Abstract
Sulfotransferase 4A1 (SULT4A1), a member of cytosolic sulfotransferases (SULT), is exclusively expressed in neurons with no known function. Severe phenotype and early postnatal death in SULT4A1 knockout mice revealed that SULT4A1 is an essential neuronal protein. Localization of SULT4A1 in different cytosolic compartments, including mitochondria, suggests multiple roles for this protein. We observed that knockdown of SULT4A1 results in the accumulation of reactive oxygen species in primary cortical neurons, suggesting a potential role of SULT4A1 in regulating redox homeostasis. Expression of SULT4A1 in the human neuroblastoma SH-SY5Y cells revealed a defused but nonuniform staining pattern in the cytoplasm, with increased density around mitochondria. Subcellular fractionation of SULT4A1 expressing SH-SY5Y cells confirms the presence of SULT4A1 in mitochondrial fractions. SULT4A1 expressing cells display significant protection against H2O2-mediated defects in mitochondrial function and loss of mitochondrial membrane potential. Expression of SULT4A1 in SH-SY5Y cells also protects against H2O2-induced cell death. These data indicate that SULT4A1 protects mitochondria against oxidative damage and may serve as a potential pharmacological target in neural diseases involving mitochondrial dysfunction and oxidative stress.
SIGNIFICANCE STATEMENT Studies on SULT4A1 knockout mice suggest that SULT4A1 plays a vital role in neuronal function and survival via yet undefined mechanisms. Our data demonstrate that depletion of SULT4A1 induces oxidative stress in neurons and expression of SULT4A1 in SH-SY5Y cells protects against oxidative-stress-induced mitochondrial dysfunction and cell death. These results suggest that SULT4A1 may have a crucial protective function against mitochondrial dysfunction and oxidative stress, and may serve a potential therapeutic target in different neurological diseases involving mitochondrial dysfunction and oxidative stress.
Introduction
Sulfotransferase (SULT) 4A1 is a member of the cytosolic SULT gene family based on its structural homology (Allali-Hassani et al., 2007). Cytosolic SULTs catalyze the transfer of a sulfonate group from 3′-phosphoadenosine-5′-phosphosulfate to their substrates (Suiko et al., 2017). SULT4A1 is highly conserved and selectively expressed in neurons in the brains of vertebrates (Garcia et al., 2018). SULT4A1 has a full-length and a splice variant transcript that is expressed in different tissues and cell types. The full-length SULT4A1 variant, found only in the neurons, encodes the complete protein sequence, whereas the splice variant transcript, found in other tissues and cells encodes an unstable protein that is undetectable. The instability of the splice variant protein is attributed to the insertion of a premature stop codon due to the failure of correctly excising an intron between exons 6 and 7 (Falany et al., 2000; Sidharthan et al., 2014). No known enzymatic activity of SULT4A1 has been reported since its initial cloning and characterization by Falany et al. (2000). SULT4A1 lacks 3′-phosphoadenosine-5′-phosphosulfate or binding properties due to the absence of a part of loop 3 that covers the substrate-binding pocket, indicating that SULT4A1 may not be a catalytically active SULT (Garcia et al., 2018). Changes in behavioral activity in SULT4A1-deficient zebrafish (Crittenden et al., 2015) and a profound neuronal phenotype and postnatal death observed in CRISPR/Cas9-generated SULT4A1 knockout mice revealed that SULT4A1 is a vital neuronal protein (Garcia et al., 2018). Unlike other cytosolic SULTs, SULT4A1 localizes with different subcellular organelles including mitochondria (Garcia et al., 2018), suggesting that SULT4A1 may have multiple novel functions in the brain.
