Abstract
Clinically observed drug interactions with cytochrome P450 (P450) enzymes have increased the need to assess drug interactions of new chemical entities early in the discovery process. To meet this need, fluorogenic substrates have been commercialized. However, only limited evaluations of their utility and comparisons to drug probes have been reported. This study examines the correlation between IC50 values obtained with fluorogenic and conventional drug probes for structurally diverse inhibitors of the five major human P450 isoforms. In general, correlations are weak, with significant numbers of compounds being missed as inhibitors by either probe. For P450s 1A2, 2C9, and 2C19, correlation coefficients were above 0.6 with slopes that ranged from 1.5 to 4.2. However, for P450s 1A2 and 2C9, about 20% of compounds were not included because an IC50 value could not be determined with one of the two probes. CYP 2C19 had the highest correlation (correlation coefficient 0.84), with a slope of 2.0 and less than 5% of compounds excluded. CYP 2D6 showed a good correlation for IC50 values less than 10 μM. However, at higher IC50 values, a high degree of scatter was observed. CYP 3A4 had the weakest correlation, and a large number of compounds were excluded with the fluorogenic probe. Overall, the study shows the care needed when selecting fluorogenic probes and the caution needed when results with fluorogenic probes are used to drive structure-activity relationships with respect to drug interactions.
Multiple drug therapy is encountered in patients with chronic ailments such as congestive heart failure, hypertension, rheumatic diseases, cancer, and human immunodeficiency. Such therapy can result in patient hospitalization due to adverse drug interactions manifested as a loss in therapeutic efficacy or acute toxicity of a drug, caused by a coadministered drug (Doucet et al., 2002). Changes in rates of in vivo metabolism because of induction or inhibition of drug-metabolizing enzymes can cause drug concentrations to fall outside their therapeutic window (Hempel and Klinger, 1976; Fuhr, 2000). A biochemical basis for drug interactions has been recognized for some time (Hussar, 1969; Hempel and Klinger, 1976); however, the specific mechanisms involved have only recently begun to be defined by identifying enzyme(s) critical in a drug's clearance mechanism. Cardiac toxicity caused by coadministering the antihistamine terfenadine with the antifungal ketoconazole or the antibiotic erythromycin is an example whereby inhibition of CYP 3A4 results in elevated terfenadine levels, resulting in prolongation of the QTc interval (Venkatakrishnan et al., 2000). Similarly, the increased bleeding in patients on warfarin therapy has been attributed to inhibition of its metabolism (Chan, 1998). The potential also exists for similar effects with herbal drugs or combinations of herbal and synthetic drugs (Henderson et al., 2002; Makino et al., 2002). Clinically significant adverse drug interactions are now presented at sites on the World Wide Web (Carlson et al., 2002; http://medicine.iupui.edu/flockhart/; http://www.projinf.org/fs/drugin.html).
The high cost associated with developing new chemical entities (NCEs1) as drugs that may fail due to adverse drug interactions has focused attention on predicting, identifying, and circumventing this potential early in the discovery process. Such considerations are now included in drug design concepts. Although other proteins such as P-glycoprotein, organic anion-transporting polypeptides, and UDP-glucuronosyl transferases are gaining increased attention as sites for the basis of adverse drug interactions, the hepatic cytochrome P450 (P450) enzymes are currently recognized as the major cause of such events (Carlson et al., 2002; Tsuji, 2002). Accordingly, promising NCEs are routinely screened for their major metabolic pathways, for their potential to inhibit the major P450 isoforms, and to correlate their in vitro inhibition to in vivo effects (Rodrigues and Lin, 2001; Yan and Caldwell, 2001; Yao and Levy, 2002).
In humans, five P450 enzymes contribute to the metabolism of greater than 90% of marketed drugs. Specific substrates are known for these P450 enzymes and are routinely used as probes for in vitro drug interaction studies (Yuan et al., 2002). For example, the inhibition of CYP 3A4 is commonly measured by the N-demethylation of erythromycin or the 6β-hydroxylation of testosterone. Similarly, probes are known for P450s 2D6, 2C9, 1A2, and 2C19. The assays require time-intensive analytical tools such as LC/UV or LC/MS. This presents a major limitation to their use in early drug discovery, where as many as hundreds of NCEs may be examined weekly to select compounds for further development. Although strategies have been developed to increase throughput by techniques such as sample pooling, multiple assay compression, high-speed LC/MS (Bu et al., 2001; Zhang et al., 2002), single-concentration IC50 projection (Gao et al., 2002), and virtual screening (Rao et al., 2000; Zuegge et al., 2002), the analytical approach still remains a challenge that limits sample throughput.
