Abstract
The effects of hepatic uptake and efflux transporters on metabolism of digoxin were examined in isolated rat hepatocytes versus microsomes. The metabolic clearance estimated from microsomes was 4.59 ± 0.69 ml/min/kg. However, the metabolic clearance estimated from hepatocytes was 15.9 ± 3.0 ml/min/kg. The former did not correlate with in vivo clearance (12.9 ml/min/kg) for digoxin. Rifampin (an organic anion-transporting peptide 2 inhibitor) or GG918 [GF120918 (N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide)] (a potent P-glycoprotein inhibitor) were used to estimate effects of uptake or efflux transporters on digoxin metabolism. Whereas both inhibitors exerted minimal effects on metabolism in microsomes, rifampin and GG918 significantly decreased and increased digoxin metabolism in hepatocytes, respectively. Concentration-time course studies further demonstrated that, compared with the area under the curve (AUC) of control (15.6 ± 0.1 μM · min), an increase of AUC (20.1 ± 0.5 μM · min, p < 0.005) was observed when digoxin was coincubated with rifampin and a decrease of AUC (14.1 ± 0.1 μM · min, p < 0.01) when GG918 was also present. Digoxin primary metabolite concentrations changed directionally in an inverse manner with parent drug concentrations, as would be expected. These results strongly suggest that the hepatic uptake and efflux transporters that are found in hepatocytes, but not in microsomes, modulate intracellular concentration of digoxin and thus affect metabolism. We conclude that the interplay of transporters and enzymes must be considered in defining the intrinsic metabolic clearance of the liver and in evaluating potential drug-drug interactions.
The liver is a major site of metabolism of many endogenous compounds and xenobiotics since hepatocytes (which comprise 80% of the liver cells) contain large amounts of smooth endoplasmic reticulum, where many metabolizing enzymes reside. These metabolizing enzymes are primarily involved in two major types of processes: redox reactions catalyzed by P450 monooxygenases (phase I) and conjugation with endogenous molecules (phase II). In recent years, much effort in drug discovery and development has focused on defining the metabolic profile and the pharmacokinetics of new chemical entities. A major portion of preclinical development involves characterizing the liver enzymes affecting drug disposition and elimination.
Liver microsome incubations are routinely carried out to survey the metabolism of potential drugs by pharmaceutical companies. Microsomes are vesicles derived from the smooth endoplasmic reticulum that contain an array of phase I and a few phase II enzymes. They are easy to prepare and store; furthermore, the enzymatic activity is relatively reproducible from one experiment to the next within a couple of months of isolation. Intrinsic clearances (Vmax/Km) of compounds are often calculated using microsome incubations in an effort to predict in vivo hepatic clearances. Although in vitro microsome studies are able to predict in vivo hepatic clearances for some compounds, numerous failed attempts have been reported (Naritomi et al., 2001). We hypothesize that hepatic transporters present in hepatocytes, but not in microsomes, are often the missing link explaining such discrepancies between microsome data and in vivo profiles.
Hepatic transporters are roughly categorized into two groups: efflux transporters that are localized on the canalicular (apical) membrane and the more recently discovered uptake transporters that are found on the sinusoidal (basolateral) membrane. Many of the well studied efflux transporters, such as P-glycoprotein (P-gp), MRP2, and BCRP are members of the ATP-binding cassette transporter superfamily. P-gp favors the transport of large hydrophobic molecules that are either neutral or positively charged (Ambudkar et al., 1999). BCRP appears to transport a smaller subset of similar compounds (Allen and Schinkel, 2002). MRP2 is known to mediate the extrusion of some negatively charged compounds, but primarily conjugated compounds such as glutathione, glucuronide, and sulfate conjugates (Renes et al., 2000).
