Application of the Relative Induction Score Approach Using Cryopreserved Human Hepatocytes
Abstract
Cytochrome P450 induction-mediated drug-drug interaction (DDI) is one of the major concerns in clinical practice and for the pharmaceutical industry. Previously, a novel approach [the relative induction score (RIS)] was developed using the Fa2N-4 immortalized human hepatocyte line and proposed as a tool for predicting magnitude of clinical DDIs caused by induction of CYP3A. The approach is based on combining in vitro induction parameters (EC50 and Emax) with the efficacious free plasma concentrations to calculate a relative induction score, which is correlated to the magnitude of clinical DDI for midazolam or ethinyl estradiol. To expand the applicability of the RIS model, we have measured induction caused by ten drugs in two different lots of human cryopreserved hepatocytes and correlated the data to clinical DDIs using the RIS. The results demonstrated that, as with Fa2N-4 hepatocytes, sigmoidal relationships can be derived between RIS and magnitude of induction of midazolam and ethinyl estradiol clearance in cryopreserved human hepatocytes. This study demonstrates the general applicability of the relative induction score approach using the human cryopreserved hepatocyte model to predict clinical DDI.
The induction of cytochrome CYP3A4 can have important clinical significance. CYP3A4 gene expression is inducible by numerous xenobiotics, resulting in altered drug metabolism and drug-drug interactions in addition to enhanced metabolism of endogenous substrates including cortisol. CYP3A4 is predominantly expressed in the liver and the small intestine and is known to metabolize the majority of drugs whose biotransformation is known (Guengerich, 1996; Rendic and Di Carlo, 1997). Induction of CYP3A activity in the clinic can result in therapeutic failure such as tissue rejection in transplant patients caused by increased clearance of cyclosporine or unwanted pregnancy caused by increased clearance of oral contraceptive agents, among others (Mannel, 2004; Huwyler et al., 2006). Therefore, different models to study CYP3A4 induction have been developed to allow the selection and development of safer compounds and aid in the design of more efficient clinical trials.
During the past several years, important advances have been made in our understanding of the mechanisms that regulate the expression of genes that determine drug clearance, including drug-metabolizing enzymes and drug transporters. Nuclear receptors, such as the pregnane X receptor (PXR) and constitutive androstane receptor (CAR), have been recognized as key mediators of drug-induced changes in the expression of CYP2B, CYP2C, and CYP3A, as well as of phase II enzymes and transporters. Moreover, it has been documented that hCAR and hPXR are both responsible for coordinating the regulation of a large number of drug metabolizing enzymes and drug transporter genes (Faucette et al., 2006; Konno et al., 2008).
Considerable effort has been expended in an attempt to predict the magnitude of in vivo metabolic drug-drug interactions using in vitro data. A number of in vitro models have been proposed to predict the likelihood of drug-drug interaction due to induction.
Currently, the cell line most often used in CYP3A4 reporter gene assays is the human hepatocarcinoma-derived HepG2 cell line (El-Sankary et al., 2001; Sinz et al., 2006). These models successfully classify and rank order known clinically CYP3A4 significant inducers for their overall ability to induce CYP3A4 transcription. Also, Kanebratt and Andersson (2007) have developed a model to predict the extent of induction using HepaRG cells.
Recently, a novel approach termed the “relative induction score” (RIS) was developed and proposed as a tool for identifying CYP3A4 inducers as well as predicting magnitude of clinical DDIs. The approach (Lin, 2006; Ripp et al., 2006) was based on combining in vitro induction parameters (EC50 and Emax) measured in the immortalized hepatocyte line Fa2N-4 and the efficacious free plasma concentrations to calculate the relative induction scores. These RIS values are correlated to known clinical induction DDIs using midazolam and ethinyl estradiol as the probe substrates to generate a curve. From this curve, predictions of DDIs caused by induction can be made by extrapolation from the RIS values derived from in vitro data.
However, while Fa2N-4 cells generally yield a reasonable response to PXR activators, a limitation is the lack of activation of CAR as well as low or absent expression of some hepatic uptake transporters (Hariparsad et al., 2008). These limitations could have a significant impact on the induction of genes that require coordinated mechanisms of PXR and CAR activation and, therefore, induction potential would likely be underestimated or potentially missed with some of the inducers if CAR is involved.
Primary cultures of human hepatocytes have been proposed as the preferred in vitro system for predicting human CYP450 induction in vitro (http://www.fda.gov/cder/drug/druginteractions). However, to this point, interbatch variability has prevented use of primary hepatocyte cultures for the quantitative prediction of induction-based clinical DDI. This is because in vitro induction parameters (i.e., Emax and EC50 when complete dose-response curves are measured or fold-induction when simpler experimental designs are used) can vary considerably among different batches of hepatocytes from different donors.
Therefore, the purpose of this study was to expand the applicability of the relative induction score model using human cryopreserved hepatocytes. We have investigated induction of ten drugs using two different lots of human cryopreserved hepatocytes and have shown that the RIS approach can be used with individual lots of human hepatocytes.
