Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins
Abstract
Grapefruit juice increases the oral availability of a variety of CYP3A4 substrates. It has been shown that recurrent grapefruit juice ingestion results in a loss of CYP3A4 from the small bowel epithelium. We now show that the reduction in intestinal CYP3A4 concentration is rapid; a 47% decrease occurred in a healthy volunteer within 4 hr after consuming grapefruit juice. To identify the specific components of the juice responsible for this effect, we used a recently developed Caco-2 cell culture model of human intestinal epithelium that expresses catalytically active CYP3A4. We found that grapefruit oil and two furanocoumarin constituents (6′,7′-dihydroxybergamottin and a closely related dimer) caused a dose-dependent fall in CYP3A4 catalytic activity and immunoreactive CYP3A4 concentration. The effect was selective in that concentrations of CYP1A1 and CYP2D6 did not fall, consistent with previous results obtained in vivo. Assays of various juices confirmed that 6′,7′-dihydroxybergamottin is the major furanocoumarin present and, although its concentration varies significantly among types and brands of grapefruit juice, it is consistently present in concentrations exceeding the IC50 (1 μM) for loss of midazolam 1′-hydroxylase activity determined in the Caco-2 cells. Studies with recombinant CYP3A4 revealed that 6′,7′-dihydroxybergamottin is a mechanism-based inactivator, which supports the idea that loss of CYP3A4 results from accelerated degradation of the enzyme. We conclude that the effect of grapefruit juice on oral availability of CYP3A4 substrates can be largely accounted for by the presence of 6′,7′-dihydroxybergamottin although other furanocoumarins probably also contribute.
Grapefruit juice has been reported to increase the oral availability of a variety of CYP3A4 substrates including cyclosporin A (1), felodipine (2), midazolam (3), terfenadine (4), verapamil (5), saquinavir (6), and ethinyl estradiol (7). Grapefruit juice has little effect on intravenously administered drugs (1,3), however, and the primary effect on orally administered drugs is to increase their peak serum concentration (Cmax) with little change in the subsequent rate of elimination as measured by half-life (2, 8). Therefore, it appears that the grapefruit juice effect is at the level of the intestine and not the liver. The grapefruit juice effect has been postulated to be the result of competitive inhibition of CYP3A4 in the enterocytes lining the small intestine (1, 2) because grapefruit juice has been shown to contain substances capable of competitively inhibiting this enzyme (9-11). However, in a recent study performed in healthy volunteers, consumption of grapefruit juice for 6 days resulted in a mean 62% decrease in enterocyte CYP3A4 immunoreactive protein concentration with no change in CYP3A4 mRNA concentration (12). The magnitude of the decrease in immunoreactive CYP3A4 correlated with the magnitude of increase in felodipine AUC when the drug was taken with grapefruit juice instead of water (12). It was suggested that the effect may be a result of a mechanism-based (“suicide”) inactivation of the enzyme, followed by its rapid intracellular degradation (12).
Flavonoids, which are major constituents of grapefruit juice, are not known to be mechanism-based inactivators. Naringin, the major flavonoid present in grapefruit juice, and quercetin do not reproduce the grapefruit juice effect when administered orally, also suggesting that flavonoids are not the active compounds (8, 13). Edwards et al. recently reported that 6′,7′-dihydroxybergamottin (DHB)1, a furanocoumarin present in grapefruit juice, is a potent inhibitor of CYP3A enzymes in rat liver microsomes (14). Furanocoumarins are known mechanism-based inactivators of cytochromes P450 (15, 16) and might be responsible for the loss of intestinal CYP3A4 protein following grapefruit juice ingestion.
In this report, we show that the fall in CYP3A4 immunoreactive protein in human enterocytes in vivo after grapefruit juice ingestion occurs rapidly. A human intestinal epithelial cell line also exhibited a rapid fall in CYP3A4 concentration when exposed to grapefruit oil. We therefore used this culture model to test the hypothesis that furanocoumarins are the major active ingredients in grapefruit juice.
