Abstract
Primary hepatocyte cultures are considered as a useful in vitro system for pharmacological/toxicological studies. Although expression of drug-metabolizing enzymes and canalicular drug transporters has been well documented in this cellular model, less information is available about sinusoidal drug transporter activities. This has led us to investigate functional expression of the major sinusoidal transporters in primary human and rat hepatocytes. Using radiolabeled substrates and chemical transporter inhibitors, activities of organic cation transporter 1, organic anion-transporting polypeptides, organic anion transporter 2, and Na+-taurocholate cotransporter were detected in cultured human and rat hepatocytes. In parallel, mRNA expression of these transporters was demonstrated using reverse transcriptase-quantitative polymerase chain reaction assays. Functional expression of sinusoidal transport proteins markedly decreased with time in primary rat hepatocyte cultures; by contrast, it remained relatively constant in primary human hepatocytes all along the culture, illustrating the fact that liver-specific functions, including drug-detoxifying pathways, are usually better preserved in cultured human hepatocytes than in their rodent counterparts. Primary hepatocytes, especially human hepatocytes, thus exhibit a pattern of sinusoidal transporter expression close to that found in vivo, highlighting the interest of hepatocyte cultures for drug detoxification studies.
Membrane transport proteins belonging to the solute carrier (SLC) superfamily of transporters play a major role in the initial sinusoidal influx of drugs to hepatocytes (van Montfoort et al., 2003). Organic cation transporters (OCT1/SLC22A1 and Oct1/Slc22a1) thus mediate the transport of small organic cations such as tetraethylammonium (TEA) (Jonker and Schinkel, 2004). Organic anion-transporting polypeptides (OATP-A/SLCO1A2, OATP-B/SLCO2B1, OATP-C/SLCO1B1, and OATP8/SLCO1B3 in human hepatocytes and Oatp1/Slco1a1, Oatp2/Slco1a4 and Oatp4/Slco1b2 in rat hepatocytes) are implicated in sodium-independent uptake of organic anions such as estrone-3-sulfate (ES), biliary acids, the antihistamine fexofenadine, and the cholesterol-lowering pravastatin (Kim, 2003); some of these OATPs also transport amphipathic compounds like digoxin, handled by OATP8 and Oatp4. Organic anion transporters (OAT2/SLC22A7 and Oat2/Slc22a7) mediate the influx of organic anions like para-aminohippurate (PAH) and salicylates (Sekine et al., 1998), whereas the Na+-taurocholic acid-cotransporting polypeptides (NTCP/SLC10A1 and Ntcp/Slc10a1) are involved in Na+-dependent uptake of biliary acids such as taurocholate (Trauner and Boyer, 2003).
Primary hepatocytes represent a major in vitro model for studying the activity and regulation of liver-detoxifying pathways and can therefore contribute to prediction of hepatic elimination of xenobiotics (Modriansky et al., 2000; Gebhardt et al., 2003). Such a liver cell culture system has been extensively characterized with respect to expression and activity of drug-metabolizing enzymes and canalicular transporters (Fardel et al., 1993; Payen et al., 2000; Gomez-Lechon et al., 2003; Annaert and Brouwer, 2005). By contrast, activity of sinusoidal membrane transport proteins has, as yet, been less studied, to our knowledge, in cultured hepatocytes, especially in primary human hepatocytes, although these influx transporters play a crucial role in xenobiotic pharmacokinetic and drug-drug interactions (Chandra and Brouwer, 2004; Shitara et al., 2005). The present study was therefore designed to investigate functional expression of the major sinusoidal transporters in primary hepatocyte cultures. Owing to known interspecies differences in liver-detoxifying pathways, this work was conducted with both human and rat hepatocytes.
Materials and Methods
Chemicals. [3H(G)]Taurocholic acid (specific activity 1.19 Ci/mmol), [6, 7-3H(N)]ES (specific activity 57.30 Ci/mmol), [3H]PAH (specific activity 4.18 Ci/mmol), and [1-14C]TEA (specific activity 2.4 mCi/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Probenecid, verapamil, and dexamethasone were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Isolation and Culture. Hepatocytes from adult male Sprague-Dawley rats weighing 150 to 200 g were isolated by a perfusion of the liver as previously described (Fardel et al., 1993), except that Liberase, a mixture of purified collagenases and proteases, was used for dissociation of liver cells instead of collagenase. Human hepatocytes from adult donors undergoing resection for primary and secondary tumors were obtained by perfusion of histologically normal liver fragments using a collagenase solution (Payen et al., 2000). All experimental procedures complied with French laws and regulations and were approved by the National Ethics Committee. Cells were seeded at a density of 105 cells/cm2 in plastic dishes in Williams' E medium supplemented with 2 mM glutamine, 1 mg/ml bovine serum albumin, 1 μg/ml bovine insulin, 10 U/ml penicillin, 10 μg/ml streptomycin, and 10% (v/v) fetal calf serum. The medium was discarded 4 h (for rat hepatocytes) or 24 h (for human hepatocytes) after seeding and cells were thereafter maintained in serum-free medium supplemented with 10-7 M dexamethasone as previously reported (Fardel et al., 1993).
