Abstract
The clinical use of carbamazepine (CBZ), an anticonvulsant, is associated with a variety of idiosyncratic adverse reactions that are likely related to the formation of chemically reactive metabolites. CBZ-10,11-epoxide (CBZE), a pharmacologically active metabolite of CBZ, is so stable in vitro and in vivo that the potential for the epoxide to covalently interact with macromolecules has not been fully explored. In this study, two glutathione (GSH) adducts were observed when CBZE was incubated with GSH in the absence of biological matrices and cofactors (e.g., liver microsomes and NADPH). The chemical reactivity of CBZE was further confirmed by the in vitro finding that [14C]CBZE formed covalent protein adducts in human plasma as well as in human liver microsomes (HLMs) without NADPH. The two GSH adducts formed in the chemical reaction of CBZE were identical to the two major GSH adducts observed in the HLM incubation of CBZ, indicating that the 10,11-epoxidation represents a bioactivation pathway of CBZ. The two GSH adducts were isolated and identified as two diastereomers of 10-hydroxy-11-glutathionyl-CBZ by NMR. In addition, the covalent binding of [14C]CBZE was significantly increased in the HLM incubation upon addition of NADPH, indicating that CBZE can be further bioactivated by HLMs. To our knowledge, this is the first time the metabolite CBZE has been confirmed for its ability to form covalent protein adducts and the identity of the two CBZE-glutathionyl adducts has been confirmed by NMR. These represent important findings in the bioactivation mechanism of CBZ.
The clinical use of CBZ, an anticonvulsant, has been associated with a variety of idiosyncratic adverse reactions, such as generalized reactions, hepatotoxicity, and hematological disorders (Hart and Easton, 1982; Shear et al., 1988; Kalapos, 2002). CBZ-induced adverse reactions have been reported in as many as 30 to 50% of all patients treated with this drug (Pellock, 1987; Durelli et al., 1989). Among these adverse reactions, about 5% can be classified as idiosyncratic (Askmark and Wiholm, 1990). Although the mechanisms behind these adverse reactions are not clear, they are proposed to result from the formation of chemically reactive metabolites (Shear et al., 1988). An arene oxide intermediate has been postulated to be responsible for the idiosyncratic toxicity of CBZ (Pirmohamed et al., 1992; Lillibridge et al., 1996; Madden et al., 1996; Amore et al., 1997; Masubuchi et al., 2001; Kalapos, 2002). However, it has been difficult to achieve definitive proof of the arene oxide due to its high instability. In contrast, CBZE, the pharmacologically active metabolite of CBZ, is so stable in vitro and in vivo that the potential for the epoxide to covalently interact with macromolecules has not been fully explored. The purpose of this study was to assess the chemical reactivity and covalent binding potential of CBZE in human liver microsomes and human plasma in vitro.
Materials and Methods
Materials. [14C]CBZ (14C-labeled in the carbonyl group; specific activity 22.6 mCi/mmol, and radiochemical purity greater than 99.5% following an in-house purification by HPLC), CBZ, CBZE, NADPH, and GSH were purchased from Sigma-Aldrich (St. Louis, MO). Human liver microsomes (pooled from 60 donors) were prepared at Pfizer (Groton, CT). Blank human plasma was received from Bioreclamation (Hicksville, NY). All other commercially available reagents and solvents were of analytical grade or better.
Chemical Reaction of CBZE with GSH. CBZE (80 μM) was incubated with GSH (5 mM) for 2 h at 37°C in 0.1 M potassium phosphate buffer (pH 7.4). After mixing with 2 volumes of acetonitrile, samples were evaporated to dryness under N2 at 30°C. The residues were reconstituted in 0.1% formic acid in water, and aliquots were injected for profiling and isolation of CBZE-glutathionyl adducts.
Microsomal Incubation of CBZ with GSH. CBZ (100 μM) was incubated with GSH (5 mM) for 2 h at 37°C in 0.1 M phosphate buffer (pH 7.4) containing HLMs (2 mg/ml) and 2 mM NADPH. Reactions were initiated by the addition of NADPH and quenched with 2 volumes of acetonitrile. Samples were vortexed, centrifuged, and supernatants dried under N2 at 30°C. Residues were reconstituted in 0.1% formic acid in water, and aliquots were injected for metabolite profiling.