Materials and Methods
Grapefruit, grapefruit juice, and frozen concentrated grapefruit juice were obtained from local markets. DHB was synthesized from bergamottin (Indofine Chemical Company, Somerville, NJ) as described (14). One sample of grapefruit oil was obtained from Berjé(Bloomfield, NJ). A second sample of grapefruit oil (Spectrum Chemical Manufacturing Corp., Garden, CA), R(+)- and S(-)-limonene, decanal, and ketoconazole were gifts from Dr. Vincent Wacher (AvMax, Berkeley, CA). Midazolam was received as a gift from Dr. Bruce Mico (Roche Laboratories, Nutley, NJ). Isopropyl β-D-thiogalactoside was purchased from Calbiochem Corp. (La Jolla, CA). NADPH, L-α-dilauroyl- and L-α-dioleyl-sn-glycero-3-phosphocholines, phosphatidyl serine, testosterone, 6β- and 11β-hydroxytestosterone, catalase, glutathione, and δ-aminolevulinic acid hydrochloride and other chemicals were purchased from Sigma Chemical Company (St. Louis, MO). NADPH-cytochrome P450 reductase and cytochromeb5 were purified from liver microsomes of phenobarbital treated Long-Evans rats as previously described (17, 18). Microsomes were prepared as described (19) from a human liver (HL4; medically unsuitable for organ transplantation) that was obtained under the auspices of the Washington Regional Transplant Consortium (Washington, DC).
Grapefruit Juice Fractionation.
Grapefruit juice frozen concentrate (354 ml) was reconstituted with water to a final volume of 1 liter. The grapefruit juice was then extracted with ten 100-ml portions of hexane, the organic layer was dried with sodium sulfate, and the hexane was removed in vacuo. The resulting residue was dissolved in ethanol, and the ethanol solution was separated into fractions by a Hewlett-Packard (Wilmington, DE) 1090 HPLC system. Grapefruit juice components were separated on a 4.6 x 150 mm M-80 C18 column (YMC, Wilmington, NC) that was eluted at 1 ml/min with a water and acetonitrile gradient as follows: (time:% acetonitrile; 0:10; 5:10; 30:80; 40:80; 45:10; 50:10). The eluate was monitored at 260 nm and UV absorption data were collected from 200–600 nm. All HPLC fractions were dried with a Savant SpeedVac system (Farmington, NY) and stored at −80°C until used in microsomal incubations.
Instrumental Analysis of Grapefruit Juice Fractions.
Isolated grapefruit juice fractions were analyzed further by LC/MS with a Hewlett-Packard 1050 HPLC system coupled to a Finnigan TSQ-7000 mass spectrometer operating in the positive ion electrospray ionization (ESI) mode with the following operating conditions: ESI spray voltage, 4.5 kV; capillary temperature, 275°C; auxiliary and sheath gas pressures, 5 and 65 psi, respectively. Conditions for LC/MS/MS analyses operating in the daughter ion scan mode were as noted and with a collision induced dissociation (CID) voltage of −60 eV and an argon CID pressure of 1 mTorr. HPLC conditions for LC/MS analyses were as noted previously except a 2.0 × 150 mm YMC M-80 C18 column was substituted for the standard column and the flow rate was reduced to 0.2 ml/min.
Microsomal Incubations with Grapefruit Juice Fractions.
Each of the HPLC fraction residues was dissolved in 40 μl of ethanol, and 10 μl of these solutions were added to 990 μl of human hepatic microsomal suspensions (0.5 mg/ml) that contained an NADPH regenerating system. Control incubations contained the same concentration of ethanol but without grapefruit juice fractions. The microsomes were incubated with 10 μM taxol for 30 min. Taxol metabolites 6α-hydroxytaxol, a marker activity for CYP2C8, and taxol metabolite B, a marker activity for CYP3A4, were quantitated as described (19,20). Fraction 39 was subsequently studied more extensively. Using its mass obtained from HPLC fractionation and its molecular weight based on LC/MS/MS, microsomal incubations were prepared that contained either 0, 0.1, 0.5, 1, 5, or 10 μM fraction 39 and either 10, 20, or 50 μM taxol. After 30 min incubations, 6α-hydroxytaxol and taxol metabolite B were quantitated.
Quantitation of Bergamottin, DHB, 6′-Epoxybergamottin, and FC726 in Grapefruit, Grapefruit Juice, and Grapefruit Oil (data in table 1).
White grapefruit juice frozen concentrate (10 ml; Kroger) was diluted with water to a final volume of 100 ml, 1 ml of 500 μM bergapten internal standard was added, and the mixture was extracted with six 100-ml portions of chloroform. The chloroform was removed in vacuo, and the resulting residue was dissolved in methanol and analyzed with a Waters 600E HPLC system.
