Abstract
Organic solvents are often used to solubilize lipophilic new chemical entities before their addition to in vitro test systems such as microsomal stability or cytochrome P-450 (CYP) inhibition. However, the effect of these organic solvents on the test systems is not usually characterized. This study was initiated to evaluate the effect of acetonitrile and acetone, in addition to other organic solvents, on the tolbutamide hydroxylation activity of CYP2C9 in both human liver microsomes and a CYP2C9-reconstituted system. Both acetonitrile and acetone significantly stimulated the NADPH-dependent tolbutamide hydroxylation by nearly 2- to 3-fold in human liver microsomes and CYP2C9-reconstituted system when incubated at 2 and 4% final solvent concentrations. When cumene hydroperoxide was used instead of NADPH, both acetone and acetonitrile significantly inhibited tolbutamide hydroxylation. This NADPH-dependent stimulatory effect was further evaluated by examining the effect of a series of other organic solvents with different carbon chain lengths and various functional groups, including hydroxyl, ketone, and aldehyde. Unlike acetone, two other ketone-containing solvents, methyl ethyl ketone (2-butanone) and diethyl ketone (3-pentanone) failed to significantly enhance tolbutamide hydroxylation. Other solvents tested, including methanol, ethanol, propanol, 1-butanol, 2-butanol, 1-pentanol, 2-pentanol, acetaldehyde, and dimethyl sulfoxide significantly inhibited NADPH-dependent tolbutamide hydroxylation. Overall, the stimulatory effect of both acetonitrile and acetone on tolbutamide hydroxylation was found to be primarily due to a consistent increase inVmax, whereas Kmwas unchanged in both human liver microsomes and the reconstituted CYP2C9 system. These data suggest that acetone and acetonitrile stimulate NADPH-mediated tolbutamide hydroxylation via the CYP reductase and not by modifying the affinity of tolbutamide for the CYP2C9 enzyme.
Cytochrome P-450 (CYP)1 inhibition studies on new chemical entities are performed routinely in the pharmaceutical industry to assess the potential for drug-drug interactions (Parkinson, 1996; Lin and Lu, 1997; Palamanda et al., 1998; Favreau et al., 1999). Organic solvents are often used to solubilize lipophilic new chemical entities before their addition to the in vitro microsomal inhibition test system. However, the exact influence of the organic solvent itself on the CYP reaction is not usually characterized. Instead, the concentration of the organic solvent in the in vitro inhibition test system is held to a minimum, in the hope of minimizing the solvent effect (Parkinson, 1996; Chauret et al., 1998).
Although it is known that many organic solvents inhibit CYP oxidative reactions (Chauret et al., 1998; Hickman et al., 1998; Busby et al., 1999), the converse effect, i.e., stimulation of CYP reaction, is rare. Examples include the stimulation of aniline (Anders, 1968) and alprazolam (Wolff et al., 1989) hydroxylation by acetone and aldrin epoxidation by ethanol (Monette et al., 1996). Also, it has been recently reported that acetone and acetonitrile slightly stimulate NADPH-dependent CYP2C9 reactions (Chauret et al., 1998; Hickman et al., 1998; Busby et al., 1999). However, the mechanisms for this apparent stimulation are not completely understood.
The current studies were initiated to further examine the effect of acetone and acetonitrile on tolbutamide hydroxylation in human liver (HL) microsomes and CYP2C9-reconstituted system, and to provide kinetic parameters and a hypothesis for the stimulation mechanism. This effect was further evaluated with series of solvents with various functional groups, including an aldehyde and dimethyl sulfoxide, and alcohols and ketones with various carbon chain lengths.
Materials and Methods
Chemicals and Reagents.