Drug Transport Assays. Activity of sinusoidal drug transporters was evaluated through determination of cellular accumulation of radiolabeled substrates in the presence or absence of transporter inhibitors as previously described (Lecureur et al., 1998). Briefly, hepatocyte monolayers were washed with transport assay buffer (Payen et al., 2000) consisting of 5.3 mM KCl, 1.1 mM KH2PO4, 0.8 mM MgSO4, 1.8 mM CaCl2, 11 mM d-glucose, 10 mM HEPES, and 136 mM N-methyl glucamine (Na+-free buffer) or 136 mM NaCl (Na+-containing buffer) and adjusted to pH 7.4. Cells were then incubated with transport assay buffer supplemented with transporter substrates, i.e., 0.2 μM [3H]taurocholate, 1.7 nM [3H]ES, 0.2 μM [3H]PAH, or 40 μM [14C]TEA handled by NTCP/Ntcp, OATPs/Oatps, OAT2/Oat2, and OCT1/Oct1, respectively (Faber et al., 2003). These incubations were performed at 37°C in the presence or absence of Na+, the OCT1/Oct1 inhibitor verapamil (Jonker and Schinkel, 2004), or probenecid, known to block organic anion transporters such as OATPs/Oatps and OAT2/Oat2 (Shitara et al., 2005). After a 30-min incubation, cells were washed twice with ice-cold phosphate-buffered saline lysed in distilled water, and accumulation of radiolabeled substrates was determined through scintillation counting. Data were then normalized to amounts of total protein quantified by Bradford's method (Bradford, 1976), using the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
RNA Isolation and RT-qPCR Analysis. Total RNA was isolated from cells using the TRIzol method. Total RNA (2 μg) was then subjected to reverse transcription-quantitative polymerase chain reaction (RT-qPCR) using the fluorescent dye SYBR Green methodology and an ABI Prism 7000 detector (Applied Biosystems, Foster City, CA), as previously described (Piton et al., 2005). Gene-specific primers, shown in Table 1, were designed with Primer3 software (http://frodo.mi.edu/cgi-bin/primer3/primer3_www.cgi); known intron-exon boundary information was taken into account for each target gene to avoid detection of genomic DNA. Moreover, the specificity of each gene amplification was verified at the end of each qPCR by analysis of dissociation curves of the PCR products. The curves of amplification were read with ABI Prism 7000 SDS software using the comparative cycle threshold method. Relative quantification of the steady-state target mRNA levels was calculated after normalization of the total amount of cDNA tested to an 18S RNA endogenous reference.
Primer sequences for RT-qPCR assays
Statistical Analysis. Data were analyzed with Student's t test or with the multirange Dunnett's t test. The level of significance was P < 0.05.
Results and Discussion
The present study was designed to investigate functional expression of sinusoidal drug transporters in cultured hepatocytes, which constitute a useful in vitro model for pharmacological and toxicological analyses (Gomez-Lechon et al., 2003). Human and rat primary hepatocytes were first cultured for 3 days, and expression of drug transporters was analyzed at mRNA levels using RT-qPCR assays. As indicated in Fig. 1, primary human hepatocytes were found to exhibit a pattern of transporter expression close to that found in freshly isolated counterparts. Indeed, they exhibited similar or only moderately reduced levels of OCT1, OATP-B, NTPC, and OAT2 mRNAs; OATP-C and OATP8 expression, although more importantly decreased when compared with those found in isolated hepatocytes, remained notable. Only OATP-A mRNAs, present in freshly isolated human hepatocytes, were not detected at all in primary human hepatocytes. Although OATP-A was initially cloned from human liver, it is noteworthy that it is predominantly expressed in cerebral capillary endothelial cells at the blood-brain barrier (Gao et al., 2000) and its substrate specificity overlaps with that of OATP-C (Kullak-Ublick et al., 2001), indicating that OATP-A may contribute, in fact, in a minor way to drug uptake in the liver. In contrast to primary human hepatocytes, primary rat hepatocytes exhibited a more altered pattern of transporter mRNA expression when compared with freshly isolated counterparts. Indeed, although remaining detectable, expression of Oct1, Oatp1, Oatp2, Oatp4, Ntcp and Oat2 mRNAs was markedly decreased in cultured hepatocytes when compared with freshly isolated cells (Fig. 1). This reduced expression of sinusoidal drug transporters in cultured rat hepatocytes has also been documented at the protein level for Ntcp and Oatp2 (Rippin et al., 2001). By contrast, mRNA levels of the biliary efflux pump MRP2 remained elevated in rat hepatocyte cultures (Rippin et al., 2001), whereas expression of the canalicular transporter P-glycoprotein has been shown to be induced (Fardel et al., 1992). Taken together, these data suggest opposite regulation of sinusoidal and biliary drug transporters in primary rat hepatocytes.