Concentration of furanocoumarins in grapefruit products
One white Florida grapefruit was halved, the fruit (juice vesicles only, not the membranes surrounding the sections) was removed and homogenized in a blender, and 1 ml of 500 μM bergapten internal standard was added. The homogenate was extracted with six 100-ml portions of chloroform. The chloroform was removed in vacuo, and the resulting residue was dissolved in methanol and analyzed with a Waters 600E HPLC system.
Samples of cold-pressed grapefruit oil obtained from two sources were each diluted 1/100 with methanol, and the resulting solutions were analyzed by HPLC.
HPLC conditions for the quantitation of bergamottin, DHB, 6′-epoxybergamottin, and FC726 in the grapefruit juice, grapefruit homogenate, and grapefruit oil were as noted above. Concentrations of bergamottin and DHB were calculated from a standard curve for bergamottin based on the assumption that bergamottin and DHB have the same extinction coefficient. Concentrations of FC726 were determined from a standard curve for bergamottin based on the assumption that the extinction coefficient of FC726 is twice that of bergamottin. The above assumptions were made because the baseline-corrected UV absorption spectra of bergamottin, DHB, and FC726 were identical (not shown) and because mass spectral analysis demonstrated that FC726 is a dimer (see Results).
Quantitation of DHB in Grapefruit Juice Preparations (data in table 1).
With fresh grapefruit, samples were prepared by hand-squeezing 3–5 fruits. Juice was homogenized with a teflon-type tissue grinder (Wheaton Instruments, Millville, NJ) prior to analysis. The samples were extracted with methylene chloride and the content of DHB was assayed by HPLC as previously described (14).
In vivo study.
Endoscopic duodenal biopsies were obtained from a healthy adult female volunteer before and 4 hr after drinking one 8-ounce glass of reconstituted Kroger brand frozen grapefruit juice concentrate. This study was approved by the University of Michigan Institutional Review Board. The biopsies were subjected to immunoblot analysis as previously described (12).
Cell Culture.
The Caco-2 cell clone P27.7 was cultured using previously described conditions under which the cells express metabolically active CYP3A4 (21).
Incubations (4 hr) with apically administered midazolam (4 μM) were performed. Grapefruit oil and constituent compounds or vehicle (absolute ethanol) were administered concurrently with midazolam to the apical medium. After the incubations, the medium from the apical and basolateral compartments of each culture were combined and assayed for the concentration of 1′-hydroxymidazolam, and the rates of 1′-hydroxymidazolam formation were calculated. One culture for each treatment was used for catalytic activity and immunoblots. A parallel set of cultures at the highest concentrations of grapefruit oil, DHB, FC726, and ketoconazole was used for paraffin embedding and preparation of H&E stained sections.
Immunoblots.
Immunoblot analysis of intestinal biopsies and Caco-2 cells was performed as previously described (12, 21). Intestinal biopsy content of CYP3A4 was expressed as a ratio to the constitutively expressed enterocyte protein villin (12, 22). Immunoblots were repeated twice, and representative blots are shown. Protein loading for intestinal biopsy homogenates was: 15 μg. Protein loading for Caco-2 cells was: CYP3A and villin, 5 μg; CYP1A1 and CYP2D6, 25 μg.
1′-Hydroxymidazolam Assay.
Quantitation of 1′-hydroxymidazolam in Caco-2 culture medium was accomplished by gas chromatography/negative ion chemical ionization—selective ion mass spectrometry as previously described (21).
Expression of CYP3A4 in E. Coli and Purification.
A full length CYP3A4 cDNA inserted into the pCW vector was obtained from Dr. Ronald W. Estabrook (University of Texas Southwestern Medical Center, Dallas, TX). The CYP3A4-containing vector was transfected into MV1304 cells. The transformed E. coli were grown in modified Terrific Broth, and the expression of CYP3A4 was induced by addition of 1 mM isopropyl β-D-thiogalactoside. δ-Aminolevulinic acid (0.5 mM) was added to increase heme synthesis. After lysozyme treatment and sonication of the bacteria, the membrane fraction was isolated by differential centrifugation. CYP3A4 was purified to homogeneity from detergent solubilized membranes on a DE52 column as previously described (23).
DHB-Mediated Inactivation of CYP3A4 in a Reconstituted System.
