Abstract
The human colon carcinoma cell line Caco-2 is often used as a model for intestinal drug absorption. To better understand xenobiotic glucuronidation in Caco-2 cells, we have examined the expression levels of different UDP-glucuronosyltransferases (UGTs) in them. The effects of two main factors were investigated, namely, passage number and cell differentiation. Hence, the mRNA levels of 15 human UGTs of subfamilies 1A and 2B were assessed in both undifferentiated and fully differentiated cells at four passage levels: P31, P37, P43, and P49. Quantitative reverse transcriptase-polymerase chain reaction was used to determine the mRNA levels of individual UGTs, and the values were normalized using β-actin as a reference gene. The results indicate that although passage number in the tested range exerts a mild effect on the expression level of several UGTs, the contribution of cell differentiation is much larger. The expression of nearly all the UGTs that were examined in this study was significantly, sometimes greatly, increased during cell differentiation. UGT1A6 was a distinct exception to this rule, however, because it was already highly expressed in the undifferentiated cells. The mRNA findings were confirmed at the enzyme activity level by measuring the glucuronidation of 1-naphthol, a very good substrate for UGT1A6, as well as estradiol that is not glucuronidated by this enzyme. The results revealed that 1-naphthol glucuronidation activity was high in both the differentiated and undifferentiated cells, whereas estradiol glucuronidation was only detected in the differentiated cells. Thus, Caco-2 cell differentiation plays a major role in UGT expression and ensuing metabolic reactions.
The epithelium of the intestinal mucosa is an important tissue for drug absorption because of its architecture and large surface area. Crossing the apical membrane of the cells lining the intestine exposes the p.o. administered drugs to the metabolic enzymes in these cells, namely, intestinal first-pass metabolism. In some cases, drug metabolism in the intestine may differ considerably from the reactions that take place in the liver cells because several metabolic enzymes, such as few of the UDP-glucuronosyltransferase enzymes (UGTs), are mainly expressed extrahepatically (Strassburg et al., 2000; Gregory et al., 2004).
The UGTs are endoplasmic reticulum enzymes that take an active part in the drug's biotransformation by catalyzing the substrate conjugation with glucuronic acid, thereby increasing its water solubility and excretion rate (Radominska-Pandya et al., 1999; Tukey and Strassburg, 2000; Wells et al., 2004). Nineteen human UGTs are described in the literature, 16 of which belong to either subfamily UGT1A or UGT2B (Mackenzie et al., 2005). Many, but not all, UGTs are expressed in the liver, the main site of glucuronidation in the human body. UGTs are also expressed in a variety of extrahepatic tissues (Tukey and Strassburg, 2001; Gregory et al., 2004).
The human colonic carcinoma cell line Caco-2 is currently widely used as a model system for the small intestine, particularly in drug absorption studies (Hilgers et al., 1990; Meunier et al., 1995; Gan and Thakker, 1997). These epithelial cells spontaneously acquire enterocyte-like histological and physiological properties during their differentiation process. Similarly to the small intestinal epithelium, fully differentiated Caco-2 cells form a monolayer of polarized cells, develop tight junctions, and are surmounted by an apical brush border (Hidalgo et al., 1989; Vachon and Beaulieu, 1992). Another inherent property of the Caco-2 cells is the expression of many enterocytic transporter systems, the expression level of which often increases during cell differentiation (Anderle et al., 1998; Siissalo et al., 2007). The passage number (the number of times the cells in the culture have been detached, diluted, and grown again to near confluence) of Caco-2 cells also affects many of their properties, such as cell viability and growth in culture (Briske-Anderson et al., 1997; Anderle et al., 1998). Thus, it is important to know and define the passage number of the cells under investigation, as well as interesting to examine its effect on UGT expression. It is worth noting in this respect that Caco-2 cell passage number was previously reported to affect glucuronidation activity (Mizuma et al., 2004), but the range of passage numbers that was examined by these authors was considerably higher than in our drug transport studies (Siissalo et al., 2007).
Comparative threshold (Ct) values for β-actin in qRT-PCR (average ± S.D., n = 3). These values were subsequently used in the normalization of qRT-PCR data from villin and the different UGT enzymes.