Materials and Methods
Hepatocyte thawing, plating, and incubation media as well as the antibiotic for the media were purchased from Celsis In Vitro Technologies (Baltimore, MD). Carbamazepine, nifedipine, phenytoin, rosiglitazone, troglitazone, phenobarbital, rifampin, Dulbecco's phosphate-buffered saline (PBS), and DMSO were purchased from Sigma-Aldrich (St. Louis, MO). Aprepitant, pioglitazone, and pleconaril were produced at Pfizer Global Research and Development (Groton, CT). All assay treatments were conducted in BioCoat Collagen I Cellware plates (Becton Dickinson, Bedford, MA). RNeasy Mini Kit and DNase 1 were purchased from QIAGEN (Valencia, California). Two lots of human cryopreserved hepatocytes, lot RCP and lot Hu4026, were obtained from Celsis In Vitro Technologies and CellzDirect (Pittsboro, NC), respectively.
Treatment of Cryopreserved Human Hepatocytes. Two lots of cryopreserved hepatocytes (lot RCP and lot Hu4026) were thawed in hepatocyte thawing medium and were seeded in collagen I-precoated 24-well plates, each well having a cell density of 3.5 to 4.2 × 105 viable cells in 0.5 ml of hepatocyte plating medium. Viability as determined by trypan blue exclusion was 85% or better for this study. The cells were maintained at 37°C in a humidified incubator with 90% atmospheric air and 5% CO2. Cells were incubated for 24 h before being treated with compounds. All drugs were dissolved in DMSO and then added to the culture medium (final DMSO concentration, 0.1%). Incubation medium containing 0.1% DMSO served as the vehicle control. The cells were treated daily for three consecutive days. The medium was removed, and the cells were washed with phosphate-buffered saline.
CYP3A4 mRNA Preparation and Analysis. Total RNA was extracted from cells using the RNeasy Mini Kit according to instructions provided by the manufacturer. Quantification of cytochrome CYP3A4 mRNA was performed using the TaqMan two-step RT-PCR method. First, reverse transcription was performed with approximately 50 ng of isolated RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Subsequently, quantitative PCR analysis was performed on RT reactions using gene-specific primer/probe sets for CYP3A4 target cDNA (Hs00604506_m1) and glyceraldehyde-3-phosphate dehydrogenase endogenous control (Hs99999905_m1) by using the Fast Universal PCR Master Mix (Applied Biosystems). RT-PCR was performed using the ABI 7500 Fast Real-Time PCR instrument. The relative quantity of the target CYP3A4 gene compared with the endogenous control was determined by the ΔΔCT method.
Results and Discussion
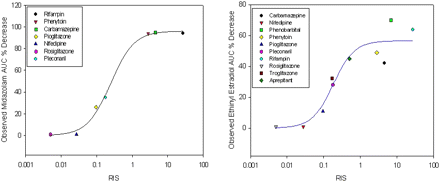
All measurements were performed in multiple wells (triplicates), and the mean value was taken as one data point to calculate the induction parameters, Emax and EC50, for each compound. RIS values were calculated as described previously (Ripp et al., 2006) by the equation [RIS = (Cu × Emax)/(Cu+ EC50)] and are presented in Table 1. For pleconaril and aprepitant, the values for the plasma concentrations (Cu) corrected for protein binding were 58 and 31 nM, respectively (Ma et al., 2006; Majumdar et al., 2007). For the other eight compounds, values for plasma concentrations are as reported previously by Ripp et al. (2006). The clinical observed changes in midazolam AUC or ethinyl estradiol were obtained from the University of Washington Metabolism and Transport drug interaction database (http://www.druginteractioninfo.org). Figure 1 shows the correlation plots between the RIS for one lot of human cryopreserved hepatocytes (Hu4026) with midazolam and ethinyl estradiol.
Summary of the in vitro induction parameters obtained with the human cryopreserved hepatocyte lots RCP and Hu4026, based on mRNA
The in vitro assessment of induction of drug metabolizing enzymes using hepatocyte cultures has been done for many years. It provides a valuable tool to identify compounds that potentially could cause clinically relevant induction and drug-drug interactions. However, using findings from in vitro induction studies to quantitatively predict the magnitude of drug interactions has been challenging. The phenomenon cannot be oversimplified since there are multiple factors contributing to induction including intrinsic binding affinity for induction receptors (e.g., PXR, AhR, etc.), the character of the receptor agonism (full or partial agonist), and the capability of the putative inducer to enter the cell. These have been recently discussed in the context of characterizing induction in a manner similar to other pharmacological responses (Smith et al., 2007). Nevertheless, combining in vitro induction data (potency, degree of agonism) with other parameters (in vivo pharmacokinetics of the inducer, target tissue penetration, free fraction, etc.) and pharmacokinetic behavior of the drug cleared by the induced enzyme(s) and transporter(s) for successful prediction of drug interactions remains elusive.