Hydroxytolbutamide was purchased from Research Biochemicals International (Natick, MA). Tolbutamide, NADPH, chlorpropamide, and cumene hydroperoxide were purchased from Sigma Chemical Co. (St. Louis, MO). Purified recombinant RECO CYP2C9 was purchased from Panvera (Madison, WI). Individual HL microsomes (five male, five female) were purchased from IIAM (Exton, PA) pooled, aliquoted, and frozen until use. Microsomal protein concentration was measured according to Lowry et al. (1951).
HL Microsomal Incubations.
All incubations were conducted under linear conditions with regard to time and protein concentration. An appropriate volume of a methanolic solution of tolbutamide to produce a final concentration of 100 μM in a 200-μl incubation volume was placed in microtubes; methanol was evaporated under a stream of nitrogen. HL microsomes (200 μg of protein in 10 μl) were added directly to the dry residue and vortexed briefly. One hundred and seventy microliters of 50 mM Tris-acetate buffer, pH 7.4, containing 150 mM KCl was added next. The reactions were initiated by the addition of 20 μl of a 10 mM NADPH solution in 50 mM Tris-acetate buffer, pH 7.4, containing 150 mM KCl (NADPH concentration in the reaction mixture was 1 mM). Incubations lasted for 45 min at 37°C and the reactions were terminated by the addition of 20 μl of 70% perchloric acid. The incubation mixtures were chilled on ice and 20 μl of a 200 μM solution of chlorpropamide (internal standard) was added, centrifuged at 2000g for 2 min, and the clear supernatant was analyzed by HPLC.
Reconstituted CYP2C9 RECO Enzyme Incubations.
All incubations were conducted under linear conditions with regard to time. Tolbutamide at 100 μM was incubated with the reconstituted CYP2C9 system in microtubes according to the manufacturer's instructions. Both buffer and enzyme mixes, supplied by the manufacturer, were thawed rapidly and kept on ice. To the dry tolbutamide residue in the microtubes, 10 μl of the concentrated enzyme mix, which contained 5 pmol of CYP2C9, 10 pmol of rat NADPH-CYP reductase, 5 pmol of human cytochrome b5and 1.0 μg of a 1:1:1 mixture ofl-α-dilauroylphsphatidylcholine,l-α-dioleoylphosphatidylcholine, andl-α-dilauroylphosphatidylserine was added. This was followed by the addition of 40 μl of a 500 mM Tris-HCl buffer, pH 7.4, 130 μl of water, and 20 μl of a 10 mM NADPH solution. The total volume of the incubation mixture was 200 μl. The reaction mixture was vortexed briefly and incubated at 37°C for 45 min. The reactions were terminated and analysis was performed as described above.
Tolbutamide hydroxylation reactions also were conducted with both HL microsomes and the CYP2C9-reconstituted system with cumene hydroperoxide (100 μM) instead of NADPH under the same conditions as those described above. Cumene hydroperoxide was used to bypass the NADPH-CYP reductase pathway (Crespi and Miller, 1997).
Concentration of Tolbutamide in Reaction Mixture in Presence of Acetone or Acetonitrile.
Ten-microliter aliquots of a 2 mM tolbutamide solution in methanol were placed in microtubes, and the methanol was evaporated under a stream of nitrogen. HL microsomes (200 μg of protein in 20 μl) were added directly to the dry residue and vortexed briefly. One hundred and sixty microliters of 50 mM Tris-acetate buffer, pH 7.4, containing 150 mM KCl followed by 20 μl of Tris-acetate buffer, pH 7.4 containing 0, 20, or 40% aqueous acetone or acetonitrile were added to each tube to produce final solvent concentrations of 0, 2, or 4% in a 200-μl total volume. The final concentration of tolbutamide was 100 μM. The mixture was vortexed and subjected to ultracentrifugation at 101,000g for 1 h to pellet the microsomal protein. The concentration of tolbutamide in the supernatant was determined at each solvent concentration by the HPLC method described below, with a calibration curve prepared in the same buffer.
Sample Analysis.