Mitochondrial vigor is crucial for neural survival and functioning as the brain on average uses nearly 20% of the energy consumed by the body even though brain mass accounts for only 2% of the total body weight (Raichle and Gusnard, 2002). Most of the ATP in neurons is used to maintain resting potential, propagation of nerve impulse transmission, and neurotransmitter release (Raichle and Gusnard, 2002). Additionally, axonal transport and mitochondrial dynamics in neurons are bioenergetically demanding processes (Raichle and Gusnard, 2002). Therefore, neuronal mitochondria may require additional factors for their efficiency and protection to support the demanding bioenergetic needs. SULT4A1 shows novel mitochondrial localization (Garcia et al., 2018), and there is an increase in oxidative stress following knockdown of SULT4A1 in mouse cortical neurons. Therefore, regulation of mitochondrial function and redox homeostasis may comprise important functions of SULT4A1 in neurons. We used SH-SY5Y neuroblastoma cells to evaluate the mitochondrial roles of SULT4A1 in normal and H2O2-induced oxidative stress conditions. Our results indicate that SULT4A1 expression in SH-SY5Y cells increases maximal mitochondrial respiration and protects against H2O2-induced mitochondrial dysfunction. These studies suggest that regulation of mitochondrial function in stress conditions and redox homeostasis are important SULT4A1 functions in neurons.
Materials and Methods
Cell Culture and Lentiviral Transduction.
SH-SY5Y cells were grown in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 5 mM glucose and 10% FBS. Human SULT4A1 was expressed in SH-SY5Y cells using a lentiviral transduction as described previously (Hossain et al., 2013). Briefly, human SULT4A1 was cloned into pLVX-Puro lentiviral vector along with psPAX2 lentiviral packaging vector pMD2.G lentiviral envelope vector cotransfected into HEK293FT cells using 25 kDa linear polyethylenimine (Polysciences, Warrington, PA) transfection reagent. Virus was collected from the media and directly added to SH-SY5Y cells. Transduced cells were selected using puromycin (Sigma, St. Louis, MO). Control virus was packaged in the same way using empty pLVX-Puro vectors. H2O2 (500 µM for 2 hours) was used to induce oxidative stress in these cells. Seahorse assay, TMRE imaging was performed immediately after H2O2 treatment, and cell death was assessed after 24 hours.
Primary Cortical Neuronal Culture and shRNA Knockdown.
Primary cortical neurons were cultured from embryonic day 15 mouse embryos as previously described (Andrabi et al., 2014; Garcia et al., 2018). Cells were plated at a density of 5 × 105 cells/ml on poly-l-ornithine (P3655; Sigma)-coated cell culture plates in neurobasal medium, supplemented with, 1 mM GlutaMax, 1 mM sodium pyruvate, B-27 and 10 mM glucose (Thermo Scientific). 5-fluoro-2-deoxyuridine (40 μM) (F0503; Sigma) was used to inhibit glial growth at DIV 2 (Days in vitro). Lentiviruses containing control shRNA (pLKO.1 GFP shRNA; Addgene) or SULT4A1 shRNA (TRCN0000103353; Sigma) were transduced (multiplicity of infection of 5) on DIV 6. Experiments were performed on DIV 10.
Reactive Oxygen Species Measurement.
Reactive oxygen species (ROS) were measured using CellRox Deep Red (Thermo Scientific) imaging. Neurons were incubated with 5 µM CellRox for 30 minutes at 37°C. Live-cell images were captured using an LSM 710 Confocal Microscope (Carl Zeiss, Germany). Fluorescence intensity was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Subcellular Fractionation and Western Blotting.
The SH-SY5Y cells were suspended in fractionation buffer containing 20 mM HEPES (pH 7.4), 10 mM KCl, 2 mM MgCl2, 1 mM ethylenediaminetetraacetic acid, and 1 mM ethylene glycol tetraacetic acid, 1 mM dithiothreitol, and complete protease and phosphatase inhibitor (Thermo Fisher Scientific). The cell suspension was passed through a 30-G needle 10 times followed by centrifugation at 750 g for 5 minutes at 4°C. The supernatant containing cytoplasm, membrane, and mitochondria was further centrifuged at 10,000 g for 10 minutes at 4°C. The resultant pellet containing mitochondria was resuspended in lysis buffer containing 25 mM Tris HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.1% SDS, 1% sodium deoxycholate, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, and complete phosphatase and protease inhibitor. Protein concentrations were determined by BCA protein assay kit (Thermo Fisher Scientific). Equal amounts of protein were separated by SDS-PAGE gel electrophoresis and transferred onto a nitrocellulose membrane. A rabbit anti-SULT4A1 polyclonal antibody (Proteintech) followed by a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Cell Signaling, Beverly, MA) was used to detect SULT4A1 via SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Glyceraldehyde 3-phosphate dehydrogenase (Cell Signaling) and TOM20 antibodies (Proteintech) were used for cytosolic and mitochondrial fractions, respectively (Dimauro et al., 2012; Wang et al., 2016).