The uptake transporters are members of the solute carrier (Slc) family and are generally categorized into three subfamilies: the organic anion-transporting peptides (OATP in humans/Oatp in rodents), the organic anion transporters (OAT/Oat), and the organic cation transporters (OCT/Oct). Oatp1 (gene symbol: Slc21a1), Oatp2 (Slc21a5), Oatp4 (Slc21a10), Oat2 (Slc22a7), Oat3 (Slc22a8), and Oct1 (Slc22a1) have been demonstrated to be expressed in the liver and localized in the basolateral membrane (Zhang et al., 1997; Shitara et al., 2002) of hepatocytes. Of all the hepatic uptake transporters, the OATP family is the most extensively studied. Whereas OATs transport mainly low molecular weight compounds, OATPs mediate the uptake of much larger and structurally diverse substrates such as digoxin (Noe et al., 1997; Kullak-Ublick et al., 2001) and bilirubin (Cui et al., 2001). Coincidentally, many compounds that are substrates for the uptake and the efflux transporters are substrates for the metabolizing enzymes as well. Our hypothesis is that microsomes alone are insufficient to estimate drug metabolic clearance, because hepatic transporter effects are not taken into account. Instead, freshly isolated hepatocytes will provide a better model to assess drug metabolic clearance and to predict correlations between in vitro and in vivo data.
Digoxin, a cardiac glycoside used clinically for more than 200 years (Antman and Smith, 1985; Heller, 1990), is excreted in humans mainly unchanged by the kidney; however, in the rat, it is extensively metabolized by cyp3a (Salphati and Benet, 1999). Biotransformation of digoxin involves a stepwise hydrolysis of digitoxosides to form digoxigenin bis- and mono-digitoxoside and the aglycone digoxigenin before conjugation and elimination (Harrison and Gibaldi, 1976). Tanigawara et al. (1992) have shown that digoxin is a good substrate for P-gp. Recently, it has been demonstrated that digoxin is a substrate for Oatp2 with a Km value of 240 nM (Shitara et al., 2002; Hagenbuch and Meier, 2003). In this study, we investigated the influence of hepatic transporter on digoxin metabolism in hepatocytes versus microsomes by comparing incubations containing inhibitors of hepatic uptake and efflux transporters versus control digoxin incubations. We also addressed the lack of specificity of many inhibitors. For example, rifampin, used here to inhibit the uptake transporter Oatp2 (Shitara et al., 2002), has recently been suggested to also inhibit P-gp (Zong and Pollack, 2003). We show here that the potent P-gp inhibitor, GG918, at higher concentrations is an effective uptake inhibitor.
Materials and Methods
Materials. Digoxin, digoxigenin (dg0), rifampin, corticosterone, and NADPH were purchased from Sigma-Aldrich (St. Louis, MO). Digoxigenin bis-digitoxoside (dg2) and digoxigenin mono-digitoxoside (dg1) were kind gifts from Professor Emil Lin, University of California, San Francisco (UCSF). [3H]Digoxin (37 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences (Boston, MA). GG918 (GF120918; GlaxoSmithKline, Research Triangle Park, NC) was graciously supplied by the manufacturer. High-performance liquid chromatography-grade methanol and tert-butyl-methyl-ether were purchased from Sigma-Aldrich. Male Wistar rats (200–300 g; Bantin and Kingman, San Leandro, CA) were housed in the UCSF animal care facility with a 12-h light/dark cycle and allowed free access to water and food. Approval of the described studies reported here was obtained from the Committee on Animal Research, UCSF.
Microsome Preparation. Rat liver microsomes were isolated as previously described (Salphati and Benet, 1999). Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) with BSA standards. P450 content was measured using a dual-beam spectrophotometer SLM-AMINCO (DW-2000, UV-visible; Aminco, Rochester, NY).
Hepatocyte Preparation. Rat hepatocytes were prepared using a modified collagenase perfusion method as described previously (Seglen, 1976). In brief, anesthesia was induced by intraperitoneal injection with a 1 ml/kg dose of ketamine/xylazine (80 mg/ml, 12 mg/ml) before surgery. The portal vein was cannulated with an i.v. catheter (catalog number 2007-04; BD Biosciences, San Jose, CA) and perfused with oxygenated liver perfusion buffer (Invitrogen, Carlsbad, CA) for 5 min at 30 ml/min, followed by perfusion with an oxygenated hepatocyte washing buffer (Invitrogen) modified with 2 mM l-glutamine, 10 mM Hepes, and 1.2 U/ml collagenase (Sigma-Aldrich) for 5 min at 20 ml/min. At the end of the perfusion, Glisson's capsule of the digested liver was excised with scissors. The liver tissue was broken down in ice-cold buffer by gentle tapping with a glass stirring rod. The hepatocytes were washed twice with an ice-cold hepatocyte wash buffer containing 2 mM l-glutamine and 10 mM Hepes and were centrifuged at 50g for 2 to 3 min. Cell viability was determined using the trypan blue exclusion method. Cells with viability of greater than 90% were used for further studies.