There is a cross-talk between drug-metabolizing enzymes and transport proteins (Wandel et al., 1999; Jeong et al., 2005a). Hence, studying drug metabolism within Caco-2 cells is important not merely for the estimation of clearance rate but also for better understanding of the transport and efflux processes. Caco-2 cells express xenobiotic metabolizing enzymes, particularly sulfotransferases, glutathione-S-transferases, and UGTs (Peters and Roelofs, 1989; Schmiedlin-Ren et al., 1997; Sabolovic et al., 2000; Meinl et al., 2008). In the case of glutathione-S-transferases, it was already reported nearly 20 years ago that differentiated Caco-2 cells expressed significantly more glutathione S-transferase than undifferentiated cells (Peters and Roelofs, 1989). Similar pattern of expression was very recently observed for sulfotransferases (Meinl et al., 2008). The expression of many UGTs of subfamily UGT1A, namely, UGT1A1, UGT1A3, UGT1A4, UGT1A5, UGT1A6, UGT1A8, and UGT1A9, was previously detected in Caco-2 cells, although the expression levels varied significantly among different Caco-2 cell lines (Sabolovic et al., 2000; Jeong et al., 2005b). Interestingly, glucuronidation activity in Caco-2 cells increased with culturing times and correlated with cell differentiation, and it was observed using reverse transcriptase-polymerase chain reaction (RT-PCR) that UGT1A3, UGT1A6, and UGT2B7 were expressed in these cells (Sabolovic et al., 2000).
In the present study, we have examined the effects of cell differentiation and passage number of Caco-2 cells on the mRNA expression of villin (as a marker for cell differentiation and formation of enterocyte-like monolayers) and nearly all the UGTs of subfamilies UGT1A and UGT2B (Chantret et al., 1988; Hodin et al., 1997). Activity assays were subsequently performed to relate the quantitative (q)RT-PCR findings with glucuronidation activity in Caco-2 cells. The results reveal a complex and interesting array of UGTs in the different stages of these cells.
Materials and Methods
Reagents and Materials. The reagents for cell culturing were purchased from Euroclone (Pero, Italy), except for fetal bovine serum, which was from Invitrogen (Carlsbad, CA). The plasticware was obtained from Corning B.V. Life Sciences (Schiphol-Rijk, Netherlands).
The materials and reagents for qRT-PCR were purchased from Applied Biosystems (Foster City, CA). Reagents for mRNA sample preparation were from Sigma-Aldrich (St. Louis, MO), Ambion (Austin, TX), Invitrogen, MBI Fermentas (Vilnius, Lithuania), GE Healthcare (Little Chalfont, Buckinghamshire, UK), and Promega (Madison, WI). The other used chemicals were of analytical grade and RNase-free.
Cell Cultures. Caco-2 (wild-type) cells obtained from American Type Culture Collection (Manassas, VA) were maintained at +37°C in an atmosphere containing 5% CO2 at 95% relative humidity. Cells were grown in a medium consisting of Dulbecco's modified Eagle's medium, high glucose 4.5 g/l; 10% heat-inactivated fetal bovine serum, inactivation at +56°C for 30 min; 1% nonessential amino acids; 1% l-glutamine; penicillin, 100 IU/ml; and streptomycin, 100 μg/ml. The medium was changed three times a week during cell growth and differentiation.
The cells were studied at two levels of differentiation: undifferentiated (cultured to approximately 80% confluency in 75-cm2 plastic flasks and harvested weekly with 0.25% trypsin) and fully differentiated, grown on polycarbonate filter membranes (pore size 0.4 μm, filter area 1.1 cm2) in 12-well Transwell insert plates at a seeding density of 6.8 × 104 cells/cm2 for at least 21 days. The studied Caco-2 cells were within the passage range P31 through P49, whereas the passages typically used in our laboratory are within P30 through P44 (Koljonen et al., 2006).
Extraction of RNA and cDNA Synthesis. Sample preparation protocol was carried out in an RNase-free environment. Control samples for each process step and genomic DNA contamination were prepared simultaneously.