Absorbance- or fluorescence-based assays that do not require metabolite separation allow for parallel monitoring of large reaction arrays on plate readers, thus enhancing sample throughput. Burke and Mayer (1974) first used the P450-catalyzed O-dealkylation of alkoxyresorufins to examine the induction of hepatic P450 activity by fluorescence. Since then, various O-alkyl derivatives of resorufin, fluorescein, 7-hydroxycoumarins, and 6-hydroxyquinolines have been examined as substrates, and some have been commercialized for use in P450-mediated drug interaction studies (Clarke, 1999; Stresser et al., 2000; Bapiro et al., 2001; Crespi and Stresser, 2001; Miller et al., 2001; Marks et al., 2002). The complex kinetic patterns known for P450 enzymes with several probe substrates and inhibitors (Tang and Stearns, 2001; Hutzler, 2002) suggested that similar complexity is to be expected with fluorogenic substrates. Consequently, the question arises whether fluorogenic probes reliably replace conventional probes for in vitro drug interaction studies in early drug discovery.
To address this question, we examined several commercial fluorogenic substrates for their potential application in high-throughput inhibition screening of P450 enzymes. In this report we compared the IC50 values obtained with drug and fluorogenic substrates for five of the human P450 enzymes (1A2, 3A4, 2C9, 2C19, and 2D6). Additionally, for two P450 enzymes, the relative rank order of inhibition within a structural series is compared.
Materials and Methods
The compounds selected for inhibition studies were either obtained commercially from Sigma-Aldrich (St. Louis, MO) and BIOMOL Research Laboratories (Plymouth Meeting, PA) or were selected from Pfizer's compound bank (Pfizer Inc., Groton, CT). These compounds have been published as inhibitors or substrates for the P450 enzymes or, in the case of proprietary Pfizer compounds, were identified as P450 inhibitors in inhibition screens conducted using conventional probe substrates. Compounds were selected based on their known potential to inhibit a particular P450 enzyme, and care was taken to increase structural diversity by avoiding repetitive homologous structures within a series. Only compounds with reported IC50 values less than 30 μM using conventional or fluorogenic probes were selected for the study. Fluorogenic substrates for P450s 1A2 (Vivid Blue), 2C19 (Vivid Blue), and 3A4 (Vivid Red) were purchased from Aurora Biosciences Corp. (San Diego, CA), and those for P450s 2C9 (MFC) and 2D6 (AMMC) were purchased from BD Gentest (Woburn, MA), respectively. NADP+, isocitrate, and isocitrate dehydrogenase used to generate NADPH were obtained from Sigma-Aldrich. All other chemicals used in the assays were commercially obtained, reagent grade, and used without purification. Insect cell microsomes containing human NADPH cytochrome P450 reductase coexpressed with human P450s 1A2, 2C9, 2C19, 2D6, and 3A4, respectively, were purchased from PanVera Corp. (Madison, WI.).
To avoid batch-to-batch variation in enzyme preparations, all IC50 determinations with a particular P450 enzyme were conducted with a single batch of membranes. Before using the membrane preparations for inhibition studies, enzyme activity was characterized for linearity with respect to time and protein, and kinetic constants (Km and Vmax) were determined for both types of substrates (fluorogenic and conventional). All IC50 values were determined using the probe substrate at a concentration equal to its experimentally determined Km except for CYP 2C9 with diclofenac, where the substrate concentration was two times Km. Typically, for each P450 study, five 96-well plates containing inhibitors were constructed. Each plate contained 12 inhibitors at eight concentrations, for a total of 56 different compounds (including one standard inhibitor that was repeated in each plate as a control). IC50 values were determined in triplicate for each inhibitor. Replicate IC50 values for each inhibitor were reduced to the median IC50 value.
Fluorogenic Assays. Fluorogenic assays were conducted in Costar 8 × 12 (96-well) white polystyrene plates. Fluorescence readings were obtained on a PerkinElmer model HTS7000+ plate reader (PerkinElmer Life Sciences, Boston, MA) using appropriate excitation and emission filters for the different fluorophores (Table 1). Rate of product formation from the fluorogenic probes was determined from the fluorescence data at eight concentrations of the inhibitor, and the IC50 values were determined from fits of the data to the Hill equation with a coefficient of one. The inhibitor concentrations were between 0.03 and 30 μM, except for ketoconazole and quinidine, which were 10-fold lower. Typically, rates were measured from less than 10% consumption of the probe substrate.