CYP3A4 (0.5 nmol) was reconstituted with 20 μg of a mixture (1:1:1) of L-α-dilauroyl- and L-α-dioleyl-sn-glycero-3-phosphocholines and phosphatidyl serine, 200 μg of cholic acid, 1 nmol of NADPH-cytochrome P450 reductase, 0.5 nmol of cytochromeb5, 500 U of catalase, 2 μmol of glutathione, 30 mM MgCl2, 0.5 mM EDTA, and 20% (v/v) glycerol in a final volume of 1 ml of 50 mM Hepes buffer (pH 7.5). Reactions with various concentrations of DHB, performed at 37°C for the times indicated, were initiated by addition of 1 mM NADPH and were terminated on ice. At each time point, 50 μl of the incubation mixture was removed and diluted to 1 ml with 50 mM Hepes buffer (pH 7.5) containing 200 μM testosterone, 500 U of catalase, 2 μmol of glutathione, 30 mM MgCl2, 0.5 mM EDTA, and 20% (v/v) glycerol. Following a 10-min incubation, the concentration of 6β-hydroxytestosterone formed was determined by HPLC on a C18 column [Microsorb-MV (Rainin, Woburn, MA), 5 μm, 4.6 × 15 cm] eluted with a mobile phase of 65% methanol at a flow rate of 1 ml/min with monitoring of the eluate by UV detection at 254 nm.
Results
To determine whether a single glass of grapefruit juice decreases enterocyte CYP3A4, small bowel mucosal biopsies were obtained from a volunteer before and after consumption of a single 8-ounce glass of regular strength grapefruit juice. As shown in fig.1, drinking the juice resulted in a 47% decrease in enterocyte CYP3A4 concentration at 4 hr, indicating that this was a rapid effect.
Effect of grapefruit juice on intestinal CYP3A4.
Endoscopic duodenal biopsies were obtained from a healthy adult volunteer before (Pre) and 4 hr after (Post) drinking an 8-ounce glass of grapefruit juice. The biopsies were homogenized and subjected to immunoblot analysis to determine CYP3A4 and villin concentrations. Villin, a constitutively expressed enterocyte protein, served as a control for the relative contribution of enterocytes to the biopsy mass (12, 22).
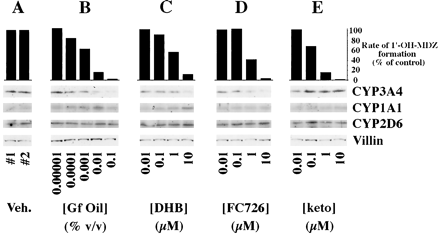
When grapefruit oil was added to the apical medium of Caco-2 cells, a concentration-dependent fall in CYP3A4 catalytic activity (midazolam 1′-hydroxylase) with 97% inhibition at the highest concentration (0.1% v/v) was observed (fig. 2B). The estimated IC50 of grapefruit oil for loss of midazolam 1′-hydroxylase activity in the Caco-2 cells was 0.0015%. The decrease in catalytic activity was accompanied by a fall in immunoreactive CYP3A4 protein. The highest concentration of grapefruit oil (0.1%) resulted in approximately a 50% reduction in CYP3A4 immunoreactive protein.
Effect of grapefruit oil and constituent compounds on CYP3A4 catalytic activity and expression of various cytochromes P450.
The Caco-2 cell clone P27.7 was cultured using previously described conditions under which the cells express metabolically active CYP3A4 (21). Shown are rates of midazolam 1′-hydroxylation by the cultured cells and representative immunoblots of cell homogenates probed with antibodies of the indicated specificities. Veh., vehicle; Gf, grapefruit; DHB, 6′,7′-dihydroxybergamottin; keto, ketoconazole; 1′-OH-MDZ, 1′-hydroxymidazolam. CYP3A4 concentrations were determined by computer aided densitometry using serial dilutions of purified CYP3A4 protein added in a second loading to the gels. Vehicle control lanes contained 20 fmol CYP3A4/μg of total protein. The CYP3A4 concentration decreased to 10 fmol CYP3A4/μg after exposure to 0.1% grapefruit oil, a 50% decrease. Thus, visual interpretation of immunoblots developed with ECL overestimates actual changes in CYP3A4 protein concentrations.