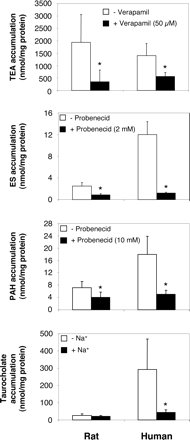
We next investigated activity of sinusoidal membrane transporters in 3-day-old primary human and rat hepatocytes through measurement of intracellular accumulation of radiolabeled substrates in the absence or presence of transporter inhibitors (for OCT1/Oct1, OATPs/Oatps, and OAT2/Oat2) or of Na+ (for NTCP/Ntcp). As indicated in Fig. 2, primary human hepatocytes exhibited activity, i.e., an inhibitor-sensitive or Na+-dependent accumulation of substrates, of OCT1, OATPs, OAT2, and NTCP. Activity of Oct1, Oatps, and Oat2 was also detected in 3-day-old rat hepatocytes (Fig. 2). However, probenecid-inhibitable uptake of the generic OATP/Oatp substrate ES was about 10-fold lower in rat hepatocytes than in human hepatocytes. Rat hepatocytes similarly displayed reduced probenecid-sensitive accumulation of PAH, a substrate for both OAT2/Oat2, when compared with their human counterparts, and Na+-dependent accumulation of the NTCP/Ntcp substrate taurocholate, present in cultured human hepatocytes, was barely, if at all, detectable in rat hepatocytes (Fig. 2). These data suggest that 3-day-old cultured rat hepatocytes displayed lower sinusoidal drug transporter activities than their human counterparts, which likely agrees with their reduced expression of transporter mRNAs described above (Fig. 1).
Expression of sinusoidal membrane transporters in 3-day-old primary human and rat hepatocytes. Total RNAs were prepared from freshly isolated hepatocytes and 3-day-old primary rat and human hepatocytes. mRNA levels of sinusoidal drug transporters were then analyzed using RT-qPCR assays as described under Materials and Methods. Data shown correspond to transporter mRNA levels in 3-day-old primary hepatocytes and are expressed as the percentage of those found in freshly isolated hepatocytes, arbitrarily set at 100%. They represent the means ± S.D. of three experiments performed on independent hepatocyte preparations from different human liver donors and different Sprague-Dawley rats.
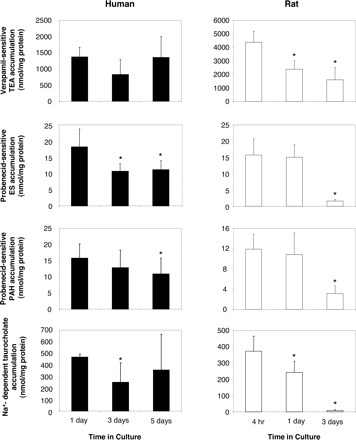
It is noteworthy that culture time has been shown to be a critical parameter for the functional expression of liver-specific functions, including drug detoxication pathways such as cytochromes P450, in primary rat hepatocytes; by contrast, human primary hepatocytes exhibit higher survival and better preservation of differentiated functions (Guillouzo et al., 1993; Mitaka, 1998; Modriansky et al., 2000). This difference may contribute to the relatively low level of sinusoidal drug transporter activities in 3-day-old cultured rat hepatocytes when compared with that found in their human counterparts. To test this hypothesis, transporter functional assays were performed in rat hepatocytes maintained in culture for 4 h, 1 day, and 3 days, whereas human primary cultures were analyzed 1, 3, and 5 days after cell seeding (Fig. 3). Verapamil-inhibitable accumulation of the OCT1/Oct1 substrate TEA was found to significantly diminish with time in culture in rat hepatocytes, but not in human counterparts. Probenecid-sensitive accumulation of the OATP/Oatp substrate ES and probenecid-inhibitable accumulation of the OAT2/Oat2 substrate PAH also markedly decreased in 3-day-old cultured rat hepatocytes when compared with ES and PAH uptake in rat hepatocytes maintained in culture for shorter times (4 h and 1 day); by contrast, they decreased only slightly, although significantly, in primary human hepatocytes along the culture (Fig. 3). Ntcp-mediated uptake of taurocholate was markedly down-regulated during primary culture in rat hepatocytes. On the contrary, Na+-related uptake of taurocholate remained relatively high in primary human hepatocytes along the culture; it was only slightly diminished in 3-day-old cultured human hepatocytes when compared with 1-day or 5-day-old primary hepatocytes (Fig. 3). These data therefore likely add sinusoidal drug transporters to the list of liver-specific functions that are better preserved with culture time in primary human hepatocytes than in their rat counterparts. This may reflect the fact that human hepatocytes are less sensitive than rat cells to the loss of their physiological environment (Mitaka, 1998). In this context, it is noteworthy that culturing rat hepatocytes in conditions mimicking their in vivo environment, i.e., in the presence of extracellular matrix components, has been demonstrated to improve expression of drug transporters (Luttringer et al., 2002; Hoffmaster et al., 2004). In the same way, we have found that the addition of dimethyl sulfoxide to culture medium, well known to favor the expression of differentiated functions in cultured hepatocytes (Isom et al., 1985), increased Ntcp and Oatp transport activities in primary rat hepatocytes (data not shown).