Isolation of [14C]CBZE. The highest conversion of CBZ to CBZE was achieved in rat liver microsomes among the human, rat, dog, and mouse liver microsomes tested (data not shown). Therefore, [14C]CBZ was incubated with rat liver microsomes to generate [14C]CBZE. All incubation procedures were the same as those described in the above section, except that no GSH was added. Reconstituted samples were injected for the isolation of [14C]CBZE using the HPLC method described below. The isolated [14C]CBZE material had a radiochemical purity of >99.5%.
Covalent Binding. [14C]CBZE or [14C]CBZ was incubated for 0 or 2 h at 37°C in 0.1 M phosphate buffer (pH 7.4) containing HLMs (2 mg of protein) or human plasma (7 mg of protein), nonlabeled CBZ (100 μM), and GSH (0 or 5 mM) at a final volume of 1 ml. Reactions were quenched with 4 ml of acetonitrile/methanol (2:1 v/v). Samples were vortexed for 5 min and centrifuged for 5 min at ∼1000 rpm. The same pellet extraction process was repeated until supernatants contained less than twice background radioactivity. Protein pellets were then dissolved in 1 N NaOH. After neutralization with HCl, the digested protein sample in each tube was measured for radioactivity by liquid scintillation counting.
NMR Analysis. All NMR spectra were acquired on a Bruker-Biospin AV700 spectrometer running TopSpin 1.3 software and equipped with a Bruker 5-mm TCI z-gradient Cryoprobe (Bruker, Rheinstetten, Germany). 1D 1H spectra were acquired with water suppression using a Watergate W5 pulse sequence with gradients and a double echo (Liu et al., 1998). 2D COSY and HSQC spectra were acquired without solvent suppression using gradient pulses for coherence selection. Chemical shifts are reported in ppm relative to tetramethylsilane.
HPLC Methods. Metabolite profiling and isolation were performed on an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA) coupled with an Agilent 1100 diode array detector, an IN/US (Tampa, FL) model 3 β-RAM radio-detector, a Gilson Medical Electronics (Middleton, WI) FC 204 fraction collector, and a Thermo Electron LCQ-Deca ion-trap mass spectrometer (Thermo Electron Corporation, Waltham, MA). Separation was achieved using a Phenomenex (Torrance, CA) Synergi Fusion-RP column (150 × 4.6 mm, 4 μm) at a flow rate of 1.0 ml/min. A linear gradient of 0.1% formic acid in water (A) and methanol (B) was initiated at 100% A for 5 min, changed to 70% A from 5 to 10 min, held at 70% A from 10 to 25 min, changed to 47% A from 25 to 48 min, changed to 20% A from 48 to 50 min, held at 20% A from 50 to 55 min, changed to 100% A from 55 to 56 min, and held at 100% A from 56 to 65 min for column equilibration. Major operating parameters for the electrospray mass spectrometry method were as follows: positive ion mode with a spray voltage of 4.5 kV, capillary temperature of 200°C, sheath gas flow rate of 80 (arbitrary), and an auxiliary gas flow rate of 20 (arbitrary). ARC data system Version 2.4 (AIM Research Company, Newark, DE) was used to control the detectors, fraction collector, and liquid chromatography-ion-trap mass spectrometry system for data acquisition and processing.
Results and Discussion
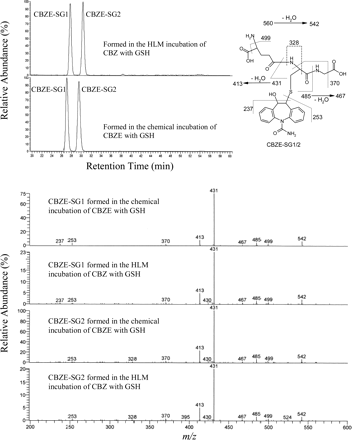
When CBZE was incubated with GSH in the absence of biological matrices and cofactors, an abundant molecular ion MH+ at m/z 560 (= 253 + 307, consistent with a direct CBZE-glutathionyl adduct) was observed. A constructed ion chromatogram of m/z 560 shows two peaks (called CBZE-SG1 and CBZE-SG2) with retention times at ∼27.5 and ∼30 min, respectively (Fig. 1), suggesting that CBZE-SG1 and CBZE-SG2 may represent two CBZE-glutathionyl adduct isomers. This is confirmed by the spectral interpretation of characteristic fragment ions observed in the MS2 ion-trap mass spectra of m/z 560 (Fig. 1). Two compounds with MH+ ions at m/z 560 were also obtained in the HLM incubation of CBZ with GSH in the presence of NADPH (Fig. 1), indicating that the doublet observed in the HLM incubation of CBZ may be the same in nature as that observed in the chemical reaction of CBZE. This is supported by the high similarity in both chromatographic retention times and MS2 mass spectra between the two doublets observed in the chemical reaction of CBZE as well as in the HLM incubation of CBZ (Fig. 1). In addition, CBZE-SG1 and CBZE-SG2 exhibited the same abundance in both sets of incubations, as indicated by radiochemical profiling (data not shown).
