Abstract
The expression levels of mRNAs for MDR1 (P-glycoprotein), multidrug resistance-associated proteins (MRP1, MRP2), and cytochrome P450 3A (CYP3A) in Caco-2 cells were quantitatively compared with those in human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Caco-2 cells (passages 36–88) were kindly supplied by several laboratories in Japan. Human duodenal enterocytes were obtained from five healthy male volunteers. Normal colorectal tissues and colorectal adenocarcinomas were simultaneously obtained from seven patients with primary colorectal adenocarcinoma. MDR1, MRP1, MRP2, and CYP3A mRNA levels were determined by real-time quantitative polymerase chain reactions (PCR). Relative concentrations of mRNAs for target proteins (MDR1, MRP1, MRP2, and CYP3A) and glyceraldehyde-3-phosphate dehydrogenase in Caco-2 cells were 1.00 ± 0.15, 1.02 ± 0.06, 0.94 ± 0.10, and 0.68 ±0.60, respectively, and those in human enterocytes were about 12-, 3-, 7-, and 8000-fold higher than in the Caco-2 cells, respectively. In contrast, MDR1, MRP1, and CYP3A mRNA levels in Caco-2 cells were comparable to those in normal colorectal tissue and colorectal adenocarcinoma.
Recent technological innovations in drug discovery (i.e., combinatorial chemistry and high-throughput screening for pharmacological activity) have enabled us to produce large numbers of potentially useful candidate drugs within a week, and the rapid assessment of their pharmacokinetic and toxicological properties, especially oral absorption, has become the bottleneck in drug development (Rodrigues, 1997; Fernandes, 1998; Tarbit and Berman, 1998). Over the last few decades, a correlation between intestinal epithelial cell permeability and the overall intestinal absorption has been demonstrated, and this has prompted us to use intestinal epithelial cell lines and to conduct in vitro transport studies (Boulenc, 1997). Among the large number of established cell lines, the Caco-2 cell line obtained from human colorectal cancer is the most useful for such studies since it is capable of morphological and biochemical differentiation in vitro to form intestinal epithelium under normal culture conditions (Pinto et al., 1983; Hidalgo and Li, 1996; Artursson and Borchardt, 1997; Boulenc, 1997; Watkins, 1997; Milovic et al., 1998). Caco-2 cells express several markers and drug-metabolizing enzymes, and several transporters were found in human enterocytes, including cytochrome P450 3A (CYP3A) and P-glycoprotein (MDR1), which have recently attracted a great deal of attention due to their barrier function against xenobiotics (Hidalgo and Li, 1996; Watkins, 1997). Recently, the contribution of the multidrug resistance-associated protein (MRP1) family to drug extrusion has also been demonstrated using Caco-2 cells (Hirohashi et al., 2000). Thus, the Caco-2 system has been shown to be useful for evaluation of the transport mechanism in addition (Hidalgo and Li, 1996; Artursson and Borchardt, 1997; Boulenc, 1997; Watkins, 1997). However, claims have sometimes been made concerning the essential characteristics of tumor cells (Milovic et al., 1998)—that these cells have a lower metabolic capacity than human enterocytes (Hidalgo and Li, 1996; Schmiedlin-Ren et al., 1997) and show alterations in expression levels of oral drug absorption-related proteins during culture and passage (Pinto et al., 1983; Hidalgo and Li, 1996). In this study, mRNA levels of MDR1, MRP1, MRP2, and CYP3A in Caco-2 cell lines were quantitatively compared with those in human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas by means of the real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) (Gibson et al., 1996;Heid et al., 1996).
Materials and Methods
Caco-2 Cell Lines and Cell Culture.
Caco-2 cells (passage 47) obtained from the RIKEN cell bank (RIKEN RCB0988; Saitama, Japan) were used as the authentic standard in each run of the assay since they were demonstrated to express sufficient levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MDR1, MRP1, MRP2, and CYP3A mRNA. Caco-2 cells (passages 36–88) were also kindly supplied by several laboratories in Japan. Caco-2 cells were grown in complete medium consisting of Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 0.1 mM minimal essential medium nonessential amino acids (Invitrogen), 2 mMl-glutamine (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) in an atmosphere of 95% air and 5% CO2 at 37°C (Tsuji et al., 1994). The cells were subcultured every 5 to 7 days using 0.02% EDTA and 0.05% trypsin. The assay was conducted 5 to 7 days after seeding of 1 × 105 cells in 10 ml of complete culture medium in 25-cm2 culture flasks (Nunclon flasks; Nalge Nunc International, Rochester, NY).
Human Duodenal Enterocytes, Normal Colorectal Tissues, and Colorectal Adenocarcinomas.
Duodenal enterocytes were obtained as proximal small-bowel mucosal biopsy samples from five healthy Japanese male volunteers ranging in age from 27 to 39 years old. The subjects took no medications and had no significant health problems. After fasting overnight, the mucosal biopsy samples were obtained by upper-intestinal endoscopy from each subject and were immediately snap-frozen and stored at −80°C. Colorectal adenocarcinomas were obtained as surgical samples from seven patients with primary colorectal adenocarcinoma diagnosed at Kobe University Hospital (four men and three women; age range, 70–80 years). Normal colorectal tissues were simultaneously taken from the resected bowel specimens but were from regions well away from the tumor. Normal colorectal tissue and adenocarcinoma samples were obtained immediately after resection, and they were quickly stripped of connective tissue, snap-frozen, and stored at −80°C until processing. Informed consent was obtained from all subjects before their participation in the study. The protocol was approved by the Institutional Review Broad of Kobe University Hospital (Kobe University, Japan).
RNA Extraction and RT.
Total RNA was extracted from confluent monolayers of Caco-2 cell lines and tissue samples using an RNeasy mini-kit (QUIAGEN, Hilden, Germany) and an RNase-free DNase set (QUIAGEN). The RT reaction was conducted in 20 μl of two-step RT reaction mix containing 4 μl of the extracted total RNA (2 μg/ml), 1× TaqMan RT buffer, 5.5 mM MgCl2, 500 μM dATP, 500 μM dGTP, 500 μM dCTP, 500 μM dUTP, 2.5 μM random hexamer, 0.4 U/μl of RNase inhibitor, and 1.25 U/μl MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA). The mixture was incubated at 25°C for 10 min and subsequently at 48°C for 30 min. RT reaction was terminated by heating at 95°C for 5 min, followed by cooling at 4°C for 5 min, giving the RT product.
Real-Time Quantitative PCR.
Primer pairs and TaqMan probes for MDR1, MRP1, MRP2, and CYP3A mRNA were designed using the Primer Express 1.0 program (Applied Biosystems) (Table 1). Primers and the TaqMan probe for GAPDH were purchased from Applied Biosystems (TaqMan GAPDH control reagent kit). The principle of real-time quantitative PCR has been described elsewhere (Gibson et al., 1996; Heid et al., 1996; Yajima et al., 1998; Latil et al., 2000). The 25 μl of reaction mixtures contained 1× TaqMan buffer A, 5.5 mM MgCl2, 400 μM dUTP, 200 μM dATP, 200 μM dCTP, 200 μM dGTP, 0.01 U/μl AmpErase UNG, 0.025 U/μl AmpliTaq Gold DNA polymerase, 200 nM each forward and reverse primer, 100 nM TaqMan probe (Applied Biosystems), and 1 μl of RT product. The reaction was performed in triplicate for each RT product. During the extension phase of PCR, consisting of an initial denaturation step at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min, the nucleolytic DNA polymerase cleaved the hybridization probe, and the resulting relative increase in the reporter fluorescent dye emission was monitored in real time using a sequence detector (ABI prism 7700 sequence detector; Applied Biosystems). The fluorescent dye emission was a function of cycle number and was determined using the sequence detector software (Applied Biosystems), giving the threshold cycle number (CT) at which PCR amplification reached a significant threshold. The value of the CT was linearly correlated with logarithmic value of genomic DNA quantity.
Sequences of oligonucleotide primers and probes used for real-time quantitative PCR
The PCR products obtained by this real-time quantitative PCR procedure were confirmed to be the expected products by electrophoresis through 3.0% agarose gels in the presence of ethidium bromide with visualization under UV illumination (data not shown). The PCR products for the target proteins were undetectable in the real-time PCR procedure without reverse transcription.
In each run of the assay, mRNAs of GAPDH and a target protein (i.e., MDR1, MRP1, MRP2, and CYP3A) were analyzed in 5-fold serially diluted samples from authentic Caco-2 cells (0.16, 0.8, 4, 20, 100 ng as total RNA determined spectrophotometrically). The standard lines were constructed by plotting mean CT values against quantity (the relative copy number). The mRNA levels of GAPDH and a target protein in Caco-2 cells from several laboratories and tissue samples were calculated from the mean CT values.
The mRNA levels of MDR1, MRP1, MRP2, and CYP3A are expressed as concentrations relative to GAPDH mRNA. Values are given as the means ± S.E. Statistical comparisons were performed by one-way analysis of variance followed by Sheffé's test. Pvalues of less than 0.05 (two-tailed) were considered significant.
Results and Discussion
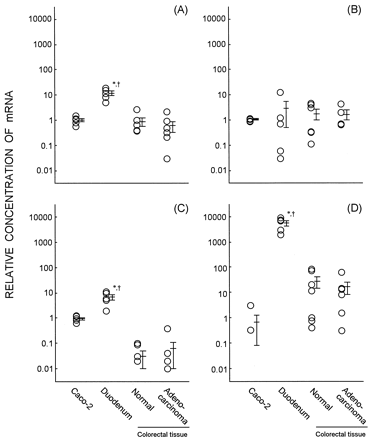
Usually, the standard lines constructed gave good linearity with excellent correlation coefficients, with minimum day-to-day or within-a-day variation. Figure 1 shows the relative concentrations of MDR1, MRP1, MRP2, and CYP3A mRNA in Caco-2 cells, human duodenal enterocytes, normal colorectal tissue, and colorectal adenocarcinoma. The relative concentration of MDR1 mRNA in the Caco-2 cells was 1.00 ± 0.15 and was significantly lower than human duodenal enterocytes (11.89 ± 2.45) but comparable to normal colorectal tissue and colorectal adenocarcinoma (0.88 ± 0.33 and 0.60 ± 0.29, respectively). Caco-2 cells tended to have a lower level of MRP1 mRNA than human duodenal enterocytes, normal colorectal tissue, and colorectal adenocarcinoma (1.02 ± 0.06, 2.86 ± 2.36, 1.74 ± 0.76, and 1.65 ± 0.69, respectively). MRP2 mRNA level in Caco-2 cells was also lower than human duodenal enterocytes, but MRP2 mRNA was barely detectable in normal colorectal tissues and colorectal adenocarcinomas. Three of five Caco-2 cell lines had no detectable CYP3A mRNA, even after 40 cycles of PCR. CYP3A mRNA level in Caco-2 cells was extensively lower than human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas (0.68 ± 0.60, 5645.76 ± 1367.73, 27.27 ± 13.36, and 16.47 ± 8.17, respectively).
Relative concentrations of mRNAs for MDR1 (A), MRP1 (B), MRP2 (C), and CYP3A (D) in the Caco-2 cell lines, human duodenal enterocytes, and human colorectal tissues.
Relative concentrations of MDR1, MRP1, MRP2, and CYP3A mRNA were determined by real-time quantitative PCR. GAPDH was selected as an endogenous RNA control to normalize for differences in the amount of total RNA. Each bar represents the average and standard error of the respective relative concentrations. ∗, p < 0.05, significantly different from Caco-2 cells. †, p < 0.05, significantly different from colorectal tissue.
Real-time quantitative PCR method in which the amplification signals are detected in real time has advantages over ordinary semiquantitative methods based on densitometry involving a competitive PCR method and Northern analysis, which contains a lengthy series of steps (mRNA preparation, electrophoresis, blotting, hybridization, and autoradiography) (Gibson et al., 1996; Heid et al., 1996; Yajima et al., 1998; Latil et al., 2000). Besides, this method makes it possible to quickly evaluate with high sensitivity the gene expression profiles for numbers of target proteins, and therefore, it is useful as a speedy assessment in advance of a functional analysis. In this article, it was suggested that Caco-2 cells expressed only low levels of MDR1, MRP1, MRP2, and CYP3A mRNA in comparison with human duodenal enterocytes. CYP3A mRNA levels varied remarkably for Caco-2 cell lines, suggesting that its content is susceptible to culture conditions. These observations strongly suggested that considerable attention should be paid to the transport data obtained from the Caco-2 cell system in determining the overall absorption kinetics. It was also suggested that the gene expression levels of these molecules in Caco-2 cells were comparable with those in normal colorectal tissue and colorectal adenocarcinoma, except for MRP2. Collectively, Caco-2 cell lines showed the gene expression profiles of oral drug absorption-related proteins closer to those of human normal colorectal tissue and adenocarcinoma rather than human duodenal enterocytes.
Acknowledgments
We thank Dr. Ken-ichi Inui and collaborators at the Department of Pharmacy (Kyoto University Hospital, Faculty of Medicine, Kyoto University) for helpful discussions and critical comments on the draft of the manuscript. We thank Drs. Akira Tsuji and Ikumi Tamai of the Department of Pharmacobiodynamics (Faculty of Pharmaceutical Sciences, Kanazawa University), Dr. Mitsuru Hashida of the Department of Drug Delivery Research (Graduate School of Pharmaceutical Sciences, Kyoto University), Dr. Shinji Yamashita of the Department of Pharmaceutics (Faculty of Pharmaceutical Sciences, Setsunan University), Drs. Toshikiro Kimura and Kazutaka Higaki of the Department of Pharmaceutics (Faculty of Pharmaceutical Sciences, Okayama University), and Dr. S. Akira Yamamoto and Takuya Fujita of the Department of Biopharmaceutics and Biochemical Pharmacology (Kyoto Pharmaceutical University) for kindly supplying Caco-2 cells and helpful suggestions.
Footnotes
- Abbreviations used are::
- MRP
- multidrug resistance-associated protein
- RT-PCR
- reverse transcription-polymerase chain reaction
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- CT
- threshold cycle number
- Received June 25, 2001.
- Accepted October 16, 2001.
- The American Society for Pharmacology and Experimental Therapeutics