Conditions for use of conventional and fluorogenic probes in assays
A typical experimental setup was as follows. Compounds dissolved in methanol were diluted to 1.5 mM in methanol/water (30%). From these solutions an inhibitor plate containing eight concentrations (0, 0.75, 2.5, 7.5, 25, 75, 250, and 750 μM), at 25-fold higher than the desired assay concentration, were made for 11 test compounds and a control inhibitor in a 96-well plate (a column of the grid). Next, to each well of three 96-well white polystyrene assay plates was added 215 μl of a mixture containing the appropriate enzyme, substrate, and buffer, in the amounts shown in Table 1. Using a Hydra 96-channel pipettor (Robbins Scientific, Sunnyvale, CA), 10 μl of inhibitor solution was transferred from the inhibitor plate to each of the assay plates. Prior to initiation of the enzymatic reaction, each assay plate was incubated for 5 to 10 min at 37°C in the plate reader. Reactions were initiated by adding 25 μl of a NADPH-generating system (NADP+, 5.2 mM; isocitrate, 62 mM; MgCl2, 112 mM; and isocitrate dehydrogenase, 0.125 unit). The assay plate was incubated at 37°C for 1 min before taking fluorescence readings every minute over 20 to 30 min of reaction time. Reaction rates were determined from the slope of the time-dependent change in fluorescence signal. Variations to this protocol were necessary for P450s 3A4 and 2D6. For CYP 3A4, the assays were conducted at room temperature (27°C) because of shorter linearity with time at 37°C. For CYP2D6, the NADP+ concentration in the regenerating system was lowered to 0.325 mM because fluorescence from NADPH caused interference in the fluorescence signal resulting from the slow turnover of AMMC.
Conventional Assays. The assay setup used with the conventional probes was similar to that for the fluorogenic assays except that the reactions were carried out in a 96-tube block of 1.3-ml Marsh tubes. Typically, to three 96-tube Marsh plates was added 215 μl of the mixture of enzyme, substrate, and buffer shown in Table 1 for the conventional probe, and 10 μl of inhibitor solutions from the inhibitor plate. The block was incubated in a 37°C water bath for 10 min, followed by addition of 25 μl of the NADPH-generating system, and incubation was continued for 20 to 30 min in the 37°C water bath. Addition of 25 μl of ice-cold methanol terminated the reactions. For P450s 1A2, 2C19, and 3A4, an appropriate internal standard was included in the methanol. The plates were shaken on a vortex mixer for 10 to 20 s and placed on ice. The samples were then filtered through a Multiscreen plate (Millipore Corporation, Bedford, MA) to remove protein. The filtrate was collected in a 96-well plate sealed with a thermally applied polypropylene membrane, and samples were analyzed by HPLC as described below for each conventional probe.
CYP 1A2. For CYP 1A2, 80 μl of sample was injected on a Waters Nova-Pak C-18 column equilibrated in 10 mM ammonium phosphate/methanol (0.9:0.1), held for 4 min, followed by ramping the methanol concentration linearly to 65% in 2 min, and then returned to equilibrium conditions in 2 min. The metabolite (4-acetamidophenol), the internal standard (3-acetamidophenol), and substrate (phenacetin) had retention times of 2.5, 3.6, and 5.0 min, respectively, under these conditions. The run time was 8 min.
CYP2C9. For CYP2C9, 280 μl of sample was injected on a Waters Nova Pak C-18 column equilibrated in 50 mM potassium phosphate (pH 7.4)/acetonitrile/triethanolamine (0.75:0.25:0.02). The flow rate was 1.5 ml/min and absorbance was monitored at 282 nm. Under these conditions 4′-hydroxy diclofenac and diclofenac had retention times of 1.2 and 2.5 min, respectively.
CYP2C19. For CYP2C19, 80 μl of sample was injected on a Supelco (Bellefonte, PA) LC-18 column equilibrated in 20 mM sodium perchlorate (pH 2.5)/methanol/acetonitrile (0.69:0.25:0.06) at a flow rate of 1.5 ml/min. Under these conditions, 4-hydroxymephenytoin, phenobarbital, and S(+)-mephenytoin, which were monitored at 204 nm, had retention times of 1.5, 2.5, and 4.0 min, respectively.