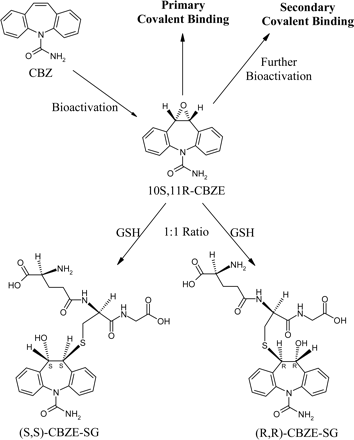
Figure 2 shows a comparison of 1D 1H NMR spectra for CBZE, GSH, and CBZE-SG1. The resonance assignments are based on the observed 1D chemical shifts along with 2D 1H-1H COSY and 1H-13C HSQC data. Several key features support the structure proposed for CBZE-SG1 (Fig. 2). First, the aromatic resonances between 7.8 and 7.2 ppm are present in the spectrum of CBZE-SG1 and integrated to 8 protons. It should be noted that the binding of the glutathionyl group to CBZE at position 10 or 11 would result in the loss of proton equivalency in the two aromatic rings of CBZE. The aromatic protons of CBZE-SG1 are more widely dispersed (Fig. 2), supportive of the GSH moiety being attached to carbon 10 or 11 of CBZE. Second, the resonances assignable to the GSH moiety are clearly present (Fig. 2). These protons were assigned by comparison with the GSH spectra and 2D COSY/HSQC data. Third, the two protons 10 and 11 (labeled as asterisks in Fig. 2) of CBZE-SG1 are shifted from 4.37 ppm in the CBZE spectrum to a crowded region between 3.70 and 3.50 ppm, as indicated by COSY and HSQC data (data not shown). This region is shown as an expanded inset in the CBZE-SG1 plot (Fig. 2). Together, these NMR data strongly support that the glutathionyl moiety is attached to one position and the hydroxyl group to the other of the two carbon atoms 10 and 11 of the CBZE moiety. Likewise, the same structural assignment was made for CBZE-SG2 (data not shown). Based on the conjugation mechanism of epoxides with GSH (Koppele and Mulder, 1991) and considering the molecular symmetry of CBZE, only two diastereomers, (S,S)-CBZE-SG and (R,R)-CBZE-SG, which should have a 1:1 formation ratio in the absence of glutathione S-transferases (GSTs), are expected to be formed (Fig. 3). This prediction is consistent with the above observations. However, it was not possible to determine the stereochemistry of CBZE-SG1 and CBZE-SG2 from the NMR data alone. GSTs are stereoselective in catalyzing GSH conjugation (Koppele and Mulder, 1991). If GSTs catalyzed the conjugation of CBZE with GSH to a significant extent, then one of the two GSH adducts might be formed preferentially. The fact that the formation ratio of the two GSH adducts was maintained at 1:1 when either CBZ or CBZE was incubated with HLMs suggested that the GSH conjugations were not appreciably mediated by liver microsomal GSTs.
