Abstract
The herb kava has recently been associated with numerous drug interactions, but its interaction with cytochrome P450 (P450) enzymes has not been investigated. In the present work the inhibition of P450 enzymes by kava extract and individual kavalactones in human liver microsomes (HLMs) was investigated. Whole kava extract (normalized to 100 μM total kavalactones) caused concentration-dependent decreases in P450 activities, with significant inhibition of the activities of CYP1A2 (56% inhibition), 2C9 (92%), 2C19 (86%), 2D6 (73%), 3A4 (78%), and 4A9/11 (65%) following preincubation for 15 min with HLMs and NADPH; CYP2A6, 2C8, and 2E1 activities were unaffected. The activities of CYP2C9, 2C19, 2D6, and 3A4 were also measured after incubation of HLMs with the major kavalactones kawain (K), desmethoxyyangonin (DMY), methysticin (M), dihydromethysticin (DHM) (each at 10 μM), and NADPH. Whereas K did not inhibit these enzymes, there was significant inhibition of CYP2C9 by DMY (42%), M (58%), and DHM (69%); of 2C19 by DHM (76%); of 2D6 by M (44%); and of 3A4 by DMY (40%), M (27%), and DHM (54%). Consistent with their potency as inhibitors, the two major kavalactones bearing a methylenedioxyphenyl moiety (M and DHM) formed “455 nm” metabolic intermediate complexes after incubation with HLMs and NADPH, but K and DMY did not. These data indicate that kava has a high potential for causing drug interactions through inhibition of P450 enzymes responsible for the majority of the metabolism of pharmaceutical agents.
Kava,Piper methysticum, is a shrub indigenous to the islands of the South Pacific (Rasmussen et al., 1979; Rouse, 1998). Inhabitants of this region prepare an aqueous extract of the root for use as a ritual beverage known for promoting relaxation and a sense of well being (Keledjian et al., 1988). The pharmacology, toxicology, and chemical characterization of kava have been reviewed extensively (Lebot et al., 1992; Jellin et al., 2002). Fifteen lactones, termed kavalactones, have been identified in kava root extract. Yangonin, desmethoxyyangonin (DMY1; 5,6-dehydrokawain), methysticin (M), 7,8-dihydromethysticin (DHM), kawain (K), and 7,8-dihydrokawain (Fig.1) are present in the highest concentrations and account for approximately 96% of lipidic extracts (Lebot and Lévesque, 1989).
Activities of hepatic microsomal cytochrome P450 enzymes after incubation of human hepatic microsomes with kava extract and individual kavalactones.
Human liver microsomes were incubated with kava extract or individual kavalactones and NADPH prior to determination of individual P450 enzyme activities. Enzymes and their activities are as follows: CYP1A2, acetanilide hydroxylase; CYP2A6, coumarin 7-hydroxylase; CYP2C8, paclitaxel 6α-hydroxylase; CYP2C9, tolbutamide hydroxylase; CYP2C19, mephenytoin 4-hydroxylase; CYP2D6, dextromethorphanO-demethylase; CYP2E1, p-nitrophenol hydroxylase; CYP3A4, dextromethorphan N-demethylase; CYP4A9/11, lauric acid ω-hydroxylase. Significantly different from control, ★, p < 0.05; ★★,p < 0.01; ★★★, p < 0.001).
Case reports detailing liver failure after ingestion of kava have been emerging over the last few years (Strahl et al., 1998; Kraft et al., 2001), and over-the-counter sales of these herbal preparations have recently been banned in Switzerland and Germany; Britain is also proposing a ban (Jellin et al., 2002). Heavy kava consumption has been linked to a characteristic dermopathy, termed kani, consisting of rough and scaly skin with light and dark bands (Keledjian et al., 1988) that is possibly attributable to altered cholesterol metabolism (Ruze, 1990;Lebot et al., 1992). There are reports of significant drug interactions when kava is used concomitantly with alprazolam (Almeida et al., 1996) and other central nervous system depressants, including barbiturates, benzodiazepines, and alcohol (Blumenthal et al., 1998; DerMarderosian, 1999).
When administered individually, kavalactones do not exhibit the degree of pharmacological activity observed after administration of whole kava extract (Lebot et al., 1992), possibly indicating modulation of transport or metabolism. Although no detailed reports of the inhibition of human cytochrome P450 (P450) enzymes by kava constituents were found in the literature, Meyer (1962) demonstrated a dramatic increase in hexobarbital sleep time, possibly indicative of CYP2C inhibition, following administration of kavalactones to mice. Additionally, methysticin analogs present in kava extract contain a methylenedioxyphenyl group. This moiety has been shown, after metabolic activation, to inhibit multiple cytochrome P450 enzymes through the formation of metabolic intermediate (MI) complexes (Hodgson and Philpot, 1974; Murray et al., 1983; Murray and Reidy, 1989). The present studies were conducted to help elucidate how the constituents of kava may interact synergistically, and to help explain and predict interactions with drugs with which kava is taken concomitantly.