Total RNA was extracted from differentiated and undifferentiated Caco-2 cells at passage levels P31, P37, P43, and P49 using TRI-Reagent (Sigma-Aldrich) protocol. The samples were DNase-treated with Ambion's DNA-Free kit, and total RNA concentrations were determined with RiboGreen RNA Quantitation Reagent and Kit (Invitrogen). Total RNA (2 μg/sample) was reverse-transcribed to cDNA with 30 U of M-MuLV reverse transcriptase (MBI Fermentas) in a reaction mixture containing 4 μl of 5× First-Strand buffer (MBI Fermentas), 1 mM dNTPs (MBI Fermentas), 3 μg of pd(N)6 primers (GE Healthcare), and 20 U of RNase inhibitor (MBI Fermentas) in a total volume of 20 μl.
qRT-PCR. The mRNA expression levels of villin, UGT1A1, UGT1A4, UGT1A5, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15, UGT2B17, and UGT2B28 were determined with ABI Prism 7500 system and FAM-labeled Assay on Demand TaqMan Gene Expression Assay reaction mixes (all from Applied Biosystems). The assays were carried out in a 96-well format so that one plate carried all the samples for a single UGT, namely, cDNA from differentiated and undifferentiated cells from four different passages, as well as all the needed controls, all of them in triplicate and simultaneously. The reaction mix was composed for each sample of 7.5 μl of TaqMan Universal PCR Master Mix, 0.75 μl of the respective TaqMan Gene Expression Assay Mix containing the primer pairs and the probe, and 1.75 μl of sterile water. To these, 10 μl of reagent mix and 5 μl of 8 ng/μl cDNA were added. The reaction profile consisted of 10-min incubation at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. Quantification of the PCR products was accomplished using the comparative Ct (threshold cycle) method, assuming equal amplification efficiencies. The Ct values of the amplified genes (low Ct values mean high expression level and vice versa) were normalized to the Ct values of β-actin (Fig. 1). Analyses of the qRT-PCR data were performed using the Qgene software (Muller et al., 2002).
Glucuronidation Assays. Differentiated and undifferentiated Caco-2 cells were cultured and collected at passage 43. The plates were washed and scraped in cold phosphate buffer saline, collected by centrifugation at 3200g, suspended in cold water, divided to aliquots, and stored at -80°C. Aliquots of both differentiated and undifferentiated cells were thawed on the day of the activity assays and centrifuged at 40,000g for 30 min. The cell pellet was suspended in 200 μl of cold water and sonicated mildly on ice (Branson sonifier 450, narrow tip, output level 4, 50% duty, six pulses with 1-min interval after the first three pulses), and the protein concentration was determined using the bicinchoninic acid method (Pierce, Rockford, IL) using bovine serum albumin as standard. The 1-naphthol glucuronidation was essentially done as previously described (Kurkela et al., 2003) in the presence of 100 μM substrate and 40 μg of protein for 120 min at 37°C. The estradiol glucuronidation assay was done similarly, except that the substrate was 100 μM 17-β-estradiol. The 1-naphthol glucuronides were detected by high-performance liquid chromatography (HPLC) as previously described (Kurkela et al., 2003), except that the column this time was Hypersil BDS-C18, 150 × 4.6 mm, 5 μm (Agilent Technologies, Palo Alto, CA). The estradiol glucuronides were separated using a Shimadzu (Kyoto, Japan) HPLC, and the column was Chromolith Speed ROD RP-18e 50-4.6 mm (Merck, Darmstadt, Germany). The glucuronides were detected by fluorescence, excitation at 216 nm and emission at 316 nm.
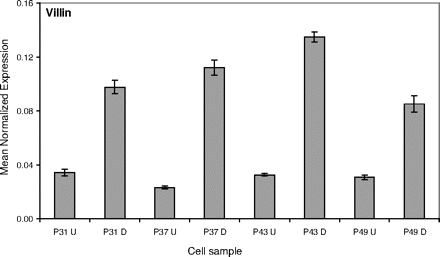
Effect of differentiation on villin expression. The expression of villin (average ± S.D., n = 3), normalized with respect to β-actin expression, in Caco-2 samples. D, fully differentiated cell samples grown on filters for at least 21 days; U, “undifferentiated” samples obtained from the culturing flasks.
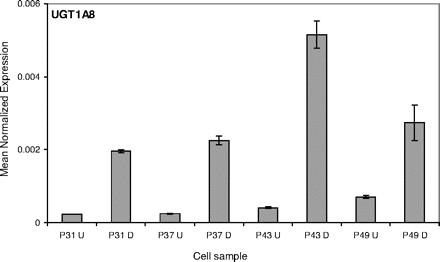
UGT1A8 expression in Caco-2 cells. The effect of differentiation and passage number on the relative expression level of UGT1A8, normalized with respect to β-actin expression. See Materials and Methods and legend to Fig. 2 for further details.
Results
The main objective of this study was to determine whether the passage number of the Caco-2 cells or their differentiation into epithelium-like cells affects the expression level of the UGTs of both subfamilies UGT1A and UGT2B. Hence, cells from four different passages, 31, 37, 43, and 49 (P31, P37, P43, and P49, respectively), were cultured either as monolayers on filters to achieve full differentiation (differentiated) or in culture flasks to near confluence (undifferentiated). The latter cells are referred to in this study as undifferentiated, even if the possible occurrence of partial differentiation in these samples could not be precluded. The polarization of Caco-2 monolayers grown on filters during the 3-week differentiation period was clearly shown by the increased expression of the protein villin compared with its level in the respective undifferentiated samples of the same passage number (Fig. 2).
The effect of differentiation and passage number, within the above-mentioned range, on the expression level of the UGTs was investigated using a large set of qRT-PCR reactions. We have examined the mRNA levels of nearly all the (human) UGTs of subfamilies UGT1A and UGT2B in these cells, namely, UGT1A1, UGT1A4 through UGT1A10, UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15, UGT2B17, and UGT2B28. The expression level of each of these UGTs was examined, in triplicate, in eight different samples, differentiated and undifferentiated cells of the four passages. Because of this multiplicity of samples, the qRT-PCR experiments were performed for each UGT separately. As a result and because of the nature of qRT-PCR assays, the expression levels of an individual UGT in the different passage numbers and differentiated versus undifferentiated cells could be compared directly, whereas the comparison of the level of two different UGTs in the same cell sample should be done much more cautiously.
The findings of this study reveal a major difference in the UGT composition of differentiated and undifferentiated Caco-2 cells (Table 1), at least within the range of passage numbers that was examined here. The results for UGT1A8 are a good example for the induction of a given UGT during cell differentiation (Fig. 3) and how its effect on the expression level of this UGT, compared with undifferentiated cells, is much larger than in the case of villin (Fig. 2). In both proteins, however, there is also a measurable amount of mRNA in the undifferentiated cells. Additionally, in both cases the expression level increase during differentiation reaches a peak at passage number 43 (Figs. 2 and 3).
The expression of different UGT enzymes in Caco-2 cells at several passage levels determined by qRT-PCR
Expression levels relative to β-actin: +, >0.0005; ++, >0.0015; +++, > 0.005; ++++, > 0.01.
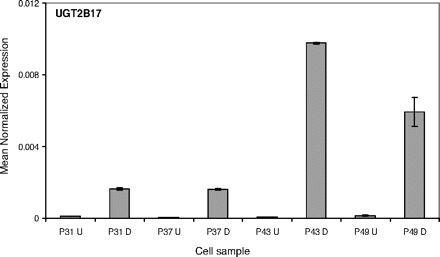
The expression results for UGT2B17 (Fig. 4) reveal a more extreme pattern than observed for villin and UGT1A8 (Figs. 2 and 3). The mRNA level of UGT2B17 in undifferentiated cells is barely detectable. On differentiation, however, it is increased dramatically, particularly in P43 cells. A similar pattern, even if not as striking as in UGT2B17, was also seen with UGT1A1, UGT1A5, UGT1A7, UGT1A9, UGT1A10, UGT2B7, and UGT2B11 (Table 1; supplemental data).
The expression of UGT1A4, UGT2B4, UGT2B10, UGT2B15, and UGT2B28 was detected in Caco-2 cells as well, and it was also strongly increased during differentiation. These results are not clearly visible in Table 1 because even the higher mRNA levels that were detected in differentiated cells fall within the lower categories used for the grading in this table. Nevertheless, inspection of the respective figures in the supplemental data will show that although the expression level of these UGTs was low, the impact of differentiation on them was significant (Supplemental Data, Fig. S1).
UGT2B17 expression in Caco-2 cells. The effect of differentiation and passage number on the relative expression level of UGT2B17, normalized with respect to β-actin expression. See Materials and Methods and legend to Fig. 2 for further details.
UGT1A6 expression in Caco-2 cells. The effect of differentiation and passage number on the relative expression level of UGT1A6, normalized with respect to β-actin expression. See Materials and Methods and legend to Fig. 2 for further details.
Although the expression of most UGTs was low or very low in the undifferentiated Caco-2 cells, UGT1A6 was highly expressed also in these cells (Fig. 5). This constitutive expression of UGT1A6 stands up as a sharp and interesting exception to all the other tested UGTs. The expression of UGT1A6 was somewhat further increased on differentiation but not that much and not in all the passage numbers (Fig. 5). These findings indicate that, in practical terms, UGT1A6 is the only UGT present in significant amounts in the undifferentiated Caco-2 in the passage number range of 31 through 49.
Our findings show that differentiation plays a major role in the induction of most UGT expression in Caco-2 cells. Nevertheless, closer inspection of the results reveals that passage number may also affect. The passage number effect was often clearer in differentiated cells, as can be seen in the case of UGT1A8 (Fig. 3) and some other UGTs (supplemental data). It is possible that this trend continues beyond P49, but this was not investigated in the present study. In differentiated cells, on the other hand, the influence of passage number appears to reach its maximum at P43, a result that is valid both for many UGTs (Figs. 3, 4, 5) and for villin (Fig. 2).
The UGTs are enzymes, and the qRT-PCR results only reveal information about their mRNA levels. To corroborate the findings of the large effect of cell differentiation on the expression level of most UGTs, but not UGT1A6, we have turned to activity assays. The objective of this experiment was to determine whether, as suggested by the qRT-PCR results, the level of most UGTs, with the exception of UGT1A6, in undifferentiated Caco-2 cells is very low and largely increases on differentiation. Therefore, we selected two different substrates for the glucuronidation assays: estradiol and 1-naphthol. Estradiol can be glucuronidated at the 3-OH and the 17-OH, and many human UGTs catalyze one or the other of these reactions (Pfeiffer et al., 2005). UGT1A6, however, does not catalyze estradiol glucuronidation (Lépine et al., 2004). The other substrate, 1-naphthol, is a good substrate for many UGTs, but its glucuronidation rate by UGT1A6 is much higher than that of the other enzymes (Kurkela et al., 2003). Hence, estradiol glucuronidation activity should reveal the presence of one or more UGTs, none of which is UGT1A6, whereas 1-naphthol glucuronidation activity, particularly in the presence of low enzyme concentration, would be in good agreement with the presence of UGT1A6.
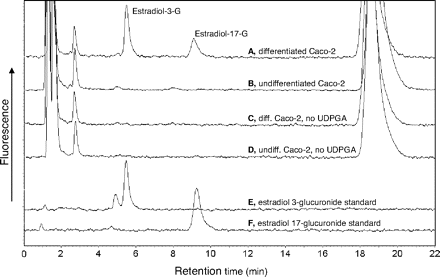
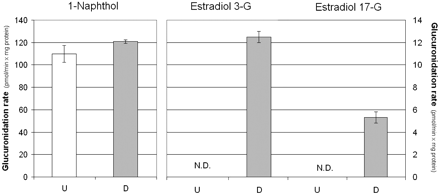
The activity results were carried out using Caco-2 cells of P43, and the chromatograms for the estradiol glucuronidation activity, including the different controls and marker glucuronides, are shown in Fig. 6. These results clearly show that in undifferentiated cells there was no detectable estradiol glucuronidation activity. On the other hand, on differentiation there was clear glucuronidation activity in the Caco-2 cells toward both the 3-OH and the 17-OH of estradiol, an indication for the presence of two or more active UGTs in these cells. In the case of 1-naphthol glucuronidation, there was an easily detectable activity in both the undifferentiated and differentiated cells (Fig. 7). Hence, the activity results are in full agreement with the qRT-PCR results for the expression of most UGTs in Caco-2 cells.
Discussion
The Caco-2 cell line serves as a model for the small intestine epithelium, and it is used as a valuable tool for screening uptake and efflux of p.o. administered compounds during drug discovery. Improving the knowledge and understanding on mechanisms affecting drug permeability, such as biotransformation, will contribute significantly to the interpretation of the results obtained from Caco-2 studies. Several UGTs, as well as glucuronidation activity, were detected in Caco-2 cells before (Münzel et al., 1999; Mizuma et al., 2004; Ishida et al., 2007), and we have now examined the expression of nearly all the human UGTs in these cells, concentrating on passage numbers that have been validated in our laboratory for drug permeability and uptake studies (Koljonen et al., 2006; Siissalo et al., 2007).
The expression values of the studied genes were normalized with regard to the expression level of β-actin (Fig. 1). The Ct values of the reference gene, β-actin, were around 20 for each sample, meaning that (above threshold) signal arising from this mRNA was recorded already at about halfway of the 40-cycle qRT-PCR runs (Fig. 1). For villin, the marker gene for Caco-2 cell differentiation, the Ct values were around 25, whereas for most UGTs they were around 30. It may be noted here that when the Ct values are more than 35, the expression quantification may be compromised by background fluorescence from the primer dimers. In the present study such high Ct values (indicating very low expression levels) were only recorded in the cases of UGT1A4 (P49D), UGT2B10 (undifferentiated samples), and UGT2B15 (P49U/D).
The results of the present study highlight the major effect of cell differentiation, or something occurring during cell differentiation, on the expression of most, but not all, UGTs. This observation is clearly shown by the qRT-PCR results for UGT1A8 and UGT2B7 (Figs. 3 and 4, respectively), as well as by the estradiol glucuronidation activity results (Figs. 6 and 7). It should be noted, however, that an increased expression level was detected by qRT-PCR for many UGTs, as can be deduced from Table 1 and additional data for the individual UGTs (Figs. 3 and 4; supplemental data). Nonetheless, it is worth pointing out that for some UGTs, such as UGT1A4, UGT2B10, UGT2B15, and UGT2B28, the observation of clear expression stimulation during differentiation does not necessarily mean that they are present in differentiated Caco-2 cells at large amounts. For these UGTs the mRNA level in the differentiated cells, although much higher than in the undifferentiated cells, was still very low (Table 1; Supplemental Data, Fig. S1).
Chromatograms of the HPLC analyses of estradiol glucuronidation in Caco-2 cells. A, differentiated Caco-2 cells from passage 43; B, undifferentiated cells from passage 43; C, control for A, reaction carried out in the absence of UDPGA; D, control for B, reaction carried out in the absence of UDPGA; E, estradiol-17-glucuronide standard; F, estradiol-3-glucuronide standard.
Glucuronidation rates of 1-naphtol and estradiol in differentiated (D) and undifferentiated (U) Caco-2 cells. See Materials and Methods for sample preparation and activity assays.
UGT1A6 is an interesting exception among the UGTs because its expression level was high also in the undifferentiated cells, not only in the differentiated ones (Figs. 5 and 7A; Table 1). It may be suggested that the reason for the large difference in the expression pattern between the UGT1A6 from most other UGTs may either stem for the presence of an additional transcription factor binding sequence in its promoter or the lack of a suppressor binding site. This question, however, is beyond the scope of the current study.
The effect of differentiation on gene expression in Caco-2 cells is not limited to UGTs. A similar effect was recently reported on the expression of many sulfotransferases (Meinl et al., 2008) and earlier on glutathione-S-transferases (Peters and Roelofs, 1989). Cell differentiation has also been observed to affect the expression profile of efflux proteins in Caco-2 (Siissalo et al., 2007). Although the detailed knowledge on the individual factors that lead to such broad increase in phase II drug-metabolizing enzymes remains unknown, our findings have clear implications on drug uptake studies that use Caco-2 cells. The UGT expression profile in undifferentiated cells differs clearly from that in the differentiated cells. Drug transport studies in Caco-2 cells are usually conducted under conditions of fully differentiated cells, a process that typically takes 21 days. It is worth noting, however, that some screening protocols are based on shorter growth times (Eneroth et al., 2001; Miret et al., 2004). This may mean that the cells are only partly differentiated and, therefore, probably lack considerable glucuronidation potential for drugs that are not conjugated by UGT1A6.