Relationship between RIS and clinical effects on coadministered midazolam and ethinyl estradiol with several inducers using human cryopreserved hepatocyte lot Hu4026. Data points were fitted to a three-parameter Hill equation. Correlations were r2 = 0.96 and 0.82, respectively.
In a recent publication, the introduction of a metric, the relative induction score, was offered as a method to predict the magnitude of drug interactions from in vitro induction data for PXR-mechanism inducers (Ripp et al., 2006). This value is calculated using in vitro induction parameters (EC50, Emax) and in vivo concentrations of the inducer and is correlated to clinical effects of known inducers on well established CYP3A cleared drugs (midazolam, ethinyl estradiol). Thus, predictions of induction DDI can be made by measuring in vitro induction parameters, calculating the RIS, and extrapolating from a curve of induction magnitude versus RIS that is established with known inducers. However, this approach was only done using the immortalized human hepatocyte line Fa2N-4. The cell line was used because, unlike batches of hepatocytes from individual donors, the Fa2N-4 hepatocytes can provide an internally consistent response from day-to-day for a supply of cells that is virtually inexhaustible. However, some shortcomings of Fa2N-4 hepatocytes have been uncovered and include the alteration of the constitutive androstane receptor mechanism of induction, which can be closely linked with PXR-mechanism induction, and alterations in the expression of some hepatic transporters that can influence intracellular inducer concentrations available to bind to the receptor (Konno et al., 2008). In the present research, an initial attempt was made to determine whether the RIS approach could be used for cryopreserved human hepatocytes and how similar or different the response-versus-RIS relationships are from batch to batch and in comparison with Fa2N-4 cells.
The data clearly demonstrates that the use of cryopreserved hepatocytes provides a greater signal-to-noise ratio for induction with a significantly higher Emax. For example, the Emax values calculated for carbamazepine and rosiglitazone using the Fa2N-4 cells (Ripp et al., 2006) were 2.5 and 2.4, respectively. However, when human cryopreserved hepatocytes were used, the Emax values for these compounds were 34 and 23, respectively. This observation can be a key point in identifying inducers in early development so that prediction of DDI is accurately evaluated and the overall confidence in identifying inducers is higher. EC50 values obtained with the Fa2N-4 cells were in many cases slightly higher than the ones obtained from the cryopreserved hepatocytes. However, it seems that CAR and PXR receptors are both important to give rise to the full potential for CYP3A4 induction, as they both seem to work in concert for some inducers. Future studies are needed to fully understand and define the contributions of each receptor, PXR and CAR separately as well as in combination.
Overall, the data based on mRNA with the human cryopreserved hepatocytes suggest that perhaps any RIS value of 0.1 or higher will likely lead to clinical DDI. Correlations can also be developed between RIS generated using enzyme activity data (testosterone 6β-hydroxylase) and clinical induction DDI magnitude (data not shown). In Table 2, it is shown that there is a high correlation between mRNA and activity increases both within individual hepatocyte lots and between the two lots. Since mRNA is an apparently more sensitive measurement of induction, we selected this endpoint for use in the RIS approach to predicting clinical induction potential. Furthermore, if a new compound were to be both an inactivator of CYP3A4 as well as an activator of PXR, then the use of activity measurements for calculating RIS and predicting induction potential may yield misleading results, especially for compounds cleared by other inducible enzymes besides CYP3A4 (e.g., ethinyl estradiol). However, in the face of mixed mechanisms, the use of a more comprehensive modeling allows investigators to accurately generate a DDI prediction that reflects the net influence of competitive inhibition, time-dependent inactivation, and induction (Fahmi et al., 2008).
Correlation coefficients of the relative induction scores
The results demonstrate that the RIS approach can be applied to human hepatocytes. The RIS scores for the set of drugs examined in this report were highly correlated for the two sets of hepatocytes (r2 values ranged from 0.82 to 0.99), but slopes of the correlations were up to 30% different from unity. Therefore, when attempting to use the RIS approach to using in vitro induction data from cryopreserved human hepatocytes to predict clinical DDI, the curve of induction magnitude for known clinical inducers versus RIS used in extrapolating data for new compounds must be generated for each batch. RIS curves generated for one batch of human hepatocytes should not be used for data generated in another batch of hepatocytes. We have demonstrated this using three batches of human hepatocytes (two cryopreserved and one immortalized) and will continue to define RIS curves for other batches.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.108.021907.
-
ABBREVIATIONS: PXR, pregnane X receptor; CAR, constitutive androstane receptor; RIS, relative induction score; DDI, drug-drug interactions; DMSO, dimethyl sulfoxide; AUC, area under the curve; RT-PCR, reverse transcription-polymerase chain reaction.
- Received April 16, 2008.
- Accepted May 30, 2008.
- The American Society for Pharmacology and Experimental Therapeutics