CYP 2D6. For CYP 2D6, 10 μl of sample was injected on a Zorbax SB-C18 column equilibrated in 10 mM potassium phosphate (pH 3.0)/methanol (0.5: 0.5) at a flow rate of 1.5 ml/min. 1-Hydroxybufuralol and bufuralol were detected by fluorescence with excitation at 252 nm and emission at 302 nm. The retention times were 1.2 and 3.5 min, respectively.
CYP3A4. For CYP3A4, 80 μl of sample was injected on a Supelco LC-18 column equilibrated in 10 mM potassium phosphate (pH3.0)/methanol (0.5: 0.5) at a flow rate of 1.5 ml/min. 6β-Hydroxytestosterone, 11β-hydroxytestosterone, and testosterone were detected at 254 nm and had retention times of 1.2, 2.5, and 5.0 min, respectively.
Statistical Methods. Upper and lower limits for the IC50 measurements were set at 30 and 0.03 μM, respectively. For statistical analysis, IC50 values <0.03 μM were designated left-censored and values ≥30 μM were designated right-censored. The designation of “censored” indicates that the IC50 value is at most 0.03 μM for left-censored data and at least 30 μM for right-censored data. The analysis of the linear relationship between the two probes was conducted using SAS Version 8.02 (SAS Institute, Cary, NC). The regression analysis was done in four parts:
-
Determination of the median IC50 value for each compound with each probe using censored and noncensored data.
-
Determination of the variance of the conventional probe data with respect to the fluorescent probe data. These data were used in a weighted regression of the two probes. The variances were used to establish a linear relationship with the fluorescent probe IC50 levels, and the results were used to estimate a smoothed variance for each point.
-
Determination of the linear relationship between the fluorogenic and conventional probes using a weighted linear regression with the inverse of the variances as the weight. The results were used to establish the 95% prediction limits.
-
Determination of the censored points that were outside the 95% prediction interval.
The first step was to determine the appropriate statistic to estimate the center of the three data replicates. A preliminary exploration of the data indicated that the uncensored data were log-normally distributed; thus, the median was an appropriate estimate of the centroid of the three data replicates. If all three data points were censored, then the information from the data was not sufficient to provide an estimate of the center of the data. Therefore, if a compound had all three replicates censored for either the fluorescent or the conventional probe, then the data for that compound for that P450 were not included in the analysis. If one or two of the three data values were not censored, then the median could be estimated using a censored analysis. When all three values were not censored, the median was the middle value of the three. The SAS procedure PROC LIFEREG was used to obtain a maximum-likelihood log-normal distribution for estimating the median for the compound.
The next step was to use the median estimates of the conventional and fluorescent probes to estimate the variance of the conventional probe as a function of the fluorescent probe IC50 values. A plot of the raw data showed that the variance increased with increasing IC50 values. Therefore, linear regression of the data should weigh the higher values less than the tighter, lower IC50 values. The fluorescent probe data were separated into bins that allowed for a sufficient amount of data in each bin (five) to get an accurate estimate of the variance. The bins were set up, and PROC UNIVARIATE was used to output the variance. Each of the P450s had a conventional probe IC50 variance that had a significant linear relationship (p < 0.05 for all five P450s). The variance for each median was estimated from the linear relationship using PROC REG, the regression routine in SAS.
The linear relationship of the conventional IC50 values to the fluorescent probe IC50 values was determined for each P450 using PROC REG and the inverse of the variance estimates as the weights. Additionally, the upper and lower 95% single point prediction limits were determined. The prediction limits differ slightly from confidence bounds and are always larger. Confidence limits show the region of confidence for the line, whereas the prediction limits used here define the 95% confidence bounds for a single point and show the boundaries where an additional point is expected to fall 95% of the time.
In the final step, the prediction limits were interpolated or extrapolated to estimate where the limits intersected the right-censored boundary (IC50 = 30). The upper limits were used for determining the intersection with the conventional probe boundary and the lower limits were used for the intersection with the fluorescent probe boundary. CYP 2C9 was the only P450 that required an extrapolation of the results to estimate the intercept. In this case, the extrapolation did not appear to result in unusual outcomes. It was obvious from observing the plots of the data that some of the censored values would not ever be within the prediction limits if able to be measured at their true values. The intersections of the upper and lower limits with the censored boundaries were used to determine these outliers.