Fraction 39 from the HPLC fractionation of grapefruit juice showed one peak by HPLC that had an electronic absorption spectrum with absorption maxima at 225, 250, and 310 nm, which is consistent with a furanocoumarin (also called psoralen). Mass spectral analyses showed a molecular ion of m/z 726 and a daughter ion spectrum consistent with a psoralen dimer [m/z (relative abundance)]: 203 (100%); 153 (90%); 215 (76%); 135 (48%); 355 (26%); 337 (25%); 709 (12%); 107 (12%); and 373 (4%). Based on electronic absorption and mass spectra, the active component in fraction 39 was identified as a geranyloxypsoralen ether dimer with mass 726 g/mol and was designated FC726. Kinetic analysis of incubations with human liver microsomes showed that FC726 was an inhibitor of CYP3A4-mediated taxol metabolite B formation with an IC50 value of less than 0.5 μM. Numerous other furanocoumarin or psoralen compounds isolated from grapefruit juice were also found to inhibit CYP3A4 and CYP2C8 activities but were excluded from further consideration because of their lack of selectivity for CYP3A4.
The effects of grapefruit oil on CYP3A4 catalytic activity and immunoreactive protein were reproduced by DHB and FC726 (fig.2C and 2D, respectively). The calculated IC50 for loss of midazolam 1′-hydroxylase activity was 1.04 μM for DHB and 1.00 μM for FC726. At the highest concentration examined (10 μM), DHB and FC726 decreased immunoreactive CYP3A4 by approximately 40% and 50%, respectively. Immunoreactive CYP2D6 and CYP1A1 concentrations were not reduced by any of the treatments (fig. 2B, C, D), and the concentration of CYP1A1 may have increased.
Ketoconazole produced a concentration-dependent inhibition of midazolam 1′-hydroxylase activity (fig. 2E) with a calculated IC50 of 0.27 μM. In contrast to the effects of the furanocoumarins, however, the CYP3A4 concentration did not decrease and may have increased (fig. 2E).
Light microscopic examination of H&E stained sections of Caco-2 cells exposed for 4 hr to 0.1% grapefruit oil, 10 μM DHB, 10 μM FC726, or 10 μM ketoconazole showed no signs of cytotoxicity (data not shown).
Limonene, a major constituent of grapefruit oil (24), and decanal, one of the principal aldehydes contained in grapefruit oil (24), in concentrations as high as 100 μM did not inhibit CYP3A catalytic activity and did not affect the concentration of CYP3A protein in the Caco-2 cells (data not shown).
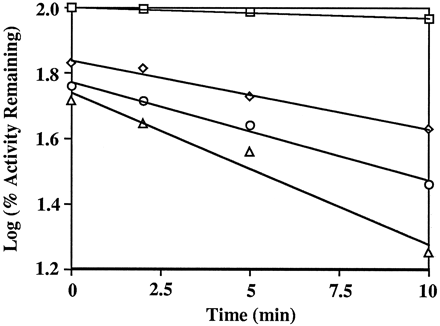
To examine further the interaction between furanocoumarins and CYP3A4, kinetic studies were conducted with recombinant CYP3A4 and DHB. As shown in fig. 3, DHB caused a time- and concentration-dependent inactivation of CYP3A4. The inactivation exhibited pseudo-first order kinetics. Linear regression analysis of the data in fig. 3 was used to determine the initial rate constants of inactivation (Kobs). The maximal rate constant (kinact) and the concentration of inactivator required for half-maximal rate of inactivation (KI) were determined to be 0.16 min−1 and 59 μM, respectively, by double-reciprocal plotting of the values ofKobs and DHB concentrations (25). These results demonstrate that DHB is a mechanism-based inactivator of CYP3A4 (25). The inactivation event appeared to occur at the active site because the inactivation rate was not affected by the presence of 2 or 3 mM glutathione in the incubation system. As can be seen from the ordinate in fig. 3, DHB-mediated inhibition of CYP3A4 activity did not require pre-incubation with NADPH. The maximum loss in activity after pre-incubation without NADPH (48.6%) was seen following incubation with 100 μM DHB. Sufficient quantities of FC726 were not available to perform similar studies.
Time- and concentration-dependent inactivation of the testosterone 6β-hydroxylase activity of CYP3A4 by 6′,7′-dihydroxybergamottin (DHB).
Recombinant CYP3A4 was pre-incubated with various concentrations of DHB in a reconstituted system for the times indicated (seeMethods). A 50 μl sample was then removed and incubated with testosterone in a reconstituted system. After a 10-min incubation, the rate of formation of 6β-hydroxytestosterone was measured. The initial concentrations of DHB in the incubations were 0 μM (□), 25 μM (◊), 50 μM (○), and 100 μM (▵), respectively.
We next determined the concentrations of bergamottin, DHB, 6′-epoxybergamottin, and FC726 in two samples of grapefruit oil, carefully excised grapefruit sections, and reconstituted frozen grapefruit juice concentrate. As shown in table 1, all of the furanocoumarins were present in all of the items examined but were most concentrated in the oil. DHB was more concentrated in the reconstituted juice than in the fresh fruit. In contrast, 6′-epoxybergamottin, bergamottin, and FC726 were more concentrated in the fresh fruit than in the reconstituted juice. Because DHB was the most concentrated furanocoumarin in the juice, its concentration was determined in a variety of commercial grapefruit juices and in hand-squeezed grapefruit juice. As shown in table 2, the concentration of DHB varied considerably among juices, but was generally highest in the reconstituted frozen concentrate.
Concentration of 6′,7′-dihydroxybergamottin in grapefruit juice from various sources
Discussion
Recurrent grapefruit juice ingestion has been shown to result in selective loss of CYP3A4 protein but not mRNA in small intestinal epithelial cells (12). This loss of enzyme is presumed to account, at least in part, for grapefruit juice-drug interactions (12). In the studies presented herein, we have shown that the in vivofall in intestinal CYP3A4 is rapid and occurs within 4 hr after ingestion of a single glass of the juice (fig. 1). In addition, this effect of grapefruit juice was also observed in vitro in Caco-2 cells exposed to grapefruit oil, DHB, and a second furanocoumarin present in grapefruit juice (FC726) (fig. 2). Each of these substances produced a dose-dependent fall in both CYP3A4 immunoreactive protein and catalytic activity (fig. 2). As previously demonstrated in vivo (12), the effect was selective in that no reduction in CYP1A1 or CYP2D6 proteins was observed (fig. 2). These data support the idea that furanocoumarins are the major active ingredients responsible for grapefruit juice-drug interactions.
Our data from incubations of recombinant CYP3A4 with DHB support the idea that the effect of furanocoumarins in grapefruit juice results, at least in part, from mechanism-based inactivation of CYP3A4 (fig. 3). Mechanism-based inactivation has been shown to result in accelerated degradation of a CYP3A enzyme in rat liver (26). The establishment of DHB as a mechanism-based inactivator of CYP3A4, therefore, supports the prior suggestion that the loss of CYP3A4 from enterocytes produced by grapefruit juice in vivo may result from accelerated degradation of the enzyme (12). Mechanism-based inactivation and loss of enzyme may account for the prolonged (≥ 24 hr) effect of grapefruit juice on oral felodipine kinetics (27).
It should be noted that concentration-dependent inhibition of CYP3A4 activity was also observed without pre-incubation with DHB and NADPH (fig. 3). A likely explanation is that DHB, carried over to the second reaction mixture, is a potent competitive inhibitor of CYP3A4. DHB was originally identified as a possible active component in grapefruit juice because of its ability to competitively inhibit a rat CYP3A enzyme (14). In addition, we have found that DHB is metabolized to several hydroxylated products by CYP3A4,2 indicating that it is a substrate for the enzyme. Competitive inhibition may in part account for the finding that greater than 90% inhibition of CYP3A4 catalytic activity occurred in Caco-2 cells in which the content of immunoreactive protein had fallen by no more than 60% (fig. 2). However, a delay between mechanism-based inactivation and subsequent enzyme degradation may be expected and could also contribute to this disparity.
Our data also support the conclusion that DHB is the major compound responsible for drug interactions caused by reconstituted frozen concentrate, the form of grapefruit juice most commonly consumed. It was the major furanocoumarin identified in frozen concentrate (table1). Moreover, the concentration of DHB present in all of the grapefruit juice preparations analyzed (tables 1 and 2) exceeded the IC50 for midazolam 1′-hydroxylation determined in the Caco-2 cells (approximately 1.0 μM). In contrast, DHB does not appear to be the major responsible component in grapefruit oil. Grapefruit oil exists in nature in the peel of the fruit and is frequently added as a flavor enhancer during commercial preparation of frozen grapefruit juice concentrate. The IC50 of grapefruit oil was determined to be approximately 0.0015%. Given the concentration of DHB determined in the oil used in the cell culture experiments (4.3 mM), the concentration of DHB was approximately 0.06 μM in 0.0015% oil. This concentration is less than 10% of the 1.0 μM IC50 estimated for DHB in the cells. It should be noted that the concentration of 6′-epoxybergamottin measured in the oil (22.4 mM, table 1) would result in a concentration of approximately 0.3 μM in 0.0015% oil. If 6′-epoxybergamottin is an inhibitor of CYP3A4 and has a potency comparable to that of DHB, the sum of the epoxide and DHB concentrations could probably account for most of the CYP3A4 inhibition produced by the oil.
The furanocoumarin dimer (FC726) we isolated from the juice appears to be a minor constituent, unlikely to substantially contribute to grapefruit juice-drug interactions. In our early studies, the concentration of this and of several other furanocoumarin compounds seemed to be more substantial relative to DHB. It was later determined, however, that this resulted from our initial use of hexane for extraction. We found that DHB is not well extracted by hexane and its abundance in the juice was not evident until a more polar solvent (methylene chloride or chloroform) was used. We remain interested in FC726 because it is more selective as an inhibitor of CYP3A4 than are other furanocoumarins present in grapefruit juice.
The addition of grapefruit oil to frozen concentrate presumably accounts for the observation that reconstituted juice generally has higher concentrations of DHB than does fresh juice (table 2). The virtual absence of the epoxide from the concentrate (table 1) was unexpected. We have found that the epoxide is converted to DHB in the presence of citric acid3 and speculate that this conversion occurs during commercial processing of the juice.
Furanocoumarins require light for their synthesis (28), and it was therefore possible that grapefruit oil (or the exterior portions of the peel) might be the sole source of these compounds in grapefruit juice. Although grapefruit oil contained the highest concentrations of furanocoumarins, these compounds were present in the fruit itself (table 1). Our data suggest that a whole grapefruit consumed as is customary in sections cut from halved fruit results in ingestion of approximately 1 mg of DHB (table 1). This amount corresponds roughly to that present in 3 ounces of an average reconstituted juice. This suggests that drinking freshly squeezed grapefruit juice or eating the fruit whole should produce an interaction with drugs similar to that observed with consumption of the reconstituted concentrate, although probably of lower magnitude. The clinical effect of fresh squeezed juice or the fruit itself has not yet been studied to our knowledge.
A final point is the variability in the concentration of DHB we determined in different brands of grapefruit juice and in different lots of the same brand of juice (table 2). This indicates that variability in the extent of the grapefruit juice effect can be expected with the consumption of different grapefruit juice preparations. This needs to be taken into account when comparing data from different institutions and indicates that the concentrations of the active ingredients, at least DHB, should probably be standardized in future studies.
In summary, at least two distinct furanocoumarins present in grapefruit and grapefruit juice are capable of decreasing CYP3A4 activity and reducing cellular concentrations of this enzyme. The presentfindings support 6′,7′-dihydroxybergamottin as the major furanocoumarin responsible for these effects after consumption of reconstituted frozen concentrate.
Acknowledgments
The authors wish to thank Dr. James W. Harris for his participation in isolating and testing grapefruit juice fractions, Elling Eidbo from the Washington Regional Transplant Consortium for his professionalism and commitment to human research, and Dr. Tony Montanari from the Florida Department of Citrus for his valuable assistance.
Footnotes
-
Send reprint requests to: Paul B. Watkins, M.D., University of Michigan Medical Center, Room A7119 University Hospital, Box 0108, 1500 East Medical Center Drive, Ann Arbor, MI 48109-0108.
-
This work was supported by the NIGMS (GM 38149, P.B.W.), the NCRR (MO1 RR00042, University of Michigan General Clinical Research Center), and the NIEHS (1-P30-ES06639, D.J.E.).
-
↵2 P. F. Hollenberg and K. He, data not shown, 1997.
-
↵3 P. B. Watkins and M. E. Fitzsimmons, unpublished observations, 1997.
- Abbreviations used are::
- DHB
- 6′,7′-dihydroxybergamottin
- FC726
- furanocoumarin of M.W. 726
- Received July 3, 1997.
- Accepted August 5, 1997.
- The American Society for Pharmacology and Experimental Therapeutics