Materials and Methods
Chemicals.
KavaPure Kava PE 40%, powdered kava extract, was obtained from PureWorld Botanicals, Inc. (South Hackensack, NJ). Individual kavalactones, including desmethoxyyangonin (5,6-dehydrokawain), 7,8-dihydromethysticin, kawain, methysticin, and yangonin, were isolated from kava and supplied by ChromaDex, Inc. (Laguna Hills, CA). All other chemicals and reagents used were of the highest commercially available quality.
Human Liver Microsomes (HLMs).
A pooled sample of human liver microsomes (lot number HHM-260) was obtained from Tissue Transformation Technologies (Edison, NJ).
In Vitro Incubations.
Microsomes prepared from human liver were incubated in the presence of kava extract or individual kavalactones and NADPH prior to determination of P450 enzymatic activities. Kava-treated incubation tubes were prepared by adding the resin, dissolved in methanol or acetone, to each tube for a final assay concentration of 1, 10, or 100 μM kavalactones (0.625, 6.25, or 62.5 μg of kava extract/ml), based on an average molecular mass of 250 atomic mass units for kavalactones. The solvent was allowed to evaporate. Kavalactone-treated incubation tubes were prepared by adding individual kavalactones, dissolved in acetone, to each incubation tube for a final assay concentration of 1 or 10 μM and allowing the acetone to evaporate. Control incubation tubes contained no kava extract or individual kavalactones. Microsomes and assay buffer were added to control and treated tubes as described below, and the tubes were preincubated at 37°C for 10 min. NADPH was then added, and the incubations were maintained at 37°C for an additional 15 min. After initiation of the reactions by addition of substrate, assays were performed as described below.
P450 Assays.
Microsomes prepared from human liver and preincubated in the presence of kava or individual kavalactones and/or NADPH were assayed for protein concentration, acetanilide hydroxylase activity (CYP1A2),p-nitrophenol hydroxylase activity (CYP2E1), and tolbutamide hydroxylase activity (CYP2C9) as previously described (Mathews et al., 1998). Lauric acid ω-hydroxylase activity (CYP4A) was measured by the method of Clarke et al. (1994). Coumarin 7-hydroxylase activity (CYP2A6) and (S)-mephenytoin 4-hydroxylase activity (CYP2C19) were determined as described by Hickman et al. (1998), using modified HPLC methods consisting of a Zorbax SB-C18 column (Agilent Technologies, Palo Alto, CA) and a flow rate of 1 ml/min. Coumarin metabolites were eluted using an isocratic mobile phase of 55% 20 mM sodium phosphate buffer (pH 4.4) and 45% methanol (v/v). Metabolites of (S)-mephenytoin were separated using a mobile phase of 3:2 water/methanol (v/v). Paclitaxel (Taxol) 6α-hydroxylase activity (CYP2C8) was determined using a modification of the method ofHarris et al. (1994). Reaction mixtures contained 100 mM potassium phosphate buffer (pH 7.4), 3.3 mM magnesium chloride, 0.2 mg of protein, 20 μM paclitaxel, and 1 mM NADPH in a final volume of 0.25 ml of paclitaxel , prepared as a 5 mM stock in ethanol, was added to each incubation to initiate the reaction. The final concentration of ethanol in each incubation was 0.4%. Reactions were allowed to proceed for 10 min at 37°C and were terminated by addition of 75 μl of acetonitrile. After centrifugation, an aliquot of each supernatant was analyzed by HPLC for 6α-hydroxypaclitaxel. Metabolites were separated using a Zorbax SB-C18 column and a linear gradient from 60 to 70% aqueous methanol over 20 min at a flow rate of 1 ml/min. 6α-Hydroxypaclitaxel was detected by measuring UV absorbance at 230 nm and quantitated using a standard curve. DextromethorphanO-demethylase (CYP2D6) and N-demethylase activities (CYP3A4) were measured at substrate concentrations of 20 μM and 500 μM dextromethorphan hydrobromide, respectively, using the method of Hickman et al. (1998) with a modification of the HPLC analysis method of Laurenzana et al. (1995). Dextromethorphan metabolites were separated by HPLC using a Microsorb MV Phenyl column (Varian, Inc., Woburn, MA). The mobile phase was: A, 0.05 M phosphate buffer, pH adjusted to 4 with phosphoric acid; B, CH3CN. The flow rate was 1.5 ml/min; the gradient started at 20% B and held at that composition for 8 min before changing linearly to 45% B over 3 min. Dextrorphan and 3-methoxymorphinan were detected and quantitated by fluorescence with the excitation and emission filters set at 270 and 312 nm, respectively. A standard curve was generated using known amounts of metabolite standards.