Results and Discussion
An ideal probe substrate used to screen for inhibition of P450 enzymes should have the following general characteristics. 1) The Km should be in the same range as the IC50valuess that are of concern, and its solubility should be significantly higher than its Km. 2) A unique product should be formed that can be accurately determined at 1% conversion of substrate. 3) The product should not be further metabolized by the enzyme system. 4) Mechanism-based inactivation of the enzyme should not occur. 5) The steady-state kinetics should preferably be saturable and monophasic. For plate reader-based chromogenic assays, additional limitations are imposed on potential substrates: a) the absorbance or fluorescence properties of the product must be distinct from those of the substrate; b) the extinction coefficient or fluorescence quantum yield of the product in an aqueous environment must be high (sensitivity); and c) for fluorogenic substrates the excitation wavelength should be in the visible range, preferably >420 nm, to avoid photo damage of the enzymes involved in catalysis. Taken together, these conditions place severe limitations on the selection of substrates suitable for plate reader-based P450 assays.
Several fluorogenic probes were first characterized for their kinetic properties to establish their usefulness based on the criteria indicated above. Figure 1, panel A shows the kinetic results for MFC with CYP 2C9. Monophasic kinetics and the kinetic parameters (Km and Vmax) make this a good probe for fluorogenic assays. Figure 1, panel B shows the kinetic results for Vivid Blue with CYP 2C19. This probe shows multiple binding events, two catalytically active (Km of 4 and 15 μM) and one inhibitory. Such a probe is limited to use at the lower Km to avoid multiple substrate binding events in the interaction of inhibitors with the enzyme. Furthermore, a low Vmax makes signal output weak at the lower Km. These limitations make the probe less than ideal for use in fluorogenic assays. Figure 1, panel C shows the v versus [S] plot and an insert of the Eadie-Hofstee plot for the CYP 3A4 fluorogenic substrate Vivid Red. At substrate concentrations above 1.5 μM, partial inhibition is observed. The Eadie-Hofstee plot at low substrate concentrations shows monophasic behavior and a Km of 0.5 μM with a high Vmax. Thus, this probe can be effectively used for IC50 determinations at 0.5 μM. Figure 1, panel D shows a probe that is unacceptable due to low signal output and nonlinearity with time. Thus, probes must be carefully evaluated for their intrinsic kinetic properties with the respective P450 enzymes prior to application in high-throughput assays.
Eadie-Hofstee plots for the probes selected for use with rP450s 2C9, 2C19, and 3A4 (A—C) (details of their use and limitations are presented in Table 1 and in the text) and an example of a probe for CYP2D6 with poor signal output that is nonlinear with time (D).
The fluorogenic and conventional probes used for our studies, and the conditions of their use with the appropriate P450 enzyme, are listed in Table 1. Table 2 shows the mean and standard deviation from the mean for IC50 values of well established inhibitors that were used as controls in each 96-well plate to monitor the intra- and interday variability of the IC50 values obtained by the two methods. In general, the standard deviations observed for either the fluorogenic or conventional probes were comparable for the inter- and intraday assays, demonstrating that the methods are highly reproducible.
Variability in IC50 values observed in intra- and interday experiments for inhibitors used as controls in each 96-well plate
Values are IC50 ± S.D.
Figure 2 shows typical data sets for different P450 enzymes in which the IC50 values were determined for compounds with the conventional and fluorogenic probes. The tighter confidence boundaries for the IC50 values determined with the fluorogenic probes show that they have a higher degree of accuracy than the conventional probes. We attribute this gain in accuracy of the fluorogenic probes to the real-time kinetic measurements provided by the fluorogenic assays in which activity is monitored concurrently with the enzyme reaction as opposed to a sequential process with the conventional probes, in which analysis by LC/UV or LC/MS is done subsequent to terminating the enzymatic reaction. In experiments not presented, we established that obtaining real-time fluorescence data gave higher accuracy of rate measurements than the endpoint quench approach (Stresser et al., 2002). Signal-to-noise ratios as low as two were well tolerated in real-time kinetic data, whereas with endpoint quench methods, a signal-to-noise ratio greater than 5 was found necessary to distinguish signal from noise. The somewhat enhanced fluorescence obtained by quenching reactions with buffers at a pH of 9.0 is not offset by the variation in signal amplitude by this approach.
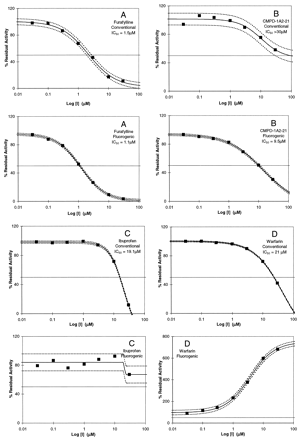
Diversity of results obtained in comparisons of the fluorogenic and conventional probes, where inhibitors show IC50values that are comparable (A) or distinct (B), and where the same compounds show either inhibition, no effect, or activation, depending on the selection of probes (C, D, and E).