Immunocytochemistry.
SH-SY5Y cells expressing SULT4A1 were fixed in 4% paraformaldehyde for 15 minutes and permeablized with 0.2% Triton X-100 for 30 minutes. Following blocking, the cells were incubated in anti-SULT4A1 and anti-TOM20 antibodies (Proteintech) overnight at 4°C. Alexa Fluor 488 donkey anti-mouse secondary antibody and Alexa Fluor 555 donkey anti-rabbit secondary antibody (Thermo Fisher Scientific) were used to detect the primary antibodies. Coverslips were mounted on glass slides using Immuno-Mount mounting medium (Thermo Fisher Scientific). LSM 710 confocal microscope (Carl Zeiss) was used for imaging.
Mitochondrial Function.
Mitochondrial function was measured as oxygen consumption rate (OCR) in SH-SY5Y cells using an XFe96 Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA). Seventy thousand cells were plated in each well of an XFe96 cell culture microplate (Agilent Technologies) and maintained overnight in a 5% CO2 at 37°C in a humidified incubator. Cells were washed and placed in serum-free, pyruvate-free, glutamine-free Dulbecco’s modified Eagle’s medium (Agilent Technologies). Oligomycin, CCCP, and rotenone/antimycin A (Sigma Aldrich) were injected into each well sequentially to determine basal respiration, maximal respiration, spare respiratory capacity ATP turnover, and proton leak as described previously (Andrabi et al., 2014).
Mitochondrial Membrane Potential.
Tetramethylrhodamine ethyl ester (TMRE) 20 nM (Thermo Fisher Scientific) was incubated with SH-SY5Y cells for 30 minute at 37°C. TMRE live-cell imaging was acquired using an LSM 710 confocal microscope Carl Zeiss). Images were captured at 10 second intervals for 1 minute to get a baseline, 20 μM CCCP was added to depolarize mitochondria and images were captured for an additional 2 minutes. Mitochondrial membrane potential (ΔΨm) was calculated as fold change in TMRE intensity before and after CCCP addition.
Cell Death Assays.
Alamar blue reagent (Thermo Fisher Scientific) was used to assess cell viability. Alamar blue reagent [10% (v/v)] was incubated in the cultures for 3 hours. One hundred microliters of the medium was collected from each well and transferred to a 96-well microplate. Victor ×5 microplate reader (Perkin Elmer) was used to measure the fluorescence using excitation and emission wavelength of 540 and 595 nm, respectively. Data were quantified and presented as percent control.
Results and Discussion
SULT4A1 is an important neuronal-specific protein (Garcia et al., 2018). Loss of SULT4A1 in SULT4A1 knockout mice results in tremors, abnormal gait, rigidity, and death at 3 to 4 weeks of age (Falany et al., 2000). Despite the apparent importance of SULT4A1 in neurons, no SULT catalytic activity has been reported for this vital neuronal protein. It is likely that SULT4A1 is a catalytically inactive member of SULT family and functions by regulating some important neural functions by yet undefined mechanisms. The distribution of SULT4A1 is diffused in the cytosol of neurons with substantial mitochondrial and microsomal localization (Garcia et al., 2018). We used shRNA-mediated knockdown of SULT4A1 via lentiviral transduction in mouse cortical neurons (Fig. 1A) and observed that ROS accumulate in these neurons (Fig. 1, B and C), suggesting that SULT4A1 may help in maintaining neuronal redox homeostasis. SULT4A1 localizes to the mitochondria, and these organelles are one of the principal sources of ROS in a cell (Nohl et al., 2005; Murphy, 2009). One possibility is that SULT4A1 protects mitochondria against oxidative damage and maintains redox homeostasis in the neural cells. To assess these functions, we used lentiviral transduction to express SULT4A1 in neuroblastoma SH-SY5Y cells (Fig. 1D). Subcellular fractionation and Western blotting revealed that SULT4A1 localizes to the mitochondrial fraction in SH-SY5Y cells expressing SULT4A1 (Fig. 1E). Likewise, immuno-fluorescence staining demonstrated that transduced SULT4A1 in SH-SY5Y cells displays diffuse cytosolic staining with a considerable colocalization with the mitochondrial outer membrane protein TOM20 (Fig. 1F). Orthogonal projections revealed that SULT4A1 colocalizes with TOM20 in XY, XZ, and YZ planes (Fig. 1F). These data confirm our previous results in mouse brain (Garcia et al., 2018) and suggest that the spatiotemporal localization of SULT4A1 expressed in SH-SY5Y cells is comparable to its endogenous localization in neurons.