Microsome Incubations. Incubation conditions were as previously described (Salphati and Benet, 1999). In brief, each reaction contained 0.5 mg/ml microsomes, 1 mM NADPH, 10 μM digoxin, various concentrations of inhibitors, and 0.1 M phosphate buffer (pH 7.4). The dimethyl sulfoxide concentration, used to solubilize substrates and inhibitors, was less than 1%. The total reaction volume was 250 μl, and the incubation period was 15 min, a time period shown previously to be in the linear region of the metabolite formation (Salphati and Benet, 1999). For each sample, the reaction was stopped by protein precipitation through addition of an equal volume of methanol containing 5 μg/ml of the internal standard, corticosterone. The supernatants were stored in -80°C before LC/MS analysis.
Hepatocyte Incubations. Hepatocyte incubations were carried out immediately after cell isolation. For Michaelis-Menten studies, 2 million hepatocytes in Krebs-Henseleit buffer (pH 7.4), containing 0.21 g/l of sodium bicarbonate and supplemented with 1% BSA and 10 mM glucose, were used in each incubation in an uncapped 20-ml glass vial. Reactions were carried out at 37°C in a water bath at a constant shaking speed of 100 rpm for 30 min. At the end of 30 min, an aliquot of hepatocytes was removed from the incubations containing the highest drug concentrations to determine drug effects on cell viability using the trypan blue exclusion method. Incubations were stopped by quick-freezing samples in a methanol/dry ice bath and stored at -80°C. Stored samples were frozen and thawed three times to lyse the cells. Twice the sample volume of tert-butyl-methyl-ether containing 5 μg/ml of corticosterone was added to each sample and vortexed. The sample mixtures were then centrifuged at 2000g for 10 min. After quick-freezing the aqueous layer in a methanol/dry ice bath, the organic layer was poured into a new tube and evaporated under nitrogen gas. Each sample was reconstituted with 300 μl of methanol for LC/MS analysis.
Hepatocyte Uptake Studies. Before each incubation, 2 million hepatocytes were prewarmed in Krebs-Henseleit buffer (pH 7.4) containing 0.21 g/l of sodium bicarbonate and supplemented with 1% BSA and 10 mM glucose for 5 min. For incubation studies, [3H]digoxin (100 nM) with and without 100 μM rifampin, 1 μM GG918, or 25 μM GG918 was added to the cells. To determine kinetic parameters of digoxin hepatocyte uptake, unlabeled digoxin (0–500 μM) was added to the incubations. After 2 min, the reactions were terminated by transferring 1 million hepatocytes into a centrifuge tube containing 150 μl of 2 N NaOH under a layer of a 500-μl mixture of silicone oil and mineral oil (Shitara et al., 2003) and centrifuged at 13,000g for 10 s. The spun down cell pellets were left at 65°C overnight to ensure complete lysis. For the inhibition studies, after removing the oil layer, 150 μl of 2 N HCl and scintillation cocktail were added to each sample, and radioactivity was measured using a scintillation counter (LS6000TA; Beckman Coulter, Fullerton, CA). For the kinetic parameter studies, after removing the oil layer, the cell pellets were resuspended in water and sonicated for 30 min. Two hundred microliters of methanol containing internal standard were added to each sample and centrifuged for 15 min at 13,000 rpm. The supernatants were transferred to high-performance liquid chromatography vials for LC/MS analysis.
Time Course Studies. Incubations were carried out in 50-ml round-bottom flasks with continuous rotation while being gassed with 95% O2/5% CO2 at 37°C (Li et al., 2002). Aliquots of 2 million hepatocytes in Krebs-Henseleit buffer (pH 7.4) containing 2.73 g/l of sodium bicarbonate and supplemented with 1% BSA and 10 mM glucose were taken at 5, 10, 15, 20, 30, 45, and 60 min. At the end of 60 min, an aliquot of hepatocytes was removed from each incubation to determine drug effects on cell viability using the trypan blue exclusion method. Each aliquot was quick-frozen in a methanol/dry ice bath to stop the reaction and stored at -80°C. Sample preparation was the same as that described earlier for hepatocyte incubations.
Assay of Digoxin and Its Metabolites. Digoxin and its metabolites were assayed using LC/MS as previously described (Lau et al., 2004).