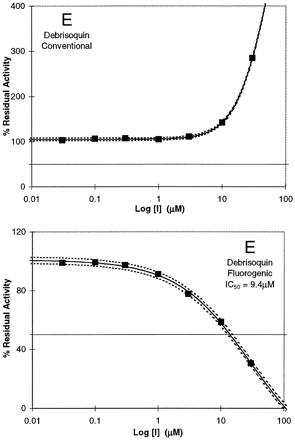
The examples shown in Fig. 2 for different P450 enzymes demonstrate the spectrum of results obtained between fluorogenic and conventional probes for the determination of IC50 values. Figure 2A shows results in which the IC50 values are comparable for the inhibition of CYP 1A2 by furafylline when probed with either Vivid Blue or phenacetin (1.1 and 1.5 μM, respectively), whereas for CMPD-1A2–21, the IC50 values are distinct with the fluorogenic and conventional probes (Fig. 2B; 9.5 and >30 μM, respectively). However, both probes do indicate the compound's ability to inhibit CYP 1A2. Figure 2, C and D, shows that ibuprofen and warfarin are inhibitors of CYP 2C9 when probed with diclofenac (IC50 = 19.1 and 21 μM, respectively), whereas when probed with the fluorogenic substrate MFC, ibuprofen showed no inhibition (Fig. 2C), and warfarin was an activator (Fig. 2D). Similarly, debrisoquin, a known CYP 2D6 substrate, is an inhibitor of CYP2D6 when probed with the fluorogenic substrate AMMC (IC50 = 9.4 ±3.9 μM), whereas with bufuralol as substrate, activation is observed (Fig. 2E).
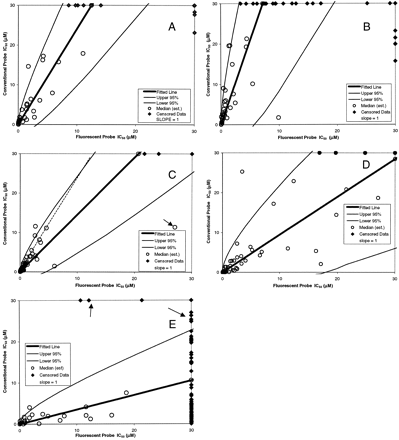
The mean IC50 values for inhibitors examined with the fluorogenic and conventional probes are listed in Table 3. The estimated medians, fitted lines, and prediction intervals are plotted for each P450 in the correlation diagrams shown in Fig. 3, panels A through E. The actual raw data for the uncensored values were not plotted since each of the three replicates did not correspond to a specific replicate from the other probe. The fitted linear equation for each figure is given in the corresponding figure legend. Although the computed intercept for 2C9 was significantly different from zero [0.9 ± 0.4 (p = 0.03)], the intercept was small and the R2 value was 0.49 in the intercept model compared to 0.59 for the no intercept model. Therefore, no intercept was used for any of the P450s. As shown in Fig. 3, A through E, there are censored values on the margins of the plots (top x-axis and right y-axis) that fall outside of the prediction intervals (hereafter referred to as outliers). For those outliers that are censored for the fluorescent probe and not for the conventional probe, it is not possible that these data would ever be within the prediction limits, even if the IC50 could be measured at its true, uncensored value. This also holds true for outliers that are censored for the conventional probe and not for the fluorescent probe. For example, in Fig. 3E, the censored fluorescent values on the right y-axis, shown by the arrow, would have true values that would have the same conventional values but a greater fluorescent value. The true value, therefore, could lie on the fitted line. On the other hand, the points indicated by the arrow on the top x-axis would have true values that would be even further outside the prediction limits. For all five P450s the censored points that could never be within the prediction limits were counted, and the results are shown in Table 4. The cell frequencies are not high enough to perform individual chi-square tests on each P450; however, the overall analysis of the totals shows no significant difference (p = 0.7). The difference between the outlier frequencies for P450s 1A2/2C9/2C19/2D6 and 3A4 is due to the very flat response seen for 3A4 in Fig. 3E compared with the other P450s.
Mean and standard deviations of the mean for three determinations of IC50 values for CYPIA2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4
Correlation diagrams for the IC50values obtained with the conventional and fluorogenic probes for P450s 1A2, 2C9, 2D6, 2C19, and 3A4, respectively (A—E).