(A) Western blot showing knockdown of SULT4A1 in primary neurons. (B) CellRox fluorescence in primary cortical neurons transduced with control shRNA or SULT4A1 shRNA. Scale bar, 20 μm. (C) Quantification of CellRox fluorescence intensity. Data represent mean ± S.E.M., n = 5, ***P < 0.001 vs. control (Student’s t test). (D) Western blots showing SULT4A1 expression in SH-SY5Y cells with or without transduction with control virus or SULT4A1 lentivirus. (E) Subcellular fractionation in SH-SY5Y showing mitochondrial localization of SULT4A1. TOM20 is used as a mitochondrial marker and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is cytosolic marker. (F) Immunostaining pictures of SULT4A1 with orthogonal projections in SH-SY5Y show that SULT4A1 (green) localize with mitochondrial marker TOM20 (red). Colocalization was determined by Pearson’s correlation coefficient, R = 0.66.
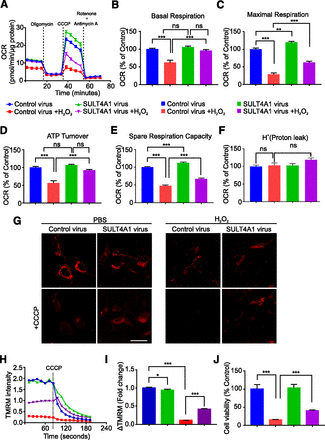
As the function of SULT4A1 in neurons has been an enigma in the field, it does appear to have an important role in regulating neuronal functions, including bioenergetics and redox balance. To determine whether SULT4A1 affects mitochondrial function and possibly protects against oxidative stress, mitochondrial function in SH-SY5Y cells that express SULT4A1 was monitored in presence or absence of H2O2 using an XF Seahorse flux analyzer. H2O2 is a potent oxidative stress agent and induces ROS generation in the cells (Wazawa et al., 1992). We monitored mitochondrial function as oxygen consumption rate (OCR) using a comprehensive Seahorse flux analysis in SULT4A1-expressing SH-SY5Y cells in presence or absence of H2O2 to assess basal OCR, maximal OCR, spare capacity, ATP turnover, and proton leak as previously described (Andrabi et al., 2014). SULT4A1 expression specifically increases maximal OCR in control SH-SY5Y cells (Fig. 2, A and C). H2O2 treatment in SH-SY5Y cells induces a drastic reduction in basal OCR, maximal OCR, and ATP turnover (Fig. 2, A–D), suggesting that H2O2-mediated oxidative stress has a severe effect on mitochondrial function. SULT4A1 expression significantly increases both basal and maximal OCR in H2O2-treated SH-SY5Y cells (Fig. 2, A–C). Spare respiratory capacity also increases significantly in SH-SY5Y cells expressing SULT4A1 (Fig. 2, A and E). The H2O2-induced decrease in ATP-turnover is almost reversed in SH-SY5Y cells expressing SULT4A1 (Fig. 2D). Proton leak in SH-SY5Y cells expressing SULT4A1 in presence or absence of H2O2 is not significantly different as compared with control (Fig. 2F). The ΔΨm generated by the activities of mitochondrial electron transport chain complexes is an important factor for oxidative phosphorylation (Murphy, 2009; Zorova et al., 2018). Loss of ΔΨm is an early feature of mitochondrial damage (Zorova et al., 2018). Mitochondria lose ΔΨm because either the electron transport chain is not functioning correctly or the mitochondrial inner membrane proton leaks (Cheng et al., 2017; Zorova et al., 2018). Nevertheless, the loss of ΔΨm is a key indicator of mitochondrial dysfunction. ΔΨm was assessed in SH-SY5Y in presence or absence of H2O2 using TMRE live cell imaging with a Zeiss LSM 710 confocal microscope (Carl Zeiss). TMRE imaging shows that H2O2 almost completely abolishes ΔΨm (Fig. 2, G–I). SULT4A1 expression in these cells significantly preserved the ΔΨm (Fig. 2, G–I). These data suggest that the recovery of ΔΨm by SULT4A1 expression in SH-SY5Y cells following H2O2 treatment and is not due to excessive proton leak but potentially due to direct regulatory effect on mitochondria function and redox homeostasis. We finally assessed cell viability by Alamar Blue assay 24 hours after H2O2 treatment in these cells. Our results indicate H2O2 induces approximately 80% cell death in SH-SY5Y cells (Fig. 2J), and SULT4A1 expression in these cells significantly protects against H2O2-induced cell death (Fig. 2J). Since SH-SY5Y cells do not express detectable levels of SULT4A1 protein, we expressed SULT4A1 using lentiviral transduction. It is important to identify the role of endogenous SULT4A1 using neurons to validate the role of SULT4A1 in regulating oxidative stress. Nevertheless, the results of this study strongly indicate that SULT4A1 may have a protective role against oxidative stress in neurons.