Clearance Calculations and Data Analysis. Microsomal and hepatocyte digoxin metabolite formation rates as a function of digoxin concentration were fitted using the Hill equation from which values for Vmax, K50, and the Hill coefficient were determined. For the microsomal studies, Vmax was converted from the measured nmol/min/mg protein to per kilogram body weight using the standard scaling factors of 44.8 mg of microsomal protein/g liver and a liver weight of 40 g/kg body weight (Naritomi et al., 2001). For the hepatocyte studies, scaling on the basis of 120 × 106 hepatocytes/g liver (Bayliss et al., 1999) was used. For the hepatocyte uptake studies, the rates of uptake (v) at different substrate (S) concentrations were fitted to the equation v = [Vmax · S/(Km + S)] + Pdif · S, where Pdif is the passive permeability value. In vivo blood metabolic clearance values were predicted from both microsome and hepatocyte Michaelis-Menten values using the well stirred model with a hepatic blood flow of 55.2 ml/min/kg (Naritomi et al., 2001) and a fraction unbound of 0.75 (Harrison and Gibaldi, 1976). Since Hinderling (1984) found in vivo blood cell to plasma partition coefficients to be 0.943 ± 0.154, the total blood to plasma ratio would not differ by more than 3% from 1.0, and no correction was utilized. p values were computed using two-tailed Student t tests.
Results
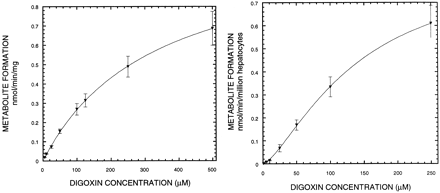
Michaelis-Menten Study of Digoxin using Rat Microsomes and Hepatocytes. Results of the Michaelis-Menten studies of digoxin using both rat microsomes and hepatocytes are shown in Fig. 1. P450 content was determined by the carbon monoxide (CO) spectrum assay (Omura and Sato, 1964) and found to be 27.4 nmol/mg microsome (data not shown). Metabolites dg2, dg1, and dg0 were monitored, but since the amounts of dg1 and dg0 contributed less than 5% of the total metabolites observed, only dg2 profiles were used for all further calculations. From the microsome studies (n = 3), Vmax and K50 of digoxin were calculated using nonlinear regression (WinNonlin; Pharsight, Mountain View, CA) of the Hill equation to be 1.12 ± 0.33 nmol/min/mg protein and 310 ± 123 μM (mean ± S.D.), respectively, with a Hill coefficient not significantly different from unity (1.03 ± 0.03). Intrinsic clearance (Vmax/K50) was 3.73 ± 0.56 μl/ml/mg protein. Using the well stirred model, the standard scaling factors of 44.8 mg of microsomal protein/g liver, liver weight of 40 g/kg body weight, liver blood flow of 55.2 ml/min/kg (Naritomi et al., 2001), and fraction unbound of 0.75 (Harrison and Gibaldi, 1976), the hepatic metabolic clearance based on the microsome results was estimated to be 4.59 ± 0.69 ml/min/kg.
Michaelis-Menten study of digoxin. Microsome incubations (A) and hepatocyte incubations (B) of digoxin. Each curve is fitted using the Hill equation. Data are depicted as the mean ± S.D., n = 3.
Comparable data for hepatocytes (n = 3) were 0.94 ± 0.34 nmol/min/106 cells for Vmax and 159 ± 83 μM for K50, with a Hill coefficient of 1.47 ± 0.25. Here a value of 6.21 ± 1.16 μl/min/106 cells was calculated for apparent intrinsic clearance. Scaling on the basis of 120 × 106 hepatocytes/g liver (Bayliss et al., 1999) and the parameters listed above provided an estimated apparent hepatic metabolic clearance of 15.9 ± 3.0 ml/min/kg, a 3.5-fold higher value than that derived from the microsomal data.
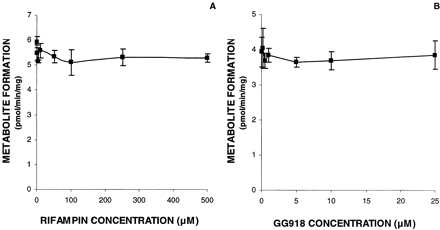
Effects of Rifampin and GG918 on Digoxin Metabolism in the Microsome System. To determine whether rifampin or GG918 inhibits CYP3A activity, microsome incubations with each inhibitor were carried out, as shown in Fig. 2. Compared with control, both inhibitors (rifampin and GG918) exerted no statistically significant effects on digoxin metabolism over 15 min.