Sinusoidal membrane transporter activities in 3-day-old primary rat and human hepatocytes. Primary 3-day-old rat and human hepatocytes were incubated with 40 μM [14C]TEA, 1.7 nM [3H]ES, 0.2 μM [3H]PAH, and 0.2 μM [3H]taurocholate, handled by OCT1/Oct1, OATPs/Oatps, OAT2/Oat2, and NTCP/Ntcp, respectively. These incubations were performed as described under Materials and Methods in the presence or absence of verapamil (an inhibitor of OCT1/Oct1), probenecid (an inhibitor of OATPs/Oatps and OAT2/Oat2), or Na+ (for NTCP/Ntcp-related transport activity). Cells were then lysed in distilled water, and intracellular accumulation of radiolabeled substrates was determined by scintillation counting and expressed as nmol/mg protein. Data represent the means ± S.D of four to six experiments realized in triplicates and performed on independent hepatocyte preparations from different human liver donors and different Sprague-Dawley rats. *, P < 0.05 when compared with substrate accumulation in the absence of inhibitor or Na+.
Time-profile of sinusoidal membrane transporter activities in primary rat and human hepatocytes. Rat and human hepatocytes, cultured for various lengths of time (4 h, 1, day, and 3 days for rat cells and 1, 3, and 5 days for human cells), were incubated with 40 μM [14C]TEA, 1.7 nM [3H]ES, 0.2 μM [3H]PAH, and 0.2 μM [3H]taurocholate, substrates for OCT1/Oct1, OATPs/Oatps, OAT2/Oat2, and NTCP/Ntcp, respectively. These incubations were performed as described under Materials and Methods in the presence or absence of verapamil (an inhibitor of OCT1/Oct1), probenecid (an inhibitor of OATPs/Oatps and OAT2/Oat2), or Na+ (for NTCP/Ntcp-related transport activity). Cells were then lysed in distilled water, and intracellular accumulation of radiolabeled substrates was determined by scintillation counting. Data are expressed as inhibitor- or Na+-sensitive accumulation of substrates, i.e., accumulation values in the absence of inhibitor or Na+ minus accumulation values in the presence of inhibitor or Na+; they correspond to the means ± S.D of four to six experiments realized in triplicates and performed on independent hepatocyte preparations from different human liver donors and different Sprague-Dawley rats. *, P < 0.05 when compared with substrate accumulation found in 4-h cultured rat hepatocytes or 1-day cultured human hepatocytes.
In addition to the functional expression of major sinusoidal transporters described in the present study, activity of main biliary drug transporters, especially P-glycoprotein and MRP2, has been previously documented in both primary rat and human hepatocytes (Fardel et al., 1993; Payen et al., 2000; Hoffmaster et al., 2004). This demonstrates that primary hepatocytes possess the two kinds of transporters present in vivo in hepatocytes, i.e., those responsible for the entry of xenobiotics from blood and those involved in xenobiotic secretion into the bile; they also express phase I and phase II drug-metabolizing enzymes. Cultured hepatocytes therefore represent a good model for analyzing interactions of drugs and their metabolites with liver transporters. Their use may be relevant with respect to drug-drug interactions, as recently demonstrated using sandwich-cultured rat hepatocytes and the P-glycoprotein substrate rhodamine 123 (Annaert and Brouwer, 2005).
Acknowledgments
We thank Dr. X. Decleves for helpful comments with respect to our study.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.004762.
-
ABBREVIATIONS: SLC, solute carrier; ES, estrone-3-sulfate; MRP, multidrug resistance protein; NTCP, Na+-taurocholate cotransporter; OAT, organic anion transporter; OATP, organic anion-transporting polypeptide; OCT, organic cation transporter; PAH, para-aminohippurate; RT-qPCR, reverse transcriptase-quantitative polymerase chain reaction; TEA, tetraethylammonium.
- Received March 21, 2005.
- Accepted July 8, 2005.
- The American Society for Pharmacology and Experimental Therapeutics