P450 Difference Spectra.
Binding spectra caused by the interaction of kava constituents with P450 were measured by a modification of the method of Elcombe et al. (1975). Treated incubation tubes were prepared by adding kava extract or individual kavalactones, dissolved in acetone, to each tube for a final concentration of 1 mM for individual kavalactones or 0.46 mM for kava extract. The acetone was then allowed to evaporate at room temperature. Control incubation tubes contained no kava extract or kavalactones. Human hepatic microsomes were diluted in 2.5 mM HEPES buffer (pH 7.4) containing 0.15 M KCl, and added to control and treated incubation tubes for a final protein concentration of 1 mg/ml in a volume of 495 μl. Microsomes were then preincubated at 37°C for 10 min. After the addition of NADPH (1 mM final concentration) to treated incubation tubes, absorbance was monitored between 400 and 480 nm at 1, 5, and 10 min.
Statistical Analysis.
The values for the enzyme activities were compared by analysis of variance followed by Dunnett's test. Statistically significant differences were determined at the p < 0.05,p < 0.01, and p < 0.001 levels.
Results
In the present work, the inhibition of human P450 enzymes was determined in vitro using hepatic microsomes. In initial experiments, HLMs were preincubated for 15 min with NADPH and kava extract normalized to 100 μM kavalactones. The activities of CYP2C9, CYP2C19, and CYP3A4 were most markedly affected, and were inhibited 78 to 92% from control (Fig. 1). There were also significant decreases in the activities of CYP1A2 (56%), CYP2D6 (73%), and CYP4A9/11 (65%), whereas the activities of CYP2A6, CYP2C8, and CYP2E1 were not significantly changed. The inhibition of the activities of CYP1A2, 2C9, 2C19, 2D6, and 3A4 were also determined after incubation with kava extract normalized to 1 or 10 μM kavalactones. Statistically significant decreases in the activity of CYP1A2 were observed at kavalactone concentrations of 1 or 10 μM, which resulted in 22 and 36% inhibition, respectively. CYP2C9, 2C19, 2D6, and 3A4 were also significantly inhibited by 53, 30, 22, and 42%, respectively, at a kavalactone concentration of 10 μM.
In subsequent experiments, those P450 enzymes that were most susceptible to inhibition by kava extract were assayed in the presence of individual kavalactones. HLMs were preincubated for 15 min with NADPH and 1 or 10 μM K, DMY, M, or DHM prior to the determination of CYP2C9, 2C19, 2D6, and 3A4 activities. Whereas K did not inhibit these enzymes, there was significant inhibition of CYP2C9 by DMY (42%), M (58%), and DHM (69%); of 2C19 by DHM (76%); of 2D6 by M (44%); and of 3A4 by DMY (40%), M (27%), and DHM (54%) when incubated at a kavalactone concentration of 10 μM.
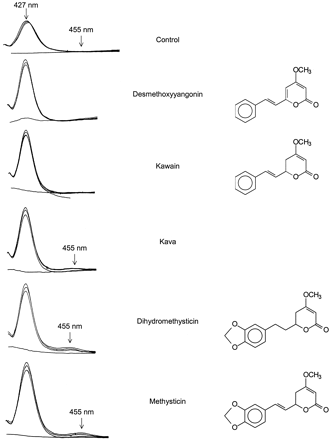
In experiments designed to determine whether kava constituents form MI complexes with P450 enzymes, human hepatic microsomes were incubated in the presence of whole kava extract or individual kavalactones, and the P450 difference spectra were measured from 400 to 480 nm (Fig.2). Microsomes incubated in the absence of any kava constituent exhibited a difference spectrum with a single peak at the absorbance maximum of 427 nm after addition of NADPH. Similar results were obtained with the kava constituents DMY and K. However, incubation of microsomes in the presence of kava extract or its major methylenedioxyphenyl-containing constituents, M and DHM, resulted in the production of a new absorption with a maximum at 455 nm. Production of the 455-nm absorbing complex was both NADPH- and time-dependent, with absorption increasing with increasing incubation time. The classic MI complex-forming agent, piperonyl butoxide (1 mM), produced the same magnitude of 455-nm complex as did DHM and M (data not shown).
Cytochrome P450 difference spectra measured after incubation of human liver microsomes with kava extract or kava constituents, and NADPH.
Discussion
The herb kava has attracted considerable recent attention due to regulatory scrutiny in Europe and the United States. These actions were precipitated by reports of liver failure and reports that the “pattern of (P450) enzymes shifts after ingestion of kava kava” (American Botanical Council, 2001). In the present work, the interaction of human P450 enzymes with kava extract and its constituents was investigated in human liver microsomes. CYP1A2, 2C9, 2C19, 2D6, and 3A4 were all markedly inhibited at a kavalactone concentration of 100 μM; this is the first literature report of the effect of kava and its constituents on P450 enzymes. A number of accounts have provided indirect indications that kava inhibits P450 in vivo, but they have not been interpreted as such. The data herein give pause for a case-by-case reevaluation of the causes of these adverse effects.
