differences between single-dose and steady-state conditions with respect to AUC orCmax. Measured half-lives of radioactivity in whole blood were approximately 5 to 6 h, congruent with ARE half-lives, and the half-lives of linezolid and metabolites. The mean plasma radioactivity half-life was about 2-fold higher than blood.
Single dose pharmacokinetic parameters for linezolid and metabolites PNU-142586 and PNU-142300 in plasma and for radioactivity in blood and plasma
Steady-state dose pharmacokinetic parameters for linezolid and metabolites PNU-142586 and PNU-142300 in plasma and for radioactivity in blood and plasma
Plasma/Blood Cell Partitioning.
Hematocrit-adjusted blood/plasma partitioning (Kp) values were about 0.7 shortly after the dose, decreasing to 0.4 to 0.5 over the 0- to 12-h period after the dose. Since a Kp value of 1 indicates equal concentrations of radioactivity in plasma and blood cell fractions, these data indicate a slight exclusion of radioactivity from the blood cell fraction. There was no evidence of irreversible uptake or retention of radioactivity by blood cells. The amount of radioactivity excluded from blood cells increased as metaboliteTmax was approached, and subjects producing lower metabolite Cmax had qualitatively less change from initial values. Greater exclusion from blood cells of the major anionic metabolites, relative to linezolid, may account for this small temporal change in Kp and the longer plasma radioactivity half-life.
Pharmacokinetic Parameters of Linezolid in Plasma.
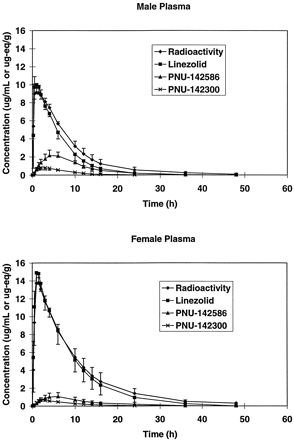
Pharmacokinetic parameters for radioactivity, linezolid, and the two major human metabolites PNU-142586 and PNU-142300 are shown in Tables 5and 6. Mean single-dose plasma concentration versus time data for linezolid and metabolites are compared with total radioactivity concentrations in Fig. 4. LinezolidCmax and AUC accounted for 106 and 78% of the radiochemical Cmax and AUC, respectively. It is apparent that radioactivity circulates mainly as parent drug.
Mean plasma concentration versus time curves for linezolid and its two major metabolites, PNU-142586 and PNU-142300, in male and female single-dose subjects.
Plasma linezolid AUC, Cmax, and CL/F values were significantly higher in females in the single-dose group. These differences are at least in part due to the 1.33 higher dose given to female subjects per kilogram of body weight, since body weight normalization of CL/F brought the difference in clearance to just above the level of significance [1.04 ± 0.21 females versus 1.53 ± 0.42 ml/min/kg (males) (p = 0.12)]. Both the half-lives and daily Cmin values of linezolid and metabolites demonstrated that steady-state conditions were achieved for linezolid and its major metabolites before the second radioactive dose on day 4.
Pharmacokinetics of Metabolites in Plasma.
The most abundant metabolite, the carboxylic acid PNU-142586, circulates at much lower concentrations and at a laterTmax than linezolid. PNU-142586 accounts approximately 26% of the mean steady-state plasma radioactivity AUC. The secondary metabolite PNU-142300 accounts for approximately 7% of the mean steady-state radioactivity AUC. The sum of metabolite and parent AUC relative to radioactivity, and the plasma radiometric HPLC profiles in Fig. 4, show that there are no other quantitatively significant circulating metabolites.
Under single-dose conditions, significantly lowerCmax and AUC for PNU-142586 were evident in females. The abundance of excreted metabolites also indicated more extensive metabolism of linezolid by males in the single-dose group. It is apparent from the inverse relation of linezolid and PNU-142586 concentrations across study volunteers that metabolism by this pathway is rate determining in clearance. Concentrations of PNU-142300 were about one-third of PNU-142586 concentrations. Since PNU-142300 accounts for only about 10% of dose, it is not abundant enough to be rate limiting and therefore cannot control the clearance of linezolid.
Discussion
Excretion Routes and Rates.
In all species examined to date, urine is a major route of excretion of linezolid-related radioactivity. The high mean total radioactivity recovery and short, monophasic semilog ARE plots indicate that radioactivity related to linezolid was not retained systemically for prolonged periods. Some of the unrecovered 5 to 7% of dose may have been exhaled as 14CO2 since a small amount of amidase-mediated hydrolysis was expected, based on rat studies (Chiba et al., 1998).
Rats and mice dosed with PNU-105368 produced linezolid as a major metabolite (J. G. Slatter, unpublished data). Therefore, any deacetylation of linezolid is probably reversible in vivo. The reacetylation of PNU-105368 is presumed to involveN-acetyltransferase and in this study would afford nonradioactive linezolid. This may be why the mean single-doseCmax for linezolid exceeded the meanCmax for radioactivity by about 6%. A similar futile deacetylation/reacetylation has been described for phenacetin (Nicholls et al., 1999). Unlike phenacetin, however, the reversible amide hydrolysis of linezolid was not quantitatively significant (>5–7% of dose), based on the high overall recovery of radioactivity in excreta.
The Lactone and Lactam Metabolism Pathways.
The hemiacetal PNU-143011 is the initial oxidation product in the lactone pathway (Fig. 1). Studies using human in vitro systems have shown that this slow oxidation step is chemical rather than enzymatic. Accordingly, the morpholine ring has weak antioxidant character, and this inefficient oxidation proceeds in the absence of more efficient metabolism options (Wienkers et al., 1999, Wynalda et al., 2000). Based on the nonenzymatic mechanism of oxidation characterized in vitro, the in vivo oxidation may occur throughout the body.