Microsome incubation of digoxin with inhibitors. Inhibition study of rifampin (A) and GG918 (B) on digoxin metabolism to dg2 in the microsome system. The digoxin concentration used was 10 μM. Lines are drawn point-to-point. Data are depicted as the mean ± S.D., n = 3.
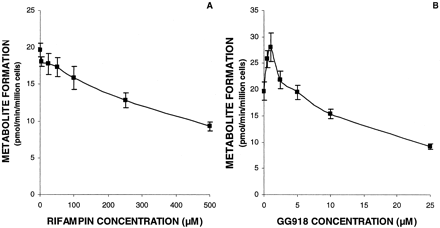
Effects of Rifampin and GG918 on Digoxin Metabolism in the Hepatocyte System. To determine the effects of hepatic uptake and efflux transporters on digoxin metabolism, rifampin (an Oatp and Oat inhibitor) and GG918 (a very potent P-gp inhibitor) were coadministered in the hepatocyte systems. Rifampin markedly reduced digoxin metabolism in the hepatocyte incubations (Fig. 3A). Interestingly, with GG918, a biphasic response was noted; at concentrations at or below 1 μM, digoxin metabolism was increased, and at concentrations above 1 μM GG918, digoxin metabolism was decreased (Fig. 3B). An IC50 value was estimated using nonlinear regression (WinNonlin). The Ki value was estimated by IC50/[1 + S/K50]. The apparent IC50 and Ki values of rifampin were 478 ± 53 μM and 451 ± 50 μM. The apparent IC50 and Ki values of GG918 (at concentrations above 1 μM) were 4.64 ± 0.49 μM and 4.38 ± 0.46 μM. Cell viability determined at the end of incubations was above 90%.
Hepatocyte incubations of digoxin with inhibitors. Inhibition study of rifampin (A) and GG918 (B) on digoxin metabolism to dg2 by hepatocytes. The digoxin concentration used was 10 μM. Lines are drawn point-to-point. Data are depicted as the mean ± S.D., n = 3.
Uptake of Digoxin in Hepatocytes. The values for Vmax, Km, and Pdif were 162 ± 10 pmol/min/million cells, 36.2 ± 8.7 μM, and 1.99 ± 0.03 μl/min/million cells, respectively.
Effects of Rifampin and GG918 on Uptake of [3H]Digoxin in Hepatocytes. The effects of rifampin and GG918 on digoxin uptake in the freshly isolated rat hepatocytes are shown in Fig. 4. Compared with the digoxin only control, coadministration of 100 μM rifampin significantly reduced uptake to 23 ± 6.5% of the control, p < 0.0005. Coadministration of 1 μM GG918 slightly increased digoxin uptake to 104 ± 4%. However, coadministration of 25 μM GG918 inhibited uptake to 42.2 ± 12.2%, p < 0.005.
Uptake study of digoxin in hepatocytes. Hepatocytes (2 × 106) were incubated for 2 min with 100 nM [3H]digoxin in the absence and presence of 100 μM rifampin (A) or 1 μM and 25 μM GG918 (B). Data are depicted as the mean ± S.D., n = 3.
Time Course Studies of the Effects of Rifampin and GG918 on Digoxin Metabolism in Hepatoyctes. Time course studies over 60 min were carried out to investigate the effects of rifampin and GG918 on digoxin metabolism (hepatocyte and supernatant) as illustrated in Fig. 5. The parent compound profile showed that coadministration of 100 μM rifampin with 200 nM digoxin resulted in a significant increase in digoxin concentration and a significant decrease in metabolite formation compared with the control without addition of rifampin. In contrast, coadministering of 0.5 μM GG918 with digoxin significantly reduced parent drug concentration and increased metabolism compared with the control without GG918. Using the parent compound profile, the areas under the curve (AUCs), over 60 min, of digoxin alone, digoxin with rifampin, and digoxin with GG918 were calculated to be 15.6 ± 0.1 μM · min, 20.1 ± 0.5 μM · min (p < 0.005), and 14.1 ± 0.1 μM · min (p < 0.01), respectively, an increase of 28.8% with rifampin and a reduction of 9.6% with GG918. At 60 min, the dg2 concentration in the presence of rifampin decreased 56.0% relative to the control versus 9.5% increase relative to the control for GG918. Cell viability determined at the end of incubations was above 90%.