In addition to liver failure, and terse “letter to the editor” reports linking hepatotoxicity to low CYP2D6 activity, there are similar reports linking lethargy and coma to concomitant use of benzodiazepines and other central nervous system depressants with kava. The prevailing hypothesis for the latter is that this is due to exaggeration of pharmacological response, consistent with the GABA agonist activity of kavalactones (Jussofie et al., 1994). Based on their observations of poor debrisoquine metabolism in two individuals reporting symptoms of liver damage following kava ingestion, Russmann et al. (2001) postulated that CYP2D6 deficiency is a risk factor for kava hepatotoxicity. However, based on the findings of the current study and the low prevalence of CYP2D6 deficiency in the general population, it would appear that inhibition of CYP2D6 expressed at normal levels, rather than low genotypic expression, alone, could account for the poor debrisoquine metabolism observed in these patients who had consumed kava.
Case reports and pharmacologic studies have indicated a potentiation of the central nervous system effects of benzodiazepines, including alprazolam, in the presence of kava extract and/or kavalactones (Jussofie et al., 1994; Almeida and Grimsley, 1996). Alprazolam clearance in humans correlates with CYP3A4/5 activity (Schmider et al., 1999; D'Souza et al., 2001; Hirota et al., 2001), as does the clearance of other benzodiazepines. Inhibition of benzodiazepine metabolism as a result of decreased CYP3A4 activity may contribute to the additive effects reported upon coadministration of kava and alprazolam. At the outset of these investigations, one of the few reports immediately suggesting inhibition of P450 arose from the observation that coadministration of kava markedly increased hexobarbital sleep time in mice, indicative of inhibition of CYP2C (Meyer, 1962). CYP2C9 metabolizes hexobarbital in humans (Guengerich, 1995), and the extensive inhibition of this family shown herein is consistent with the effects found in mice. CYP2C9 is also important in the metabolism of other barbiturates, nonsteroidal anti-inflammatory drugs, and the anticoagulant warfarin. The risk of severe bleeding due to concomitant ingestion of kava with warfarin, which has a narrow therapeutic index, is but one example of the potential consequences of the inhibition of this and other P450 enzymes.
By 1984, in Vanuatu alone, 72 different cultivars of kava had been identified (Ellis, 1984). Because these cultivars vary greatly in chemical composition and physiological effects, Lebot and Lévesque (1989) characterized them based on their relative concentrations of six kavalactones: desmethoxyyangonin, 7,8-dihydrokawain, yangonin, kawain, dihydromethysticin, and methysticin. Among the cultivars characterized was a variety known in the vernacular of Vanuatu as tudei (“two days”), renowned for its intense physiological effects and an intoxication that lasts for 2 days. This cultivar was found to contain particularly high concentrations of methysticin and dihydromethysticin, kavalactones that contain a methylenedioxyphenyl functional group. In the present studies, incubation of HLMs with either M or DHM resulted in the time- and NADPH-dependent formation of an MI complex with a characteristic absorbance maximum at 455 nm, indicating the inactivation of P450 enzymes. However, the magnitude of the 455-nm absorbance was not pronounced. Since DMY, as well as DHM and M, caused the most pronounced enzyme inhibition, it may be that competitive inhibition is a major contributor to the overall inhibition of P450 activities. Alternatively, displacement of MI complexes by the presence of high concentrations of unmetabolized kavalactone (initial concentration, 1 mM), as has been shown for other lipophilic displacers (Elcombe et al., 1975), may have diminished the amount of complex ultimately measured.
The profound, long-term, and broad spectrum inhibition of human P450 enzymes responsible for the metabolism of >90% of pharmaceuticals poses a significant risk for consumers. The authors suggest that fractionation or commercial development of cultivars containing lesser amounts of methysticin and 7,8-dihydromethysticin, and higher kawain content, could alleviate drug interactions associated with this herb, while preserving many of its beneficial properties.
Acknowledgments
We thank Kathy Ancheta for assistance in preparation of the manuscript, and Drs. Nicholas Oberlies and David Kroll for review of the manuscript.
Footnotes
-
This work was performed under National Institute of Environmental Health Sciences Contract NO1-ES-75407.
- Abbreviations used are::
- DMY
- desmethoxyyangonin
- M
- methysticin
- DHM
- dihydromethysticin
- K
- kawain
- P450
- cytochrome P450
- MI
- metabolic intermediate
- HLM
- human liver microsome
- HPLC
- high-performance liquid chromatography
- Received April 2, 2002.
- Accepted August 5, 2002.
- The American Society for Pharmacology and Experimental Therapeutics