The covalent binding potential of a radiolabeled compound in liver microsomes is usually assessed using incubations without NADPH as negative controls. However, such negative controls become invalid when NADPH is not necessary in a covalent binding assessment. This was the case for the covalent binding study of [14C]CBZE in HLMs and human plasma. For this reason, [14C]CBZ was used as a negative control for evaluating the covalent binding potential of [14C]CBZE. It was assumed that any nonspecific binding in either HLMs or human plasma was similar for [14C]CBZE and [14C]CBZ when the same amount of radioactivity was used in the covalent binding incubations. In addition, nonlabeled CBZ (100 μM) was added to all [14C]CBZE and [14C]CBZ incubations to mimic the biological environment where CBZ and CBZE coexist (i.e., CBZ has higher concentrations than CBZE) during in vitro or in vivo metabolism of CBZ. In HLM proteins, the unextractable radioactivity of [14C]CBZ was low (14–20 pmol Eq/mg protein) and did not show significant dependence on incubation time or presence of GSH (Table 1), suggesting that [14C]CBZ itself is not reactive and should be an appropriate negative control. In contrast, the unextractable radioactivity of [14C]CBZE was dependent on both the incubation time (23.2 and 162 pmol Eq/mg protein for time 0 and 2 h, respectively) and the presence of GSH (162 and 57.8 pmol Eq/mg protein without GSH and with 5 mM GSH, respectively; Table 1), indicating that [14C]CBZE does form covalent protein adducts with HLM proteins (called primary covalent binding; Fig. 3), and the covalent protein binding can be significantly prevented by GSH. In addition, the presence of NADPH increased the unextractable radioactivity of [14C]CBZE in the HLM incubation (162 and 241 pmol Eq/mg protein without NADPH and with 2 mM NADPH, respectively; Table 1), suggesting that [14C]CBZE can be further bioactivated in the formation of covalent protein adducts (called secondary covalent binding; Fig. 3). In human plasma, the unextractable radioactivity of [14C]CBZ was dependent on incubation time (3.8 and 7.5 pmol Eq/mg protein for time 0 and 2 h, respectively) but not on GSH (Table 1). The increase in unextractable [14C]CBZ with time may suggest that more radioactivity is trapped by protein clusters (nonspecific binding) during a longer incubation time. It was more difficult to extract trapped radioactivity when more proteins were present (7 mg of plasma proteins/ml used in the study; data not shown). The unextractable radioactivity of [14C]CBZE in human plasma was also dependent on incubation time (4.3 and 21.5 pmol Eq/mg protein for time 0 and 2 h, respectively) and the presence of GSH (21.5 and 9.8 pmol Eq/mg protein without GSH and with 5 mM GSH, respectively; Table 1), suggesting that [14C]CBZE also forms covalent protein adducts with human plasma proteins.
Covalent binding of [14C]CBZE with HLMs and human plasma
Representative ion chromatograms, MS2 mass spectra, and proposed ion fragmentation pathways of CBZE-SG1 and CBZE-SG2 (both MH+ ions at m/z 560) formed in the chemical reaction of CBZE with GSH or in the HLM incubation of CBZ with GSH.
In conclusion, CBZE forms two glutathionyl adducts with GSH in the absence of biological components and cofactors (e.g., liver microsomes and NADPH). The chemical reactivity of CBZE has been further confirmed by the formation of covalent protein adducts of [14C]CBZE with human plasma and HLMs in the absence of NADPH. With either matrix (HLMs or human plasma), the covalent binding of [14C]CBZE is significantly attenuated by the presence of GSH, demonstrating that GSH can compete with nucleophilic residues of proteins for trapping the chemically reactive epoxide. It is clear that the 10,11-epoxidation represents a bioactivation pathway of CBZ. In addition, [14C]CBZE can be further bioactivated by HLMs in the presence of NADPH. Confirming the potential of CBZE to form covalent protein adducts and identifying the regiochemical structures of the two CBZE-GSH adducts represent important findings in the bioactivation mechanism of CBZ. In plasma, CBZE levels are usually 15 to 55% and 5 to 81% of CBZ levels in adults and children, respectively (Bertilsson and Tomson, 1986; Liu and Delgado, 1994). Because CBZE is a chemically reactive metabolite having high exposure, it may form covalent protein adducts anywhere in the body. It is readily speculated that the broad covalent binding potential of CBZE may play a role in the high incidence of the adverse reactions associated with the clinical use of CBZ (Askmark and Wiholm, 1990).
Representative 700 MHz 1H NMR spectra of CBZE, GSH, and CBZE-SG1.
Chemical reactivity and bioactivation of CBZE in the HLM incubation.
Acknowledgments
We acknowledge Drs. Deepak Dalvie and Ellen Wu for helpful scientific discussions and critical review of the manuscript.
Footnotes
-
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
-
doi:10.1124/dmd.105.006866.
-
ABBREVIATIONS: CBZ, carbamazepine; CBZE, carbamazepine-10,11-epoxide; GSH, glutathione; HLM, human liver microsome; HPLC, high-performance liquid chromatography; 1D, one-dimensional; 2D, two-dimensional; COSY, correlation spectroscopy; HSQC, heteronuclear single quantum correlation; MS2, tandem mass spectrometry; GST, glutathione S-transferase.
- Received August 10, 2005.
- Accepted September 30, 2005.
- The American Society for Pharmacology and Experimental Therapeutics