Data points shown on the top x-axes and the right y-axes are censored and are not included in the linear equations. The linear relationships for uncensored data are presented in the text.
Count of IC 50 values that lie outside of the linear bounds
As shown in Fig. 3A for CYP1A2, for those compounds that fall within the boundary limits, there appears to be a linear relationship (R2 = 0.7), although the variance increases with increasing IC50 values. The high slope of the line (∼2.4) indicates that the fluorogenic probe provides a lower IC50 value than the conventional probe, and it is more likely that the conventional probe readings will exceed the boundary limits and be censored. However, although the conventional censored data are within the prediction limits, the fluorescent-censored data are not, and do not obey the observed linear relationship from noncensored data. Ten of the 60 compounds tested were censored either for the fluorogenic or conventional probes. This corresponds to 17% of the total tested.
The relationship observed for CYP 2C9 is similar to that for CYP 1A2 (Fig. 3B). However, the slope is higher (∼4.2) and the likelihood of getting a censored value is correspondingly greater. This is evident in the number of conventional censored data shown on the top y-axis. Of the 55 compounds examined, 23 resulted in all censored data and were not included in the regression analysis. The majority of these compounds (18) were excluded because the IC50 values with diclofenac were greater than 30 μM. Linear regression analysis of the uncensored data shows an R2 value of 0.59. Although the IC50 values obtained with MFC are generally lower than those obtained with diclofenac, a statistical extrapolation of the values between the two systems is not advisable. Particularly relevant to an early drug discovery setting is the observation that a larger number of compounds would be considered as noninhibitory to P450s 1A2 and 2C9 if only phenacetin and diclofenac were used as the probe substrates for these isoforms.
With CYP 2C19, the data are concentrated, for the most part, in the lower IC50 range (Fig. 3C). If the data point indicated by the arrow in Fig. 3C is removed from the analysis, then the equation becomes [IC50 Conventional = 2.0522 · IC50 Fluorescent] R2 = 0.84, and is shown as the dotted line. Although this point is a valid, noncensored point, the second equation should be used if the fluorescent IC50 values are below 15 μM. The data for CYP 2C19 show the highest correlation between the conventional and fluorogenic probes, with the fluorogenic probe showing approximately a 2-fold lower IC50 value than the conventional probe at the lower IC50 range. Remarkably, only three compounds had IC50 conventional probe data that were all censored.
For CYP 2D6 a linear trend for the IC50 data correlation appears to be followed below an IC50 value of 6 μM (Fig. 3D). However, there is a significant amount of scatter beyond this value, and the prediction interval grows rapidly. One interesting result is that there are no data points where only the fluorescent probe has been censored. Given that the calculated slope is very nearly 1, it may be that the conventional probe does not obey a linear relationship at high values and increases faster than the fluorescent probe. There are not enough data, however, to evaluate the possibility of a nonlinear relationship between the two systems.
As shown in Fig. 3E, the low R2 value for CYP 3A4 is primarily due to the small change in IC50 values for the conventional probe and the resulting low slope. A significantly larger number of IC50 values are censored with the fluorescent probe than with the conventional probe (right y-axis). Despite the low IC50 values obtained with the conventional probe, there are three conventional-only censored points that are designated as outliers (top x-axis). Given the known complexity of CYP 3A4 with respect to classes of substrates, this should not be considered a surprising result (Wang et al., 2000; Lu et al., 2001). The correlation diagram suggests that Vivid Red does not reflect the interaction of compounds with the 3A4 active site as does testosterone, and that this fluorogenic probe for CYP 3A4 would provide poor guidance for compound selection based on a drug interaction issue.
In a discovery setting, if inhibition of a P450 enzyme cannot be eliminated in a structural series, compounds will often be rank ordered for their potential to inhibit the P450 enzyme with the intent of selecting the structure having the least potential for inhibition. To determine whether fluorogenic substrates rank order compounds in a manner similar to that of the corresponding conventional probes, we compared IC50 values for two series of compounds obtained with the fluorogenic probes (using recombinant enzymes) to Pfizer's database of IC50 values obtained with the conventional probes and human liver microsomes. The results for P450s 2D6 and 3A4 are shown in Table 5. Since the data were not obtained in directly comparable experiments, the IC50 values were classified based on the degree of inhibition, as potent (A, <3 μM); moderate (B, >3–<10 μM), and weak (C, >10 μM). As seen in Table 5 for 2D6, the rank ordering of compounds was not similar between the two probes. Surprisingly, for CYP3A4, the rank ordering appears to be similar. The results for CYP 3A4 should be taken with caution, inasmuch as all compounds were potent inhibitors that had an imidazole ligand and, as determined for ketoconazole, a good correspondence exists between the IC50 values obtained with the two systems.