Time course studies of digoxin with and without inhibitors. A time course study of digoxin metabolism with and without transporter inhibitors in rat hepatocytes. A, profile of the parent compound. ○, digoxin alone; □, coadministration of digoxin and rifampin; ▵, coadministration of digoxin and GG918. B, profile of metabolite (dg2). •, digoxin alone; ▪, coadministration of digoxin and rifampin; ▴, coadministration of digoxin and GG918. Data are depicted as the mean ± S.D., n = 3. ⋆, p < 0.05; ⋆⋆, p < 0.01; ⋆⋆⋆, p < 0.001.
Discussion
With increasing awareness of the effects of hepatic transporters on drug disposition, our laboratory has investigated their role and importance in the overall drug metabolism process. Based on a model cellular system containing human P-gp and CYP3A4 (Cummins et al., 2001), we showed that inhibiting P-gp, with no effect on CYP3A activity, reduces CYP3A metabolism in the apical to basolateral direction, mimicking the gut, but increases CYP3A metabolism in the basolateral to apical direction, mimicking the liver (Cummins et al., 2002a, 2004; Benet et al., 2003). We suggested that this inverse orientation (i.e., the drug encounters the enzyme CYP3A before the efflux transporter P-gp in the liver, which is reversed in the intestine) could explain the increased metabolic clearance observed for CYP3A/P-gp dual substrates in women versus men (Cummins et al., 2002b). In that paper, we reviewed the seminal studies of Lan et al. (2000), who showed that in P-gp knockout mice, CYP3A metabolism of erythromycin was increased when P-gp was absent; and we reported that increased hepatic metabolism was also observed for paclitaxel, vinblastine, and doxorubicin in knockout mice versus controls. More recently, using the isolated perfused rat intestine (Cummins et al., 2003) and isolated perfused rat liver (IPRL) (Wu and Benet, 2003), we confirmed the results hypothesized from the cellular system described above. The increased metabolism of digoxin in the IPRL during P-gp inhibition and the decrease of digoxin metabolism in the IPRL during Oatp2 inhibition (Lau et al., 2004) were confirmed.
The results reported here demonstrate that the conventional way of defining liver intrinsic clearance using a microsome incubation profile is not sufficient. Here we demonstrate that the freshly isolated hepatocytes that maintain uptake and efflux transporters should be a better model for defining intrinsic clearance. A pharmacokinetic study conducted in our laboratory (Salphati and Benet, 1998) measured total clearance of digoxin to be 37 ± 11 ml/min/kg. An earlier study by Harrison and Gibaldi (1976) reported a total digoxin clearance to 28.0 ml/min/kg, with a metabolic clearance of 12.9 ml/min/kg. The apparent metabolic clearance predicted using hepatocytes (15.9 ± 3.0 ml/min/kg) in the present study appears to be a much better approximation of the measured in vivo value. This hepatocyte value is 3.5-fold greater than the metabolic clearance calculated using microsomes (4.59 ± 0.69 ml/min/kg), which is a counterintuitive finding since it would be expected that drug access to the metabolizing enzymes would be enhanced in microsomes.
To demonstrate the effects of hepatic uptake and efflux transporters on digoxin metabolism, rifampin, an inhibitor of Oat/Oatp, and GG918, a well established P-gp inhibitor, were administered. The results of the digoxin uptake study using freshly isolated hepatocytes showed that 100 μM rifampin effectively inhibited the uptake of [3H]digoxin by more than 75%. The remaining 25% of digoxin that is found intracellularly is probably due to passive diffusion or uptake mediated by other transporters. Figure 3A demonstrates that increasing the concentration of rifampin decreased digoxin metabolism in hepatocytes, whereas no effect of rifampin was observed in microsomes (Fig. 2A). GG918 also had no effect on digoxin metabolism in microsomes (Fig. 2B), whereas in hepatocytes, GG918 concentrations up to 1 μM increased digoxin metabolism, but higher concentrations caused decreased metabolism (Fig. 3B). Many studies have documented the potent inhibition by GG918 of the efflux transporter P-gp, with a Ki < 0.5 μM (Annaert et al., 2001; Luo et al., 2002; Savolainen et al., 2002; Wu and Benet, 2003), but the results here suggest that GG918 may also effectively (Ki = 4.38 ± 0.46 μM) inhibit Oatp2, or another hepatic uptake transporter for digoxin. This lack of specificity of transporter inhibition does not appear to be unique to GG918, and our study should provide a caution to investigators to test alternate inhibitory (and activating) potential effects beyond that being investigated. Here, we chose to utilize 0.5 μM GG918 in the time course studies as our efflux inhibitory example, but we recognize that some Oatp2 inhibition may occur. The determination of apparent IC50 and Ki values validate that the metabolism differences observed between microsome and hepatocyte incubations are due to uptake and efflux transporters that are present in hepatocytes but not in microsomes.
