Abstract
We applied physiologically based pharmacokinetic (PBPK) modeling to study the dose-dependent metabolism and excretion of verapamil and its preformed metabolite, norverapamil, to unravel the kinetics of norverapamil formation via N-demethylation. Various initial verapamil (1, 50, and 100 μM) and preformed norverapamil (1.5 and 5 μM) concentrations, perfused at 12 ml/min, were investigated in the perfused rat liver preparation. Perfusate and bile were collected over 90 minutes, and livers were harvested at the end of perfusion for high-performance liquid chromatography analysis. After correction for the adsorption of 10%–25% dose verapamil and norverapamil onto Tygon tubing and binding to albumin and red blood cell, fitting of verapamil and formed and preformed norverapamil data with ADAPT5 revealed nonlinearity for protein binding, N-demethylation (
 nmol/min;
nmol/min;
 μM), formation of other metabolites (
μM), formation of other metabolites (
 nmol/min;
nmol/min;
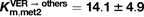 μM), as well as biliary excretion (
μM), as well as biliary excretion (
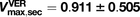 nmol/min;
nmol/min;
 μM). The hepatic clearance of verapamil (
μM). The hepatic clearance of verapamil (
 ) decreased with the dose (8.16–10.2 ml/min), with values remaining high relative to perfusate blood flow rate among the doses. The hepatic clearance of preformed norverapamil (11 ml/min) remained unchanged for the concentrations studied and approximated perfusate blood flow rate, suggesting a high norverapamil extraction ratio. The fractional formation of norverapamil and biliary excretion of verapamil based on fitted constants were 31.1% and 0.64% of
) decreased with the dose (8.16–10.2 ml/min), with values remaining high relative to perfusate blood flow rate among the doses. The hepatic clearance of preformed norverapamil (11 ml/min) remained unchanged for the concentrations studied and approximated perfusate blood flow rate, suggesting a high norverapamil extraction ratio. The fractional formation of norverapamil and biliary excretion of verapamil based on fitted constants were 31.1% and 0.64% of
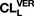 , respectively. Enantiomeric disposition and auto-inhibition of verapamil failed to perturb these estimaties according to PBPK modeling, due to the low values of the Michaelis-Menten constant, K
m, and inhibition parameter, k
I.
, respectively. Enantiomeric disposition and auto-inhibition of verapamil failed to perturb these estimaties according to PBPK modeling, due to the low values of the Michaelis-Menten constant, K
m, and inhibition parameter, k
I.
Footnotes
- Received November 20, 2014.
- Accepted February 3, 2015.
-
↵ 1 Current affiliation: School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, P.R. China.
-
↵ 2 Current affiliation: Pharmacy School, Shihezi University, Shihezi City, Xinjiang, P.R. China.
-
Q.J.Y. and L.S. contributed equally to this work.
-
L.S. and H.T. were supported by the China Scholarship Council (CSC), and K.S.P. is supported by Canadian Institute of Heath Research (CIHR).
-
The work was presented in part, at the 2014 AAPS Annual Meeting; San Diego, CA.
-
↵
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
- Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics
DMD articles become freely available 12 months after publication, and remain freely available for 5 years.Non-open access articles that fall outside this five year window are available only to institutional subscribers and current ASPET members, or through the article purchase feature at the bottom of the page.
|