Comparison of rank order between fluorogenic and conventional probes for P450s 2D6 and 3A4
A common concern about the use of fluorogenic substrates for in vitro drug-drug interactions is that they do not bear structural resemblance, or they have physicochemical properties that are comparable to those of drug probes. Since fluorogenic substrates must undergo oxidative metabolism to manifest a fluorescence signal, and since the crystal structures of several P450 isoforms indicate a single heme center where oxidative metabolism of substrates occurs, it can only be concluded that fluorogenic and drug probes must bind to the same active site. The parameters of concern with respect to these probes is their affinity constant, as reflected by their respective Km values, and their ability to occupy similar three-dimensional space within the active site. Although the affinity is dependent on structural and physicochemical properties of the molecules that provide hydrophobic, hydrogen bonding, and/or ionic interactions within the active site under consideration, it is not dependent on those characteristics that impart drug characteristics to a molecule. Hence, concerns that fluorogenic probes do not resemble drug molecules are not justified. A greater concern must be the ability of the fluorogenic probe to more adequately mimic the binding of the conventional probe drug within the active site. This consideration also applies to structurally diverse drug substrates that are used to probe a single P450 isoform for drug interactions. It is the architecture of the active sites of drug metabolizing P450 isoforms that permits a broad diversity of structures to bind within them. These characteristics of the active sites are also most likely responsible for the complex kinetic behavior of substrates and inhibitors with multiple molecules capable of simultaneously binding to the active site, resulting in either activation or inhibition. Thus, it may be predicted that no two structurally diverse probes for a particular P450 isoform will yield the same IC50 values for a given set of inhibitors, unless the inhibition is due to coordination of the inhibitor to the heme iron and effectively interferes with the activation of molecular oxygen. Indeed, with CYP3A4, such observations have been reported (Wang et al., 2000; Kenworthy et al., 2001).
The results presented in this report strongly suggest that translation of IC50 values obtained using the fluorogenic substrates to corresponding values for conventional probes currently used to establish inhibition by NCEs is not advisable. This is in contrast to the results observed by Bapiro et al. (2001) in the study of in vitro drug interactions with a series of antiparasitic drugs. A question that naturally arises is what value such potentially conflicting data can give chemists for follow-up lead optimization. This issue is not confined to differences between fluorogenic and conventional probes. As stated earlier, studies with CYP 3A4 using conventional drug probes have shown that the IC50 values are dependent on the probe substrates (Wang et al., 2000; Kenworthy et al., 2001). This suggests that in any drug development program, when selection of NCEs has been narrowed down, multiple probes are likely to be necessary to obtain a comprehensive view of the NCE's potential for drug interactions. Thus, fluorogenic probes, which provide significant enhancements in throughput over conventional probes, are ideally suited at very early stages of a drug discovery program in which selection of NCEs is not uniquely confined to a drug-drug interaction issue. However, when considering a compound or a structural series as lead candidate for further drug development, it is advisable to examine multiple probes to gain a better appreciation for the in vitro results to predict potential in vivo effects.
In summary, fluorogenic assays with P450 enzymes provide a useful means for rapidly determining the potential of NCEs to interact with the P450 family of enzymes. These assays are robust and highly reproducible. However, since the results do not generally support a direct correlation between fluorogenic and conventional probes, follow-up studies with conventional probes are recommended when either lead series or a few compounds are being considered for further development as potential drugs.
Acknowledgments
We thank Theodore Liston, Scott Obach, and Karthik Venkatakrishnan for critical review of the manuscript; and Theodore Liston for support and encouragement during the study.
Footnotes
-
↵1 Abbreviations used are: NCE, new chemical entity; P450, cytochrome P450; LC/MS, liquid chromatography/mass spectrometry; MFC, 7-methoxy-4-(trifluoromethyl)-coumarin; AMMC, 3-[2-(N,N-diethyl-N-methylammonium)ethyl]-7-methoxy-4-methylcoumarin; CPMD, compound.
-
This study was supported by funds from Pfizer Inc.
- Received February 14, 2003.
- Accepted April 25, 2003.
- The American Society for Pharmacology and Experimental Therapeutics