Rapid oxidation of PNU-143011 to the major carboxylic acid metabolite PNU-142586 and minor diol metabolite PNU-143010 by high capacity cytosolic aldehyde and alcohol dehydrogenases is ensured in vivo. There are many examples of these types of morpholine ring metabolites (Tocco et al., 1980; Wilson et al., 1987; Jauch et al., 1990), although nonenzymatic initiation of this metabolic process has not been previously documented. Once formed, PNU-142586 exists in a pH dependent equilibrium with the lactone PNU-142620, where only acidic conditions (pH < 5) will favor the lactone. PNU-142586 is also unstable in acidic solution and irreversible N-dealkylation to afford PNU-142618 is favored over lactone formation. 19F NMR results showed that PNU-142618, at least in part, may be a chemical degradation artifact rather than metabolite of PNU-142586. PNU-173558 could be a metabolite of PNU-142586 or PNU-142618.
Metabolites in the lactam pathway included PNU-142300, PNU-144089, and PNU-143131. These three compounds arise from an intermediate carbinolamine in a mechanistically similar manner to the lactone pathway. The initial carbinolamine oxidation product was not observed in this study, even though the nonbasic nitrogen atom in linezolid could impart some stability to this intermediate.
Cross-Species Similarity in Metabolism.
To facilitate cross-species comparisons, the total percentage of dose that follows the lactone and lactam pathways were summed for each pathway by excretion route, sex, and dose group. Lactone pathway/lactam pathway ratios were 4.5:1, except in the single-dose female group where the ratio was 2.5:1. Metabolites in dog and rat excreta were qualitatively similar to humans, however the relative proportions of the lactone and lactam pathways were different (Chiba et al., 1998; J. G. Slatter, unpublished data). The dog metabolized linezolid by the lactone and lactam pathways about equally, and the rat favored the lactam pathway (lactone/lactam ratio 1:4). Dogs, rats, and humans all excreted a similar amount of intact linezolid. Cross-species comparisons of linezolid exposure have shown that AUC at any given milligram per kilogram dose decreases in the order human > dog > rat (J. G. Slatter, unpublished data). Therefore, at a given milligram per kilogram dose in rats and dogs, higher metabolism by the lactam pathway may account for a lower AUC in these species, relative to humans. Based on metabolite abundance in excreta, the rate-limiting step in clearance in rats, unlike humans, is likely to be PNU-142300 formation.
Rate-Limiting Processes in Total Clearance.
It is evident from the similarity of the linezolid concentration and radioactivity concentration plots that linezolid circulates mainly as parent drug. The most abundant metabolite, the inactive carboxylic acid anion PNU-142586, circulates at much lower levels than linezolid, and has a much later Tmax. The high ratio of linezolid to metabolites in plasma, and the relatively high abundance of anionic metabolites in excreta, is due to renal tubular reabsorption of linezolid, which prolongs systemic exposure to the neutral parent drug. This allows the slow formation of anionic metabolites to proceed. As they are formed, the metabolites are excreted rapidly in urine, presumably by filtration and secretion, with no reabsorption.
Pharmacokinetic Comparison with the Linezolid Database.
Mean pharmacokinetic parameters agreed with data from other clinical studies (Pharmacia Corporation, 2000). In a separate single-dose study, powered to test for sex differences, female human subjects had about a 20% lower body weight-normalized clearance than males, in accord with the results in this study (Sisson et al., 1999). Sample sizes in this excretion and metabolism study were not large enough to adequately define a sex difference in metabolism and clearance.
Variability in Linezolid Total Clearance.
Overall, the degree of clearance variability (in ml/min) in this study was 37 and 50% CV in the single-dose and steady-state groups, respectively. Corresponding percentage CV values for clearance in the package insert were 38 and 36%, for single and steady-state doses. Based on clearance values and variability, the 15 subjects in this study are representative of the general population. The data in this study show the full range and interdependence of linezolid and PNU-142586 excretion, and define renal excretion of intact linezolid and formation of PNU-142586 as the two main sources of intersubject variability in linezolid clearance. Dose adjustment has not been necessary, based on the wide range of linezolid concentrations that have been well tolerated and effective in large clinical studies.
In conclusion, linezolid circulates in plasma mainly as parent drug. Linezolid and two major, inactive metabolites account for the major portion of linezolid disposition, with urinary excretion representing the major elimination route. Formation of PNU-142586 was the rate-limiting step in the clearance of linezolid.
Acknowledgments
We are grateful to study contributors Timothy L. Popp, John Easter, Brian E. Bothwell, Maria Courtney, K. Susan Cathcart, Michael T. Verburg, Hung Ren-Lin, Ann E. Zieve, Nancy K. Hopkins, Dorothy Wenzel, Dave Seybert, Barbara Gulotti, Karle Tackwell, Amy Manchester, Louise DeYoung, Denis A. Avery, M.D. (deceased), and the staff at Pharmacia and Upjohn Clinical Research Unit.
Footnotes
-
This study was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, 1998, pp 17, San Diego, CA.
- Abbreviations used are::
- CV
- coefficient of variation
- LSC
- liquid scintillation counting
- ARE
- amount remaining to be excreted
- HPLC-APCI-MS
- high-performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry
- SPE
- solid-phase extraction
- AUC
- area under the curve
- CL/F
- total apparent oral clearance
- CLr/F
- renal clearance
- Received January 26, 2001.
- Accepted April 27, 2001.
- The American Society for Pharmacology and Experimental Therapeutics