Alongside differentiation, the passage number of the Caco-2 cells also affects UGT expression (Fig. 3; Table 1). It is important to note that some of the previous studies on the UGT expression in Caco-2 cells (Mizuma et al., 2004; Köhle et al., 2005; Sabolovic et al., 2006) were conducted at the passage number range of 80 to 120, much higher than in the present work. It seems that higher passage numbers within the range of 31 to 49 lead to increased expression level of UGTs in undifferentiated cells (Fig. 3; Table 1; Supplemental Data, Fig. S1). If this trend continues to passage numbers around 100 in undifferentiated cells, our results of UGT and glucuronidation activity might be easier to detect and study in these cells when using not fully differentiated cells.
Passage number also affected UGT expression level in the differentiated Caco-2 cells (Figs. 3, 4, 5). It is difficult to know at this stage if the mechanism of this effect is similar to the one in the undifferentiated cells at higher passage number. One difference between the two types of cells was that in the undifferentiated cells the expression of UGT1A8 and few other UGTs kept growing and did not reach a maximum within the range of this study. In the differentiated cells, on the other hand, the UGT expression peaked at passage 43 and then started to decline (Figs. 3, 4, 5). Hence, there may be a link between the factors affecting UGT expression during differentiation to factors influenced by the cell passage number, even if for the time being we are unable to pin down the identity of these factors.
Based on the qRT-PCR studies, the main UGT expressed in the undifferentiated Caco-2 cells was UGT1A6, whereas in the differentiated cells the major UGTs were UGT1A6, UGT1A8, and UGT2B17 (Table 1). The activity results (Figs. 6 and 7) fully support the finding that mainly UGT1A6 is present in the undifferentiated cells at passage 43, whereas many other UGTs are expressed in these cells on differentiation. The glucuronidation of estradiol at both hydroxyls is a good indication for the expression of multiple UGTs but not UGT1A6. Human UGTs that were already implicated in estradiol glucuronidation at either the 3-OH or 17-OH include UGT1A1, UGT1A8, UGT1A10, and UGT2B7 (Lépine et al., 2004; Guillemette et al., 2004), and our recent studies reveal that several other enzymes could catalyze either of these reactions (Itäaho et al., 2008). UGT1A6, an enzyme that is not able to catalyze estradiol glucuronidation, is the human UGT with the highest activity toward 1-naphthol (Kurkela et al., 2003; Uchaipichat et al., 2004). Therefore, the results of our activity assays not only support the qRT-PCR findings but also explain why 1-naphthol glucuronidation activity was often selected as an indication for glucuronidation activity in Caco-2 cells, including studies that did not focus on differentiated cells (Mizuma et al., 2004; Naganuma et al., 2006).
In conclusion, we have shown, both at the levels of mRNA and enzymatic activity, that differentiation has a major effect on the expression of most, but not all, UGTs in Caco-2 cells. Thus, it may be suggested that the most suitable Caco-2 cells for UGT research, or studies on the uptake of drugs that may undergo glucuronidation during first-pass metabolism in the intestine, are differentiated cells of passage number in the range of 37 to 43.
Acknowledgments
We thank Dr. Marika Ruponen at the University of Kuopio for help with qRT-PCR.
Footnotes
-
This study was financially supported by TEKES (Finnish Funding Agency for Technology, Project No. 40186/04), Orion Pharma, European Union (LIINTOP, LSH-2005-1.2.3-4), and the Academy of Finland (Project No. 210933).
-
doi:10.1124/dmd.108.022335.
-
ABBREVIATIONS: UGT, UDP-glucuronosyltransferase; RT-PCR, reverse transcriptase-polymerase chain reaction; qRT-PCR, quantitative RT-PCR; HPLC, high-performance liquid chromatography; Ct, threshold cycle.
-
↵
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material. - Received May 13, 2008.
- Accepted August 7, 2008.
- The American Society for Pharmacology and Experimental Therapeutics