Hydroxytolbutamide was quantified with reversed phase HPLC according toMiners et al. (1998), with minor modifications. The mobile phase consisted of 22% acetonitrile and 78% 10 mM sodium acetate, pH 4.3, at a flow rate of 1.2 ml/min. The stationary phase was a Zorbax-CN column (4.6 mm × 15 cm). The analytes were quantified by UV absorbance at 230 nm. The retention times for hydroxytolbutamide, chlorpropamide, and tolbutamide were 4, 8, and 11.5 min, respectively.
Determination of Kinetic Parameters.
Michaelis-Menten parameters for tolbutamide hydroxylation were calculated by varying tolbutamide concentration from 25 to 600 μM. Kinetic parameters were determined from substrate concentration and reaction velocity data by nonlinear regression with the Slidewrite graphic program (Advanced Graphic Software, Inc., Sunnyvale, CA).
Statistics.
Significant differences (P < .05) were estimated with Student's t test. The data are the mean of three separate determinations and the activity was expressed as a percentage of control with percentage of coefficient of variation (CV) or ±S.D.
Results
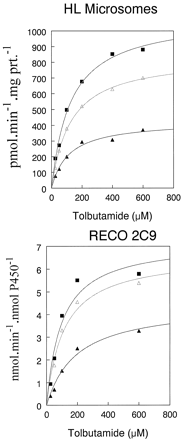
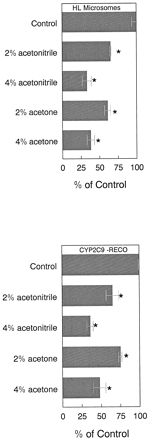
Acetone stimulated NADPH-dependent tolbutamide hydroxylation by 75 and 90% in HL microsomes relative to control values at 2 and 4% concentrations, respectively, without altering the concentration of tolbutamide in the reaction mixture (Tables1 and 2; Fig. 1). Most other organic solvents inhibited the reaction (Fig. 2). Similarly, acetonitrile stimulated tolbutamide hydroxylation by 78 and 87% relative to control values at 2 and 4% concentrations, respectively, also without altering the concentration of tolbutamide in the reaction mixture (Tables 1 and 2; Fig.3). The stimulation of tolbutamide hydroxylation by acetone and acetonitrile was not unique to HL microsomes because this effect also can be observed in a reconstituted CYP2C9 system over a wide range of tolbutamide concentrations (Figs. 1and 3).
Effect of acetonitrile and carbonyl-containing solvents on HL microsomal tolbutamide hydroxylation
Concentration of tolbutamide in reaction mixture in presence of 0, 2, and 4% acetone/acetonitrile
Stimulation of tolbutamide hydroxylation in HL microsomes and reconstituted CYP2C9 system by acetone.
Data are means of three separate determinations. ▴, 0% acetone; ▵, 2% acetone; and ▪, 4% acetone.
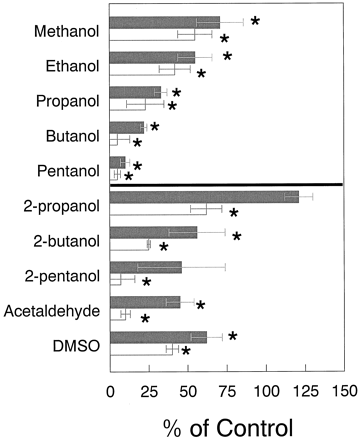
Inhibition of tolbutamide hydroxylation as a function of carbon chain length.
Data are means ± S.D. of three separate determinations. ∗, significantly different from controls with Student's ttest (P < .05). Tolbutamide concentration was 100 μM. Closed and open bars represent 1 and 2% solvent concentrations, respectively.
Stimulation of tolbutamide hydroxylation in HL microsomes and reconstituted CYP2C9 system by acetonitrile.
Data are means of three separate determinations. ▴, 0% acetonitrile; ▵, 2% acetonitrile; and ▪, 4% acetonitrile.
The clearance (CLmet =Vmax/Km) of tolbutamide to hydroxytolbutamide by HL microsomes increased by 51 and 83% over control values in the presence of 2 and 4% acetone, respectively (Table 3) when NADPH was used to stimulate the reaction. Corresponding increases in tolbutamide CLmet for the reconstituted CYP2C9 enzyme system closely paralleled that of the HL microsomal system in magnitude (Table3). This increase in tolbutamide CLmet by acetone, in both HL microsomes and the reconstituted CYP2C9 enzyme system could be primarily attributed to a consistent increase inVmax without a consistent change in the apparent Km for tolbutamide hydroxylation (Table 3).
Effect of acetone on tolbutamide hydroxylation in HL microsomes and in the CYP2C9-reconstituted system
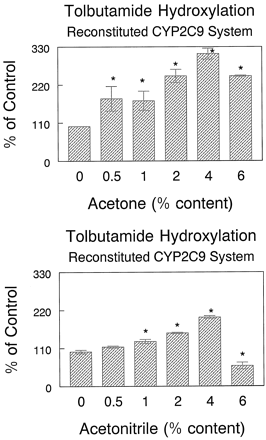
Tolbutamide CLmet by HL microsomes increased by 79 and 129% over control values in the presence of 2 and 4% acetonitrile, respectively (Table 4). The gain in tolbutamide CLmet in the reconstituted CYP2C9 enzyme system was 140 and 193% over control values at 2 and 4% acetonitrile concentrations, respectively (Table 4). Both acetone and acetonitrile at concentrations up to 4%, stimulated NADPH-dependent tolbutamide hydroxylation in the reconstituted CYP2C9 system (Fig.4). However, both acetonitrile and acetone inhibited cumene hydroperoxide-mediated tolbutamide hydroxylation in both HL microsomes and CYP2C9-reconstituted systems (Fig. 5).
Effect of acetonitrile on tolbutamide hydroxylation in HL microsomes and in the CYP2C9-reconstituted system
Effect of acetone and acetonitrile concentrations on tolbutamide hydroxylation in the CYP2C9-reconstituted system.
Data are means of three separate determinations. ∗, significantly different from control, with Student's t test,P < .05. Tolbutamide concentration was 100 μM.
Inhibition of tolbutamide hydroxylation by acetone and acetonitrile in a cumene hydroperoxide (100 μM)-dependent reaction.
Data are means of three separate determinations. ∗, significantly different from control, with Student's t test,P < .05. Tolbutamide concentration was 100 μM.
With the exception of 2-propanol, 2-butanone, and 3-pentanone, other organic solvents tested, including methanol, ethanol, 1-propanol, 1-butanol, 2-butanol, 1-pentanol, 2-pentanol, acetaldehyde, and dimethyl sulfoxide significantly inhibited HL microsomal tolbutamide hydroxylation at 1 and 2% final concentrations (Table 1 and Fig. 2). Interestingly, tolbutamide hydroxylation decreased by increasing the number of carbon atoms in the alcohol series, with pentanol inhibiting the most and methanol inhibiting the least (Fig. 2). Secondary alcohols were less potent inhibitors of tolbutamide hydroxylation than their corresponding primary analogs (Fig. 2). 2-Propanol, 2-butanone, and 2-pentanone showed low stimulatory effects on tolbutamide hydroxylation at the lower solvent concentrations (statistically insignificant); however, the three solvents were inhibitors at higher concentrations (Table 1 and Fig. 2).
Discussion
The stimulatory effects of acetone on aniline (Anders, 1968) and alprazolam (Wolff et al., 1989) hydroxylation reactions have been previously documented. Aniline hydroxylation in rat liver microsomes is primarily mediated by CYP2E1 and CYP1A2 (Labella and Queen, 1995) and to a lesser extent by CYP2B1 and CYP2C11 (Guengerich et al., 1982;Favreau et al., 1987; Ryan and Levin, 1990; Labella and Queen, 1995). Alprozolam hydroxylation is mediated by CYP3A4 (Monette et al., 1996).
Previously, both acetone and acetonitrile have been reported to slightly stimulate NADPH-dependent CYP2C9 reactions (Chauret et al., 1998; Hickman et al., 1998; Busby et al., 1999). We have confirmed those original findings and report that both acetone and acetonitrile stimulate tolbutamide hydroxylation, a reaction mediated primarily by CYP2C9 (Miners et al., 1988; Relling et al., 1989; Hall et al., 1994). However, the extent of stimulation of tolbutamide hydroxylation in our reaction system is greater than that previously observed by other investigators. Although other investigators have seen up to a 45% increase in the reaction velocity, we have seen nearly a doubling of the reaction velocity in the presence of acetone or acetonitrile in HL microsomal incubation at 2 and 4% solvent concentrations. The reason for the discrepancy could be due to the differences in buffer composition, buffer strength, microsomal protein concentrations, length of incubation, or the presence cosolvents in the incubation mixture used by different investigators.
Our studies show that the mechanism of this stimulation appears not to be due to a consistent enhancement of the affinity (Km) of tolbutamide for HL CYP2C9 by either solvent. A similar lack of a concentration-dependent change by acetone on the Km for alprazolam hydroxylation has been previously documented, despite an overall increase inVmax (Wolff et al., 1989). In the instance of aniline hydroxylation, however, there was a concentration-dependent increase in both Km andVmax (Anders, 1968). Thus, it appears that acetone can enhance a variety of CYP-mediated reactions perhaps by slightly different mechanism(s).
Further insights to the understanding of the mechanism of stimulation were obtained in the CYP2C9-reconstituted system. Both acetone and acetonitrile increased tolbutamide hydroxylation in the CYP2C9-reconstituted system in a concentration-dependent manner (Fig.4). The stimulation of tolbutamide hydroxylation occurred across all concentrations of tolbutamide (Figs. 1 and 3). Similar to the HL microsomal system, the Vmax of tolbutamide hydroxylation consistently increased without consistent changes in theKm (Tables 3 and 4).
This stimulation of tolbutamide hydroxylation in the reconstituted system that is devoid of other CYP isoforms or other microsomal proteins, with the exception of cytochromeb5 and NADPH-CYP reductase, suggests that acetone and acetonitrile might be acting directly on CYP2C9 and/or cytochrome b5 and/or NADPH-CYP reductase to facilitate this effect. Alternatively, acetone and acetonitrile could be enhancing the fluidity of the membrane components and facilitate some sort of a protein/protein type interaction between CYP2C9 with CYP reductase and/or cytochrome b5. This explanation is consistent with the fact that other carbonyl-containing solvents with longer chain length (2-butanone and 3-pentanone) showed some enhancement (although not statistically significant) of tolbutamide hydroxylation.
When cumene hydroperoxide was used instead of NADPH, thereby bypassing NADPH-CYP reductase (Crespi and Miller, 1997), both acetone and acetonitrile inhibited tolbutamide hydroxylation. This finding suggests that both solvents enhance CYP2C9 activity via the interaction with the NADPH-CYP reductase. The solvents could be enhancing the flow of electrons from the reductase to the CYP, by altering membrane fluidity and/or facilitating an interaction between the two proteins. This also appears to be consistent with the fact that both solvents do not appear to directly modify the affinity of the CYP2C9 for tolbutamide as evidenced by the lack of effect on the Km(Tables 3 and 4).
Maximum stimulation of tolbutamide hydroxylation (Vmax) in the reconstituted CYP2C9 system was achieved at an acetone concentration of 4%. The amplitude of the stimulation of the Vmax in reconstituted CYP2C9 was greater than in HL microsomes at 4% acetone concentration (Table 3). This difference could be due to optimization of the environment required for CYP2C9 activity in the reconstituted CYP2C9 system. Alternatively, in the case of acetone, it could be that a product of acetone metabolism by CYP2E1 (Johansson et al., 1988) in HL microsomes might have inhibited tolbutamide hydroxylation. This also suggests that acetone and not its metabolite is directly capable of stimulating CYP2C9 reaction.
All of the other solvents tested with the exception of 2-propanol, 2-butanone, and 3-pentanone inhibited HL microsomal tolbutamide hydroxylase activity at 1 and 2% concentrations. This inhibition phenomenon is consistent with the literature describing the inhibitory effects of organic solvents on CYP reactions (Wolff et al., 1989;Chauret et al., 1998). In the alcohol series, the inhibition potency increased as the chain length increased. This effect is more pronounced if the molar concentration of the alcohol is considered rather than the v/v percentage of solvent in the reaction mixture (1% methanol = 247 mM; 1% pentanol = 92.5 mM). In addition, secondary alcohols are much less potent inhibitors than their primary counterparts. In fact, 2-propanol showed some stimulatory effect at 1% concentration (∼20% stimulation), whereas propanol caused >50% inhibition of tolbutamide hydroxylation at the same concentration in HL microsomes (Fig. 2).
In an attempt to explain the anesthetic effects of alcohols, it has been hypothesized that at nerve-blocking concentrations, alcohols occupy membrane space, thus resulting in expanding biological membrane and increasing membrane fluidity that leads to physical disordering of membrane structure (Seeman et al., 1969; Seeman 1974). Using electron paramagnetic resonance technique, Lyon et al. (1981) have shown that the disordering potency of a series of alcohols on neuronal membrane increased logarithmically with the increase in the number of methylene groups (hydrophobicity). Also, the membrane disordering potencies by structural isomers were in the order of straight chain primary > iso-primary > secondary > tertiary, again consistent with hydrophobicity, which is primarily a reflection of the ability of an alcohol to partition into the membrane bilayer (Lyon et al., 1981). In a more recent study, McKarns et al. (1997) have reported that increasing the number of carbon atoms in primary alcohols increased the extent of damage of cellular membrane as measured by lactic dehydrogenase leakage from rat liver cells.
The present study demonstrates that the inhibitory potency of a series of primary alcohols on CYP2C9 increases with increased carbon chain length, and primary alcohols are more potent inhibitors than their secondary counterparts, consistent with the increase in hydrophobicity, and concordant with the data of Lyon et al. (1981) andMcKarns et al. (1997). These results suggest that the inhibitory effects of alcohols (and probably other solvents) on the activity of CYP2C9 are due to biophysical processes on the microsomal membrane that affect its structure/fluidity and therefore the interactions between CYP2C9, NADPH-CYP reductase and cytochromeb5.
In summary, the results of the present study show that acetone and acetonitrile stimulate the hydroxylation of tolbutamide in both HL microsomes and CYP2C9-reconstituted system. This stimulatory effect appears to involve the NADPH-CYP reductase and not directly with CYP2C9. Most other organic solvents tested inhibit tolbutamide hydroxylation especially at the higher concentrations, probably by biophysical disruption of the microsomal membrane.
Acknowledgments
We thank Drs. Ronald White and Mitchell N. Cayen for their support and for reviewing the manuscript.
Footnotes
-
Send reprint requests to: Amin A. Nomeir, Ph.D., Department of Drug Metabolism and Pharmacokinetics, K-15-2, Mail Stop 2880, Schering-Plough Research Institute, 2015 Galloping Hill Rd., Kenilworth, NJ 07033. E-mail: Amin.Nomeir{at}spcorp.com
- Abbreviations used are::
- CYP
- cytochrome P-450
- HL
- human liver
- CV
- coefficient of variation
- Received June 18, 1999.
- Accepted September 29, 1999.
- The American Society for Pharmacology and Experimental Therapeutics