Mitochondrial function in SH-SY5Y cells expressing SULT4A1. (A) Representative data showing mitochondrial OCR in SH-SY5Y cells transduced with control or SULT4A1 virus in presence or absence of H2O2. Quantification of basal respiration (B), maximal respiration (C), ATP turnover (D), spare respiration capacity (E), and proton leak (F). Data are mean ± S.E.M., n = 18–22 for each group. Experiments were repeated three times with similar results. (G) Confocal images showing TMRE intensity in SH-SY5Y cells transduced with control and SULT4A1 virus in presence or absence of H2O2, Scale bar, 20 μm. (H) Time-lapse quantification of TMRE fluorescence intensity before and after CCCP addition. (I) Bar graph showing quantification of TMRE intensity change (∆TMRE) before and after CCCP addition in SH-SY5Y cells. Data represents mean ± S.E.M., n = 3. (J) Cell viability in SH-SY5Y cells in presence or absence of H2O2. Data are mean ± S.E.M., n = 8. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control, was calculated using one-way ANOVA and Tukey’s post hoc test.
Mitochondrial function is critical to meet high bioenergetic demands in neurons as defects in mitochondrial function lead to neurodegeneration (Gan et al., 2018; Zhao et al., 2019). Mutations in mitochondria-related genes often result in neurologic defects (Reeve et al., 2008; Keogh and Chinnery, 2015), and these defects are similar to those observed in the SULT4A1 knockout mice. It is likely that SULT4A1 is required at neural mitochondria to meet high-energy demands and possibly regulate ROS generated by the mitochondria (Murphy, 2009). Our data suggest that there is a functional link between SULT4A1, mitochondria, and oxidative stress. How these functions of SULT4A1 are mediated remain unknown. Because of the importance of SULT4A1 in the brain (Garcia et al., 2018), elucidation of mechanisms and pathways that SULT4A1 uses to impart its essential functions in neurons is crucial. Further experiments assessing the identification of SULT4A1-protein interaction network will provide important insights into the roles of SULT4A1 in regulating mitochondrial function, redox homeostasis, and other neuron-specific processes.
Authorship Contributions
Participated in research design: Hossain, Marcus, Lee, Garcia, Andrabi.
Conducted experiments: Hossain, Marcus.
Performed data analysis: Hossain, Marcus, Lee, Garcia, Gagné, Poirier, Falany, Andrabi.
Wrote or contributed to the writing of the manuscript: Hossain, Marcus, Lee, Garcia, Gagné, Poirier, Falany, Andrabi.
Footnotes
- Received May 17, 2019.
- Accepted June 27, 2019.
This work was supported by the National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grant NS086953].
Abbreviations
- CCCP
- carbonyl cyanide m-chlorophenyl hydrazine
- LSM
- laser scanning microscope
- ΔΨm
- mitochondrial membrane potential
- MOI
- multiplicity of infection
- OCR
- oxygen consumption rate
- ROS
- reactive oxygen species
- SULT
- cytosolic sulfotransferase
- SULT4A1
- sulfotransferase 4A1
- TMRE
- tetramethylrhodamine ethyl ester
- TOM20
- translocase of the outer membrane of mitochondria 20
- Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics