Abstract
Extrahepatic glucuronidation, such as that in the central nervous system (CNS), may play a very important role in xenobiotic disposition and may serve to protect the CNS from potentially toxic xenobiotics. UDP-glucuronosyltransferase (UGT) 1A6 is an important catalyst for phenol and polycyclic aromatic hydrocarbon glucuronidation. Studies were designed to determine the immunohistochemical localization of UGT1A6 and the steroid-reactive UGTs 2B2 and 2B3 in brain regions throughout the rat development. Neuronal cells, such as pyramidal cells, in sections from cerebral cortex and hippocampus displayed intensive UGT1A6-specific staining. UGT1A6-specific staining was also found in neuronal cells throughout the cerebral cortex, as well as in the cerebellar white matter. Glial cells revealed no apparent staining. In addition, staining for UGT1A6 was seen in choroid plexus at a later developmental stage. Although UGT1A6 staining was evident, brain sections analyzed for UGT2B2 and UGT2B3 immunoreactivity showed no significant staining. These results provide the first definitive evidence for the presence and cellular localization of UGT1A6, in neurons of developing rat brain, whereas UGT2B2 and UGT2B3 were not detected.
Glucuronidation is an important process that serves a significant role in the metabolic disposition of many endogenous substances and xenobiotics. Although the liver is recognized as a major site of glucuronidation within the body, various studies (Hook et al., 1975; Aitio and Marniemi, 1980; Gherzi-Egea et al., 1986; Minn et al., 1991; Suleman et al., 1993) have reported that a variety of tissues such as kidney, intestine, lung, adrenal gland, heart, and brain possess the ability to convert endobiotics and xenobiotics to glucuronides. This reaction is catalyzed by the numerous members of the UDP-glucuronosyltransferase (UGT1) gene superfamily (Mackenzie et al., 1997).
Glucuronidation in rat and human brain tissue have been determined by several other investigators (Gherzi-Egea et al., 1986;Wahlström et al., 1988). Several studies have shown that substrates for a phenol-type UGT, UGT1A6, are glucuronidated quite well in rat brain and regional differences in glucuronidation rates were determined experimentally (Leininger et al., 1991;Gherzi-Egea et al., 1994). These workers have suggested that the blood brain interfaces and circumventricular organs are major sites where xenobiotic glucuronidation might occur (Gherzi-Egea et al., 1994).
Our laboratory has several polyclonal antibody preparations that are specific in recognizing rat UGT1A6 and the steroid-reactive UGTs 2B2 and 2B3. Immunohistochemical localization of UGTs 1A6, 2B2, and 2B3 in livers from untreated rats has been shown previously (Knapp et al., 1988). In the liver, staining for UGT1A6 was more intense in centrilobular hepatocytes than in periportal hepatocytes. Specific staining for UGT1A6 was also observed in the epithelium of the bile duct and the endothelium of the hepatic artery and portal vein. In contrast, hepatocytes throughout the liver lobule exhibited uniform staining of UGT2B2 and UGT2B3, but no staining for UGT2B2 and UGT2B3 in nonparenchymal periportal structures was observed.
The current study was designed to determine the possible localization for UGT1A6, UGT2B2, and UGT2B3 in the brain of developing rat. UGT1A6 staining was found in brain tissue throughout development, and was confined primarily in neurons. Brain sections analyzed for UGT2B2 and UGT2B3 showed no specific staining.
Materials and Methods
Tissue Preparation.
Pregnant Sprague-Dawley rats were obtained from Harlan Sprague-Dawley (Houston, TX). The pups (postnatal day 0, 7, 14, 21, and 28) were anesthetized with xylazine (5–8 mg/kg body weight) before the transcardial perfusion performed with 4% paraformaldehyde in 0.10 M phosphate buffer. Brains removed from these animals were first immersed in 15% sucrose for 24 hr (4°C), and then stored in 30% sucrose (4°C) until use (Neymeyer et al., 1997).
Immunohistochemical Procedure.
Brain sections (10 μm thick) were cut from frozen blocks using a cryostat (−20°C). The sections were collected on positively charged glass slides. Immunoperoxidase staining was achieved by subsequent incubations with primary and secondary antibodies followed by the application of Vectastain Elite kits (Vector Laboratories, Burlingame, CA) and subsequent use of diaminobenzidine hydrochloride as the chromogen. Polyclonal antibody preparations specific for UGT1A6 or UGTs 2B2 and 2B3 have been described previously (Knapp et al., 1988). Primary antibody dilution (1:500,000 for UGT1A6 and 1:10,000 for UGTs 2B2 and 2B3) was made in phosphate-buffered saline containing 1% normal serum and 0.75% Triton X-100. Incubations with nonimmune serum were used as a control (Martinasevic et al., 1996).
Results
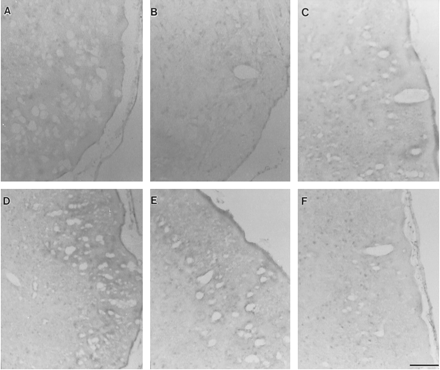
Various brain regions obtained from rats during the early period of their development (postnatal days 0–28) were analyzed for UGT1A6 immunoreactivity. Fig. 1 shows neuronal cells of cerebral cortex stained for this enzyme. An apparent age-related difference was not seen in staining from day 0 to day 28 after birth (fig. 1, A–E). Staining in glial cells was not observed, whereas glial localization for glial fibrillary acidic protein and 10-formyltetrahydrofolate dehydrogenase was observed as reported by Neymeyer et al. (1997) (data not shown). Incubations with nonimmune serum revealed no staining (fig.1F). Pyramidal cell staining (fig. 1, A–E) was particularly intense, and strong immunoreactivity was seen in the somata of these cells. Slightly weaker staining was noticed in their processes (fig. 1, A–E).
Immunohistochemical expression of UGT1A6 in cerebral cortex during rat development.
Photomicrographs (A, postnatal day 0; B, postnatal day 7; C, postnatal day 14; D, postnatal day 21; E, postnatal day 28, F, nonimmune control, day 28) show positive staining representing UGT1A6-specific immunoreactivity observed primarily in pyramidal cells. Cell bodies demonstrate dense staining, whereas slightly weaker staining could be noticed in their processes. Scale barrepresents 200 μm.
UGT1A6-specific immunoreactivity was also detected in cerebellum. Sections from early stages of rat development revealed positive staining primarily in neuronal cells throughout the granular cell layer (fig. 2). In addition to the staining visible in the granule cell layer, cerebellar sections from later stages of development (postnatal days 21 and 28) showed comparable staining in cell bodies of Purkinje cells (fig. 2, D andE). Furthermore, moderate staining was observed in neuronal cells located throughout the cerebellar white matter at every stage of brain development. Incubations with nonimmune serum revealed no staining (fig. 2F).
Immunohistochemical expression of UGT1A6 in cerebellar region during rat development.
Photomicrographs (A, postnatal day 0; B, postnatal day 7; C, postnatal day 14; D, postnatal day 21; E, postnatal day 28, F, nonimmune control, day 28) show positive staining representing UGT1A6-specific immunoreactivity observed primarily in neuronal cells thoughout the granular cell layer (arrows). Lighter staining is also visible in neuronal cells throughout the white matter. Images from later stages of development (D and E) indicate presence of specific immunoreactivity in cell bodies of Purkinje cells (arrowheads). Scale bar represents 50 μm.
In contrast to the staining observed for UGT1A6, brain sections revealed no staining specific for UGT2B2 and UGT2B3 (figs.3 and 4) throughout the period of rat development.
Immunohistochemical expression of UGT2B2 in cerebral cortex during rat development.
Photomicrographs (A, nonimmune serum, day 28;B, postnatal day 0; C, postnatal day 7;D, postnatal day 14; E, postnatal day 21;F, postnatal day 28) reveal images that show insignificant UGT2B2-specific immunoreactivity. Scale bar represents 50 μm.
Immunohistochemical expression of UGT2B3 in cerebral cortex during rat development.
Photomicrographs (A, nonimmune serum, day 28;B, postnatal day 0; C, postnatal day 7;D, postnatal day 14; E, postnatal day 21;F, postnatal day 28) reveal images showing insignificant immunostaining specific for UGT2B3. Scale bar represents 50 μm.
Discussion
Staining specific for UGT1A6 was found in brain sections analyzed throughout the early period of rat development. This staining was observed only in the neuronal cells and specific staining was not seen in glial cells. Thus, UGT1A6 expressed in neuronal cells appears to account for the glucuronidation activity seen in other studies (Gherzi-Egea et al. 1987, 1993; Leninger et al., 1991). Positive staining was not detected for the UGT2B2 and UGT2B3 enzymes, which react with steroid substrates (Green et al.1985; Leninger et al., 1991).
Several studies (Gherzi-Egea et al. 1987, 1993; Leningeret al., 1991) have suggested that UGT activity exists in circumventricular organs, microvessels, and choroid plexus. Our results do not support the presence of UGTs 1A6, 2B2, or 2B3 in the microvasculature and the circumventricular organs of the rat CNS. Further experiments are needed to explore the rat choroid plexus for the presence of UGT1A6.
Neuronal cells, especially the pyramidal cells of the cortex and the granular cells throughout the cerebellum, displayed intense immunoreactivity for UGT1A6. The pattern of staining determined in Purkinje cells of the cerebellum seemed to be age-dependent. Minimal staining of Purkinje cells was found in cerebellum sections from the early postnatal stages, whereas cerebellum sections obtained from postnatal days 21 and 28 revealed dense staining in the Purkinje cell somata.
UGT1A6 has been characterized as a UGT that is reactive with simple and planar phenols (Burchell, 1996). Certain hydroxylated benzo-(a)-pyrene metabolites, especially the quinols, are known to be very reactive with this isoform as well as the simple phenols. In addition, 3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo-pdioxin have been shown to bind to the arylhydrocarbon receptor and produce induction of hepatic UGT1A6 (Münzel et al., 1996). Rat UGT1A6 may also be involved in catalyzing the glucuronidation of 5-hydroxytryptamine in vivo (Leakey, 1978). However, questions such as the presence of UGT1A6 in human brain, or the induction of UGT1A6 in CNS, have not been adequately addressed. Preliminary studies from our laboratory have shown mRNA transcript for UGT1A6 in human brain.
Acknowledgment
The authors gratefully acknowledge the assistance of Dr. M. Miller for supplying the animals for this study.
Footnotes
-
Send reprint requests to: Thomas R. Tephly, MD, PhD, Department of Pharmacology, University of Iowa College of Medicine, Iowa City, IA 52242.
-
This work was supported by National Institutes of Health Grant GM26221 and Monsanto (Skokie, IL).
- Abbreviation used is::
- UGT
- UDP-glucuronosyltransferase
- Received March 30, 1998.
- Accepted June 1, 1998.
- The American Society for Pharmacology and Experimental Therapeutics