Time course studies demonstrated that over 60 min, metabolism was impaired by 100 μM rifampin and enhanced by 0.5 μM GG918 (Fig. 5). Since rifampin limits the amount of digoxin entering the hepatocytes, we expected that less drug would be available for metabolism and more parent drug would be found in the cell suspension compared with the control without addition of rifampin. Conversely, we expected that inhibition of P-gp by GG918 would result in an accumulation of digoxin intracellularly, thus increasing its exposure to the metabolizing enzyme and leading to an increase in metabolite formation and a decrease in parent drug concentration compared with that of digoxin-alone control. We see that 100 μM rifampin does decrease hepatic uptake markedly at 2 min (Fig. 4A), consistent with the decreased metabolism of digoxin over the 60-min time course (Fig. 5). A slight but nonsignificant increase in digoxin hepatic uptake is noted with 1 μM GG918 at 2 min (Fig. 4B). This lack of a significant increase is not surprising due to the relatively small overall change found for digoxin and dg2 (∼9.5%) over 60 min, and the fact that the intracellular increase is counteracted by the increased metabolism. However, the recognition that GG918 also possesses hepatic uptake inhibitory properties (Figs. 3 and 4) further complicates the analysis.
The total AUC (hepatocytes plus supernatant) for coadministration of rifampin with digoxin is significantly (p < 0.005) higher (20.1 ± 0.5 μM) than that of digoxin alone (15.6 ± 0.1 μM). The AUC for coadministration of GG918 with digoxin is significantly (p < 0.01) lower (14.1 ± 0.1 μM) than that of the control (15.6 ± 0.1 μM). These directional AUC changes for parent digoxin and the inverse findings for the dg2 metabolite are consistent with the results from ex situ perfused rat liver studies by our laboratory (Lau et al., 2004). These results strongly support our hypothesis that the hepatic uptake and efflux transporters, Oatp2 and P-gp, are responsible for the altered digoxin metabolism.
In conclusion, hepatic uptake and efflux transporters play important roles in drug disposition and metabolism. Hepatic intrinsic clearance may be better defined by using hepatocytes (or other methodology that preserves the transporter/enzyme architecture of the liver) rather than microsomes, so that transporter effects are taken into account. Uptake and efflux transporters, such as Oatp2 and P-gp, may modulate a compound's metabolism by altering its accessibility to the metabolizing enzymes. Furthermore, inhibitors of an efflux transporter may also affect uptake. The interplay of transporters and enzymes must be considered in defining the intrinsic metabolic clearance of the liver (and other organs such as intestine and kidney) and in evaluating potential drug-drug interactions.
Acknowledgments
We appreciate the assistance of Drs. Carolyn Cummins and Muhammad Baluom with analytical methods. We thank Professor M. Almira Correia for helpful discussions.
Footnotes
-
This study was supported in part by National Institutes of Health Grants HD40543 and GM61390 and in part through facilities of the University of California, San Francisco Liver Center (DK 26743).
-
ABBREVIATIONS: P450, cytochrome P450;P-gp, P-glycoprotein; Slc, solute carrier; Oatp, organic anion-transporting polypeptide; Oat, organic anion transporter; Oct, organic cation transporter; GG918, GF120918 (N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide); BSA, bovine serum albumin; LC/MS, liquid chromatograph/mass spectrometry; AUC, area under the curve; dg2, digoxigenin bis-digitoxoside; dg1, digoxigenin mono-digitoxoside; dg0, aglycone digoxigenin; IPRL, isolated perfused rat liver.
- Received December 1, 2003.
- Accepted August 17, 2004.
- The American Society for Pharmacology and Experimental Therapeutics